Abstract
The preclinical studies on circRNA are the most popular topic these days, including many latest reports about diabetic retinophathy (DR) for both treatment and diagnosis. This review aims to investigate the treatment effects of circRNAs on DR, its potential mechanism and cell targeting in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, and finally discover the optimal targeting sequence of circRNAs for further clinical application. A literature search for all potential reference articles published online until 2021 was conducted in electronic databases of Pubmed, ScienceDirect and China National Knowledge Infrastructure. This systematic review has given rise to many impressive findings of circRNAs on the treatment of DR by regulating the proliferation, migration, tube formation, apoptosis, and inflammatory response of various cells in the retina. The functional exploration of circRNAs in DR will provide new ideas or target molecules for the prevention and control of DR development. According to their effects on rodent DR model, circZNF532 and circPWWP2A were the most hopeful targets with major effects on pericytes, followed with circZNF609 and circHIPK3. Many more circRNAs would be prospectively promised to optimize the clinical outcomes of circRNAs in DR.
ABBREVIATIONS: DM: diabetes mellitus; DR: diabetic retinopathy; HRVECs: human retinal vascular endothelial cells; IL-6: interleukin 6; IL-1β: interleukin 1β; TNF-α: tumor necrosis factor α; VECs: human retinal vascular endothelial cells
Introduction
Diabetic retinopathy
Vascular disorders are the major diabetic complications induced by hyperglycemic stress of diabetes mellitus (DM). Diabetic retinopathy (DR) is one of the common microvascular complications among DM people in the late phase, and may finally cause visual damage and blindness (Rask-Madsen and King Citation2013). Hyperglycemic stress can induce excessive proliferation of vascular endothelial cells (VECs) and unordered angiogenesis, which may be associated with vascular exudation and macula edema (Duh et al. Citation2017). Besides, many other factors such as oxidative stress, inflammatory cytokines, apoptosis, and dysfunction of pericytes are also involved in the underlying vascular dysfunction in DR (Dehdashtian et al. Citation2018; Rubsam et al. Citation2018). The dysfunctions of both pericytes and VECs are the leading pathologic feature of DR at the initial stage. Meanwhile, the unbalanced pericyte-VEC crosstalk also induces the out-of-order situation of the retinal microvasculature system in DR (Armulik et al. Citation2005; Hammes Citation2005).
Features of circRNA and its differential expression in DR
circRNA is produced by special alternative splicing with a closed circular structure. The main features of circRNA are differential from common linear RNAs due to its circularization without 5′ 7-methylguanosine (m7G) cap and 3′ poly(A) tail, which is more stable and not degraded by exonuclease easily. circRNA is commonly conserved in nucleotide sequences across species. Although most circRNAs are noncoding, they can still regulate gene expression and epigenetic modification at the transcriptional and post-transcriptional levels by acting as the sponge of miRNAs or proteins (Hansen et al. Citation2013; Chen Citation2020).
A series of studies have demonstrated the altered expression profiles of circRNAs in serum samples and the vitreous humor of DR patients, which is the fundamental evidence for the involvement of circRNAs in DR (Gu et al. Citation2017; He et al. Citation2020). Many findings suggest that circRNAs play an extremely significant role in the progress of DR and other diabetic complications by regulating the angiogenesis, proliferation, apoptosis, and inflammatory response of various cells in the retina (Boeckel et al. Citation2015; Memczak et al. Citation2015; Cheng et al. Citation2019). It was claimed that downregulation or knockdown of hsa_circ_0068087 induced by hyperglycemia stress reversed the dysfunction of vein endothelial cells and inflammation by ameliorating the TLR4/NF-kappaB/NLRP3 inflammasome signaling pathway and sponging miR-197 (Cheng et al. Citation2019). Hsa_circ_0047814 was a kind of cricRNA only found in pericytes but not in human retinal vascular endothelial cells (HRVECs), the expression of which was also enhanced under hyperglycemia stress. It was reported that hsa_circ_0047814 had resembled regulation on pericytic functions through the CSPG4/LOXL2/CDK2 signaling pathways acting as miR-29a-3p sponge (Jiang et al. Citation2020). Thus exploring the role of circRNAs in DR will provide new ideas for its potential application as the target molecules in the treatment of DR.
The association between differential expression of circRNA and retinal microvascular dysfunction of DR
In most current literature, the effects of many circRNAs in DR-induced retinal microvascular dysfunction were mainly focused on their roles of miRNA sponge.
Endothelial angiogenesis and microvascular leakage: The expression of circHIPK3 was distinctly upregulated in HRVECs under hyperglycemia conditions. circHIPK3 could sponge endogenous miR-30a-3p to promote the cell viability, proliferation and tube formation of HRVECs by inducing the overexpression of endothelial proliferation dysfunction-related genes, which resulted in the increment of microvascular leakage and acellular capillary, and final exacerbation of retinal microvascular dysfunction (Shan et al. Citation2017). Besides, the upregulation of cPWWP2A under diabetic stress can alleviate functional damage of retinal microcirculation in DR and obviously improve visual function (Liu et al. Citation2019). The expression of circDNMT3B was declined in HRMECs under hyperglycemia stress. Conversely, the upregulated expression of its target miRNA miR-20b-5p could positively stimulate capillary growth and endothelial angiogenesis (Zhu et al. Citation2019).
Inflammation and apoptosis: It was affirmed that the downward modulation of hsa_circ_0068087 in hyperglycosemia-incubated HRVECs attenuated TLR4/NF-κB/NLRP3 inflammasome-mediated inflammation and subsequently endothelial dysfunction by acting as a miR-197 sponge (Cheng et al. Citation2019). As another upregulated non-coding RNA in hyperglycosemia-incubated HRVECs, the silencing of has_circ_0010729 could inhibit proliferation and boost apoptosis concurrently (Dang et al. Citation2017). Nevertheless, the overexpression of circHIPK3 could inhibit hyperglycosemia-induced endothelial injury and cell apoptosis (Cao et al. Citation2018). As stated above, the roles of several circRNAs were completely different in the progress of retinal microvascular dysfunction of DR. Hence, it is necessary to summarize the variable functions of circRNAs in DR in this systematic review. Even though many evidences proved the hypothesis that circRNAs participated in the pathological progress of DR, the specific signaling pathway was still uncertain, with the conflicts that a certain number of circRNAs might play critical roles in those disorders, and that the potential risk of circRNA interfere did not be evaluated currently. For healthy people, these circRNA levels may affect their susceptibility, which can be classified as susceptible or insusceptible.
Systematic review on the studies of circRNA against DR
This review mainly summarized the modulation and related mechanism of circRNAs on several DR-associated cells, which were the critical factors of retinal microvascular dysfunction in DR. This review included almost all preclinical studies on circRNAs against DR in English. Cellular studies were about the effects of circRNAs on cytobiological functions of several DR-associated cells against DR in vitro. Animal studies focused on the effects of circRNAs on acellular capillary and microvascular leakage in DR animal models. All data of circRNAs were qualified and compared with each other to identify their functional difference in DR. This systematic review has given rise to many impressive findings of circRNAs on the treatment of DR, and finally, discover the optimal targeting sequence of circRNAs for further clinical application.
Methods
Restriction of screening
The strict literature search was based on three mainstream electronic databases (PubMed, ScienceDirect, and China National Knowledge Infrastructure) in the date range of 1990–2021. The keywords were composed of ‘diabetic retinopathy’ and (‘circRNA’ or ‘circular RNA’). The scope of the search setting was based on full text without other special limitations.
Brief introduction of screening procedure in the flowchart
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline as shown in the designed flowchart (Figure ). The titles or abstracts were checked in the initial screen and full texts were read subsequently. The eligible articles would be included according to the following restrictions: (1) the full text was in English; (2) The target molecule was circRNA; (3) The in vitro or in vivo model must be related to DR; and (4) The study design must contain circRNA, DR, and vascular dysfunction as a whole. According to these criteria, 12 cell studies and 5 animal studies were included conditionally. These preclinical studies were systematically reviewed and critically analyzed, whilst meta-analysis was not performed due to the limitation of eligible studies.
Results
Effects of circRNAs on HRVECs
A total of 7 studies reported effects of these circRNAs on human retinal vascular endothelial cells under hyperglycemic stress, and 85.7% (6 out of 7) circRNAs (hsa_circ_0005015, circHIPK3, circZNF609, hsa_circ_0001879, hsa_circ_0002570 and circCOL1A2) were considerably increased in both hyperglycemic medium in vitro and clinical samples in DR patients compared to nondiabetic control (Table ). Only circDNMT3B exhibited the pronounced downregulation in DR patients. Besides, 71.4% (5 out of 7) of those circRNAs (hsa_circ_0005015, circHIPK3, hsa_circ_0001879, hsa_circ_0002570 and circCOL1A2) positively regulated the functions of HRVECs, whilst only circDNMT3B and circZNF609 negatively inhibited their abilities.
Table 1. Effects of circRNAs on cell activities in cellular level.
For cell proliferation of HRVECs, the effects of silencing 5 positive and 2 negative regulator circRNAs presented with obvious differences, including the top 3 circZNF609, hsa_circ_0005015 and circHIPK3 with changes above 80%, and the rest with changes in the range of 15–20%.
For migration and tube formation of HRVECs, the effects of silencing 5 positive and 2 negative regulator circRNAs presented with obvious differences, including the top 3 circZNF609, hsa_circ_0005015 and circHIPK3 with changes above 50–70%, and the rest with changes in the range of 20–50%.
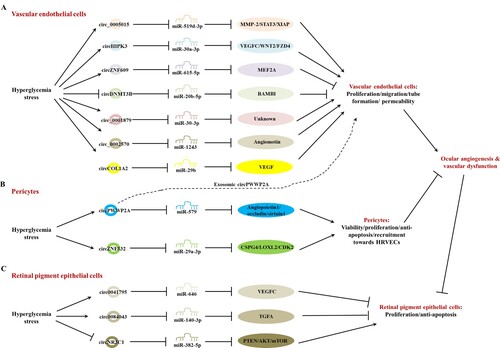
All data mentioned above were relatively in accordance with the effect of each circRNA on HRVECs ability. Meanwhile, most of their mechanisms seemed distinct and the related information were also studied and presented in Figure .
Effects of circRNAs on human retinal pericytes
In all available studies screened from databases, only cPWWP2A and cZNF532 were verified as potential targets for the treatment of pericytic dysfunction and the related retinal disorder DR (Liu et al. Citation2019; Jiang et al. Citation2020), since these two cricRNAs were only found in pericytes but not HRVECs, with enhanced expressions under hyperglycemia-related stresses and in clinical DR samples (Table ). The upregulated expressions of cPWWP2A and cZNF532 were valid to promote the viability, proliferation, and anti-apoptosis of pericytes, which was approved to antagonize the DR-related vascular leakage and retinal microvascular dysfunction (Figure ) (Hu et al. Citation2013; Liu et al. Citation2014; Sohn et al. Citation2016).
In pericytes, cPWWP2A could make miR-579 gathered to sequester its binding sites with mRNA of angiopoietin1, occludin, and sirtuin1, which led to their raised expression and the increment of cell viability, proliferation, inhibited cell apoptosis, and finally alleviated retinal microvascular permeability by targeting pericyte-EC crosstalk (Liu et al. Citation2019). Regulating the cPWWP2A level had no effect on HRVECs directly, but modulated endothelial angiogenesis through the paracrine of exosomes from pericytes.
cZNF532 had resemblance to regulation on pericytic functions based on another specific network of cZNF532-miR-29a-3p-CSPG4/LOXL2/CDK2. cPWWP2A, cZNF532 could directly regulate endothelial angiogenesis by recruitment toward HRVECs (Jiang et al. Citation2020).
Effects of circRNAs on human retinal pigment epithelial cells (HRPECs)
More importantly, three latest reports introduced the modulation of circRNAs on human retinal pigment epithelial cells directly (Table ) (Chen et al. Citation2020; Li et al. Citation2020; Sun and Kang Citation2020). For DR patients, the apoptosis of retinal pigment epithelial cells and the impairment of visual function are the final outcomes of ocular angiogenesis and microvascular dysfunction of DR (Ibrahim et al. Citation2015; Zeng et al. Citation2019). Two circRNAs (hsa_circ_0041795 and hsa_circ_0084043) negatively regulated the proliferation and anti-apoptosis of HRPECs, whilst only circNR3C1 positively promoted cellular functions. Both hsa_circ_0041795 and hsa_circ_0084043 were considerably increased in both in vitro studies and samples from DR patients compared to nondiabetic control (Figure ). Only circNR3C1 exhibited pronounced downregulation in DR patients.
In the aspects of proliferation and anti-apoptosis in HRPECs, silencing hsa_circ_0084043 could enhance proliferation and inhibit apoptosis by 73.6% and 63.9%, respectively, compared to model control against high glucose insult by acting as a miR-140-3p sponge (Li et al. Citation2020). Besides, oxidative stress could increase the permeability of HRVECs and attribute to retinal edema and inflammation, which is the hallmark of DR progression. Knockdown of hsa_circ_0084043 resulted in the downregulation of oxidative stress including the reduction of malondialdehyde and enhancement of superoxide dismutase and glutathione peroxidase, and the repression of inflammatory cytokines, such as tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), and COX-2 in hyperglycemia-induced ARPE-19 cells.
The second one with subordinate effect was hsa_circ_0041795, knockdown of which remarkably enhanced proliferation and inhibited apoptosis by 38.9% and 41.5% respectively compared to model control by acting as a miR-646 sponge (Sun and Kang Citation2020). Silencing hsa_circ_0041795 significantly repressed inflammatory cytokines, such as TNF-α, interleukin 1β (IL-1β) and IL-6 in hyperglycemia-induced ARPE-19 cells.
The last one circNR3C1 positively promoted cellular proliferation but had no effect on anti-apoptosis of HRPECs. Silencing circNR3C1 could enhance proliferation by 50.0% compared to model control against high glucose insult by acting as a miR-382-5p sponge (Chen et al. Citation2020).
Effects of circRNAs in a rodent model of DR
A total of 5 studies reported effects of these circRNAs in a rodent model of DR, and 80.0% (4 out of 5) circRNAs (circHIPK3, circZNF609, circPWWP2A, and circZNF532) were considerably increased in both hyperglycemic medium in vitro and clinical samples in DR patients compared to nondiabetic control (Table ). Only circDNMT3B exhibited pronounced downregulation in DR patients. Besides, 60.0% (3 out of 5) of those circRNAs (circHIPK3, circZNF609 and circDNMT3B) positively regulated retinal microvascular dysfunction of DR, whilst only circPWWP2A and circZNF532 negatively inhibited their abilities.
Table 2. Effects of circRNAs on angiogenesis and endothelial leakage in rodent model of DR.
Considering the effects of most of the circRNAs on acellular capillaries number were relatively similar without statistical significance, only retinal vascular leakage was evaluated by Evans blue assays. For Evans blue infusion in a rodent model of DR, the effects of silencing circRNAs presented with obvious differences, in the rank of circZNF532 > circPWWP2A > circZNF609 > circHIPK3, whilst that of circDNMT3B was not reported in the study.
Of all five studies including both cellular studies and a rodent model of DR, the results of silencing circZNF609 on HRVECs and rodent DR model were self-contradictory, as the significant stimulation of HRVECs on proliferation, migration, and tube formation was accompanied by great alleviation on DR-induced retinal vascular dysfunction and inflammatory response (Liu et al. Citation2017). It was inconsistent with the common theory that the over-stimulation of endothelial proliferation and tube formation would aggravate the increment of microvascular leakage and acellular capillary, and final exacerbation of retinal microvascular dysfunction.
Discussion
This systematic review introduced the effects and potential mechanisms of the latest circRNAs in DR based on preclinical studies for the first time, including total of 12 cellular reports and 5 animal studies. According to all the outcomes, it could be summarized that under the hyperglycemia stress, the modification of circRNAs could promote multifunctions of vascular endothelial cells (proliferation, migration, tube formation, permeability, etc.) and pericytes (viability, proliferation, recruitment towards HRVECs), but prohibit proliferation or anti-apoptosis of retinal pigment epithelial cells.
This evidence-based review supported the point that circRNAs indeed play an important role in the progress of retinal microvascular dysfunction at least partly by acting as a miRNA sponge. Literature about the relationship between oxidative stress and miRNA in the carcinogenesis model was summed up and indicated that miRNA was involved in oxidative stress signaling pathways, and could not only be induced by reactive oxygen species (hydrogen peroxide, NO, superoxide, etc.) but also adjust the reactive oxygen species activation in carcinogenesis (Akbari et al. Citation2020). The functions and regulations of circRNAs can be summarized as (1) stimulating or inhibiting the biological functions of endothelial cells, (2) promoting the viability, proliferation, and anti-apoptosis of retinal pericytes, (3) promoting or depressing the proliferation and anti-apoptosis of retinal pigment epithelial cells, and (4) regulating the expression of inflammatory cytokines and oxidant stress. Despite the current studies on the potential mechanism of circRNAs in DR being superficial with some controversial results and only based on their role as a miRNA sponge, many impressive findings on the effect of circRNAs in DR gave rise to the attention of many scientists in whole biomedical field, which indicated that circRNA was crucial in the biopathological progression of DR by regulating the proliferation, migration, tube formation, apoptosis, and inflammatory response of various cells in the retina. The functional exploration of circRNAs in DR will provide new ideas or target molecules for the prevention and control of DR development.
Many limitations are necessary to be explained. First of all, the quality of study design or original data in all listed studies was not evaluated or discussed in detail, even though the conclusions of a few studies were contradictory. Hence the primary results of those circRNAs need to be verified by many other scientists. Secondly, the mechanisms of circRNAs were too complicated to be studied in all aspects, whilst the current studies were only based on their effects as a miRNA sponge. Next, another limitation was that there was no PROSPRO registration code in this study, thus the methodology might not be followed with the protocol of PROSPRO strictly. Lastly, the potential safety of circRNA interference also should be warranted and interpreted in further studies. The selection criteria using the ‘PICO’ (Population, Intervention, Comparison, Outcomes) present flaws mainly in relation to population and comparison. In this review, the population (cell type or the experimental rodents) and comparisons were identical, but the experimental outcomes might be impacted by the cell-culture medium or differentiated treatment strategies. Besides, the sample size was small and hence the supporting evidence might be affected by many occasions, which must be claimed as the limitation of those in vitro and in vivo studies.
In total, the results of both in vitro and in vivo reports supported circRNAs as a candidate target for DR treatment. According to their effects on the rodent DR model, circZNF532 and circPWWP2A were the most hopeful targets with major effects on pericytes, followed by circZNF609 and circHIPK3. Various signal pathways were involved in the beneficial effects of circRNAs in DR. Many more circRNAs look promising and encouraging to optimize the clinical outcomes of circRNAs in DR.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Akbari A, Majd HM, Rahnama R, Heshmati J, Morvaridzadeh M, Agah S, Amini SM, Masoodi M. 2020. Cross-talk between oxidative stress signaling and microRNA regulatory systems in carcinogenesis: focused on gastrointestinal cancers. Biomed Pharmacother. 131:110729.
- Armulik A, Abramsson A, Betsholtz C. 2005. Endothelial/pericyte interactions. Circ Res. 97:512–523.
- Boeckel JN, Jae N, Heumuller AW, Chen W, Boon RA, Stellos K, Zeiher AM, John D, Uchida S, Dimmeler S. 2015. Identification and characterization of hypoxia-regulated endothelial circular RNA. Circ Res. 117:884–890.
- Cao Y, Yuan G, Zhang Y, Lu R. 2018. High glucose-induced circHIPK3 downregulation mediates endothelial cell injury. Biochem Biophys Res Commun. 507:362–368.
- Chen LL. 2020. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol. 21:475–490.
- Chen X, Jiang C, Sun R, Yang D, Liu Q. 2020. Circular noncoding RNA NR3C1 acts as a miR-382-5p sponge to protect RPE functions via regulating PTEN/AKT/mTOR signaling pathway. Mol Ther. 28:929–945.
- Cheng J, Liu Q, Hu N, Zheng F, Zhang X, Ni Y, Liu J. 2019. Downregulation of hsa_circ_0068087 ameliorates TLR4/NF-kappaB/NLRP3 inflammasome-mediated inflammation and endothelial cell dysfunction in high glucose conditioned by sponging miR-197. Gene. 709:1–7.
- Dang RY, Liu FL, Li Y. 2017. Circular RNA hsa_circ_0010729 regulates vascular endothelial cell proliferation and apoptosis by targeting the miR-186/HIF-1alpha axis. Biochem Biophys Res Commun. 490:104–110.
- Dehdashtian E, Mehrzadi S, Yousefi B, Hosseinzadeh A, Reiter RJ, Safa M, Ghaznavi H, Naseripour M. 2018. Diabetic retinopathy pathogenesis and the ameliorating effects of melatonin; involvement of autophagy, inflammation and oxidative stress. Life Sci. 193:20–33.
- Duh EJ, Sun JK, Stitt AW. 2017. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. 2:e93751.
- Gu Y, Ke G, Wang L, Zhou E, Zhu K, Wei Y. 2017. Altered expression profile of circular RNAs in the serum of patients with diabetic retinopathy revealed by microarray. Ophthalmic Res. 58:176–184.
- Hammes HP. 2005. Pericytes and the pathogenesis of diabetic retinopathy. Horm Metab Res. 37(Suppl 1):39–43.
- Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. 2013. Natural RNA circles function as efficient microRNA sponges. Nature. 495:384–388.
- He M, Wang W, Yu H, Wang D, Cao D, Zeng Y, Wu Q, Zhong P, Cheng Z, Hu Y, Zhang L. 2020. Comparison of expression profiling of circular RNAs in vitreous humour between diabetic retinopathy and non-diabetes mellitus patients. Acta Diabetol. 57:479–489.
- Hu Y, Chen Y, Ding L, He X, Takahashi Y, Gao Y, Shen W, Cheng R, Chen Q, Qi X, et al. 2013. Pathogenic role of diabetes-induced PPAR-alpha down-regulation in microvascular dysfunction. Proc Natl Acad Sci USA. 110:15401–15406.
- Ibrahim AS, Tawfik AM, Hussein KA, Elshafey S, Markand S, Rizk N, Duh EJ, Smith SB, Al-Shabrawey M. 2015. Pigment epithelium-derived factor inhibits retinal microvascular dysfunction induced by 12/15-lipoxygenase-derived eicosanoids. Biochim Biophys Acta. 1851:290–298.
- Jiang Q, Liu C, Li CP, Xu SS, Yao MD, Ge HM, Sun YN, Li XM, Zhang SJ, Shan K, et al. 2020. Circular RNA-ZNF532 regulates diabetes-induced retinal pericyte degeneration and vascular dysfunction. J Clin Invest. 130:3833–3847.
- Li Y, Cheng T, Wan C, Cang Y. 2020. circRNA_0084043 contributes to the progression of diabetic retinopathy via sponging miR-140-3p and inducing TGFA gene expression in retinal pigment epithelial cells. Gene. 747:144653.
- Liu C, Ge HM, Liu BH, Dong R, Shan K, Chen X, Yao MD, Li XM, Yao J, Zhou RM, et al. 2019. Targeting pericyte-endothelial cell crosstalk by circular RNA-cPWWP2A inhibition aggravates diabetes-induced microvascular dysfunction. Proc Natl Acad Sci USA. 116:7455–7464.
- Liu C, Yao MD, Li CP, Shan K, Yang H, Wang JJ, Liu B, Li XM, Yao J, Jiang Q, Yan B. 2017. Silencing Of Circular RNA-ZNF609 ameliorates vascular endothelial dysfunction. Theranostics. 7:2863–2877.
- Liu G, Zhou S, Li X, Ding X, Tian M. 2020. Inhibition of hsa_circ_0002570 suppresses high-glucose-induced angiogenesis and inflammation in retinal microvascular endothelial cells through miR-1243/angiomotin axis. Cell Stress Chaperones. 25:767–777.
- Liu JY, Yao J, Li XM, Song YC, Wang XQ, Li YJ, Yan B, Jiang Q. 2014. Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis. 5:e1506.
- Memczak S, Papavasileiou P, Peters O, Rajewsky N. 2015. Identification and characterization of circular RNAs as a new class of putative biomarkers in human blood. PLoS One. 10:e0141214.
- Rask-Madsen C, King GL. 2013. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab. 17:20–33.
- Rubsam A, Parikh S, Fort PE. 2018. Role of inflammation in diabetic retinopathy. Int J Mol Sci. 19:942.
- Shan K, Liu C, Liu BH, Chen X, Dong R, Liu X, Zhang YY, Liu B, Zhang SJ, Wang JJ, et al. 2017. Circular noncoding RNA HIPK3 mediates retinal vascular dysfunction in diabetes mellitus. Circulation. 136:1629–1642.
- Sohn EH, van Dijk HW, Jiao C, Kok PH, Jeong W, Demirkaya N, Garmager A, Wit F, Kucukevcilioglu M, van Velthoven ME, et al. 2016. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci USA. 113:E2655–E2664.
- Sun H, Kang X. 2020. Hsa_circ_0041795 contributes to human retinal pigment epithelial cells (ARPE 19) injury induced by high glucose via sponging miR-646 and activating VEGFC. Gene. 747:144654.
- Zeng Q, Liu J. 2020. Silencing circ_0001879 inhibits the proliferation and migration of human retinal microvascular endothelial cells under high-glucose conditions via modulating miR-30-3p. Gene. 760:144992.
- Zeng Y, Cao D, Yu H, Yang D, Zhuang X, Hu Y, Li J, Yang J, Wu Q, Liu B, Zhang L. 2019. Early retinal neurovascular impairment in patients with diabetes without clinically detectable retinopathy. Br J Ophthalmol. 103:1747–1752.
- Zhang SJ, Chen X, Li CP, Li XM, Liu C, Liu BH, Shan K, Jiang Q, Zhao C, Yan B. 2017. Identification and Characterization of Circular RNAs as a New Class of Putative Biomarkers in Diabetes Retinopathy. Invest Ophthalmol Vis Sci. 58:6500–6509.
- Zhu K, Hu X, Chen H, Li F, Yin N, Liu AL, Shan K, Qin YW, Huang X, Chang Q, et al. 2019. Downregulation of circRNA DMNT3B contributes to diabetic retinal vascular dysfunction through targeting miR-20b-5p and BAMBI. EBioMedicine. 49:341–353.
- Zou J, Liu KC, Wang WP, Xu Y. 2020. Circular RNA COL1A2 promotes angiogenesis via regulating miR-29b/VEGF axis in diabetic retinopathy. Life Sci. 256:117888.