Abstract
Because boar semen is highly susceptible to lipid peroxidation process, sperm capacitation and early acrosomal reaction is important the employment of antioxidant agents for seminal preservation, which is why in the present investigation the evaluation of natural extracts of Cymbopogon citratus and Hypericum perforatum is proposed as a new alternative to maintain the spermatozoa viability up to 70% for 72 h. For those reasons, three different concentrations (0.250, 0.125 and 0.0625 µl) of each extract and BTS were mixed with 5 ml of fresh boar semen. The extracts were characterized by DPPH*, total phenolics and HPLC for the determination of antioxidants activity and total sugars. Seminal motility was analyzed by optical microscopy and viability using a Seminal Quality System and the morphology by a specific staining kit. The results obtained of viability were compared with a short-term extender (BTS). For Cymbopogon citratus, 73% vs. 76% and, for Hypericum perforatum 73% vs. 85%, in a period of 3 days. By using these natural extracts, it was possible to maintain sperm viability above 70% for 72 h of storage.
Introduction
The development of new assisted reproduction techniques has evolved throughout the years, being one of these, artificial insemination (AI). Between the principal advantages of this technique are the genetic selectivity and the improvement of the fertilization rate in animals, having then as benefit the current supply of per capita consumption.
This methodology was first implemented in cows by Milanov in 1938, using specific bull semen diluting fluids to maintain the seminal viability through long periods (Foote Citation2002). However, a low seminal viability and consequentially, a low in the fertilization rate and prolificacy were shown when applied in other species. It was until the middle of the twentieth century that said the technique was applied in the swine area with the development of specific commercial extenders for boar semen, amongst where we found the Betsville Thawing Solution (BTS), including sugars as source of energy, buffers for pH regulation, electrolytes for osmotic pressure control and antibiotics, thus achieving the preservation and storage of boar semen for a period of 3–7 days at a temperature between 15°C and 17 °C (Rodríguez-Gil and Estrada Citation2013).
Nevertheless, damage to the sperm membrane has been observed due to two major problems, the first, due to poor manipulation from sample collection to its preservation, and the second, due to the low amount of antioxidant agents present in commercial extenders, which can lead to the death of sperms and, from the degradation of its structure. These problems can generate free radicals causing an accumulation of reactive oxygen species (ROS), which are responsible for damage to the structural and functional integrity, affecting proteins and lipids due to the oxidation of their sulfhydric groups, DNA breakdown and consequently, an accelerating process of cellular apoptosis. Due to the high percentage of nitric oxide generating the cascade in the mitochondria being in charge of the cellular respiration process (Bathgate Citation2011; Tsantarliotou and Sapanidoi Citation2018).
According to the report by Valdebenito (Citation2007), the use of caffeine in Rainbow trout water (Oncorhynchus mykiss) increased sperm motility as well as fertility rate (Azam et al. Citation2003). The use of magnesium fumarate as an antioxidant in boar semen doses increased sperm viability, besides, the addition of polyunsaturated fatty acids in their food improved its characteristics (Szczęśniak-Fabiańczyk et al. Citation2003). Therefore, it has been investigated the incorporation of antioxidant agents to the seminal doses is a new alternative to preserve the spermatozoids for an extended time, decreasing the ROS offering bigger protection to the sperm membrane. As Desroches et al. (Citation2005) reports, the use of Lowbush blueberries (Vaccinium angustifolium Aiton) resulted positive as a retardant of early sperm capacitation for approximately a week.
In the present investigation, it proposed the use of natural antioxidants from Cymbopogon citratus (Cc), composed of antioxidant agents such as vitamin C, myrcene, limonene and geraniol; and Hypericum perforatum (Hp), which contains antioxidant flavonoids such as quercetin, glycosidic kaempferols and bi-flavonoids, phenolic acids and isomeric caffeoylquinic acids. In addition, their chelating activity to divalent ions has been reported for both extracts, preventing those metals from participating in the initialization of lipid peroxidation and oxidative stress through a metal-catalyzed reaction, protecting the sperm membrane, reducing cell apoptosis, as well as sperm abnormalities (Benedıí et al. Citation2004; Orčić et al. Citation2011; Balakrishnan et al. Citation2014).
Considering that boar sperms are highly susceptible to lipidic peroxidation and early capacitation, the use of antioxidant agents must decrease the production rate of free radicals, and cellular apoptosis, achieving a better sperm viability, morphology and motility within the diluting media. The methodology proposed would provide great benefits to the seminal conservation for periods of approximately 3 days, and due to the easy accessibility of these herbs, for Cymbopogon citratus, although it is naturally grown in tropical climates we chose a commercial brand of tea; and for Hypericum perforatum from Europe it is currently cultivated worldwide for its multiple benefits, ensuring the accessibility of both plants for this work. This could become a practical alternative to the actual short-term extenders, for its later use in AI techniques (Szczęśniak-Fabiańczyk et al. Citation2003).
Materials and methods
Synthesis of the samples
Natural extracts
For the natural extract’s preparation, we used an infusion of Lagg’s® Cymbopogon citratus and previously dehydrated Hypericum perforatum flowers. These extracts were prepared by steeping one Cymbopogon citratus infusion bag (1.5 g) and one Hypericum perforatum (1 g), in 60 mL of double distilled water at 50°C for 30 min, respectively. Then these extracts were left to stand at room temperature for approximately 1 h. Separately, a Zoitech Lab® short-term BTS solution in 1 l of double distilled water was prepared and kept in constant agitation for 30 min as a control sample.
Antioxidant activity by DPPH* (2,2-diphenyl-1-picrylhydrazyl hydrate) assay of natural extract solutions
The antioxidant activity of the samples was measured by preparing a solution of 180 µM of DPPH* in methanol, weighing 1.775 mg of DPPH* and diluting it in a 25-mL volumetric flask with methanol at 80%. As a reference, we used a synthetic antioxidant Trolox (6-hydroxy-2,5,7,8- tetramethylchroman-2-carboxylic acid), starting from a stock solution 1600 µM (which was prepared weighing 10 mg of Trolox and diluting it in 25 mL of methanol at 80%) we obtained a calibration curve. Then, parting from the stock solution, a series of solutions with different concentrations were prepared and stocked in amber flasks as is shown in Table .
Table 1. Concentration series for calibration curve of Trolox.
The samples were prepared by adding 20 µl of each extract in a 96-well microplate, then adding 260 µl of DPPH*. The readings were taken every 24 h, by triplicate with UV–Vis spectroscopy at a wavelength of 510 nm; the results are expressed in percent of inhibition (%).
Determination of total phenolics using Folin Ciocalteu method
A 100 µl of each extract, standard or methanol reference at 95%(v/v) was added by duplicate in 2 ml microtubes. Then, 200 µl of Folin–Ciocalteu (FC) 10% (v/v) were added and stirred at a Vortex. Afterward, 800 µl of Na2CO3 (700 mM) were added to each tube and incubated at room temperature for 2 h. Finally, 200 µl of each extract, standard or reference was transferred to a 96-well microplate and read at a 765 nm absorbance.
For the determination of total phenols present, the values obtained for the gallic acid equivalents (GAE) were compared to a standard curve corrected by a reference at an absorbance of 765 nm. Then, the total phenolic content was calculated as equivalents of gallic acid using linear regression between GAE and A765.
Determination of total sugars by high performance liquid chromatography (HPLC)
Total sugars from the natural extracts were determined by centrifuging the samples at 10,000 rpm for a period of 10 min to separate the suspended solids present in each solution and facilitate its filtration. Then, the filtered samples were passed through Waters Sep-Pak C18 cartridges, which had been previously reactivated with 3 ml of methanol and 6 ml of water, HPLC grade. Finally, samples were filtered through a Millipore™ 0.45 µm pore-size membrane.
For the analysis, a Waters Alliance® e2695 separation module and a Waters Alliance® 2414 refractive index detector were used, as well as a Bio-Rad fermentation monitoring (150 × 7.8 mm) column, with HPLC grade water as mobile phase at a fixed flow rate of 0.8 ml/min at 60 °C. Additionally, the software package Empower Pro was used for the acquisition of the resulting data.
Semen samples
Semen samples were collected once a week using the gloved-hand manual collection method (n = 12) from a 1 1/2-year-old Yorkshire boar housed in a corral of 51 m2 equipped with a feeder and a drinking trough in complying with the animal welfare standards. The animals used in the present investigation were cared for by acceptable practices and experimental protocols reviewed and approved by the campus ‘Centro Universitario de Ciencias Biológicas y Agropecuarias (CUCBA)’ at the University of Guadalajara.
Dilution of the seminal samples with the natural extracts and BTS
The seminal solutions for each different system were prepared by adding three different concentrations (0.250, 0.125 and 0.0625 µl) of each extract and the BTS solution to 5 ml of freshly collected semen (T1 = BTS 0.250 µl, T2 = BTS 0.125 µl, T3 = BTS 0.0625 µl, T4 = Hp 0.250 µl, T5 = Hp 0.125 µl, T6 = Hp 0.0625 µl, T7 = Cc 0.250 µl, T8 = Cc 0.125 µl and T9 = Cc 0.0625 µl). After 1 hour, the samples were stored at 17 °C and analyzed for three consecutive days (24, 48 and 72 h). Semen sample motility and viability were evaluated thermalizing the samples in a water bath until gradually reaching a temperature of 37°C for further analysis.
Motility
The rate of moving sperm was evaluated from 0% to 100%. 5 µl of each sample were put into a microscope slide previously templated at 37°C and immediately covered with a coverslip. By the use of an optic microscope with a 40× objective, for 3 consecutive days (24, 48 and 72 h).
SQS
The SQS® is a new compact and high throughput advanced system for automated semen quality analysis in boar semen doses production. The system is based on cell counting, using dual fluorescent staining. The dual staining method allows differentiating live (green) and dead (red) sperm cells. As a result of analysis a text report with dose calculation is provided. It’s a spermatozoa counter, based on fluorescence microscopy using high-resolution CMOS camera technology, light-emitting diode and an advanced image analysis system with new autofocus technology (Zoitech® Lab).
All collected seminal doses had their viability evaluated by an SQS® at the moment of the collection and after being diluted with the natural extracts and BTS for the control group. The evaluation samples consisted of 20 µl of the previously diluted semen with the natural extracts or BTS (1:9) and were prepared in a fluorescent stain which was subsequently mounted on a 4-well slide system and inserted into the equipment chamber for later analysis.
Seminal morphology
The seminal morphology was evaluated by a quick morphology kit (Espermaform by MegaFértil®) performing a smear with 10 µl of each sample and left to dry at room temperature. Subsequently, the samples were introduced to a specific fixative for 3 min, allowing them to dry. Then, they were immersed in dye A for a period of 2 min until they were dried by rinsing them with double distilled water. Finally, the dried smears were placed in dye B for 4 min following the above procedure. 200 sperm were counted in duplicate by light microscopy through a 100× objective. observing the following regions of the sperm stained with specific colorations, acrosome: light purple, nucleus: dark purple, middle piece and flagellum: dark green and cytoplasmic residue: light violet color.
Statistical analysis
A completely randomized trial design was used, where 9 treatments and 12 replicas each had their viability evaluated against a control group of boar semen diluted with a conventional extender. Three variables were considered: concentration of the natural extracts (0.250, 0.125 and 0.0625 µl) of Cymbopogon citratus, Hypericum perforatum and BTS (0.250, 0.125 and 0.0625 µl), seminal viability and seminal morphology.
For the data analysis, we ran the data through Minitab 18® statistical software. The data obtained from the treatments were analyzed by means of Turkey’s test with a 95% confidence level, because there are different markers for each studied object.
Results
DPPH* essays
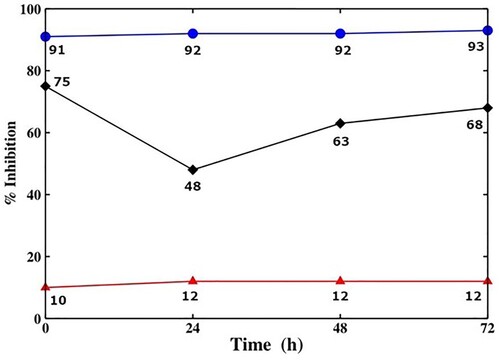
Figure shows the characterization results from natural extracts’ antioxidant properties and BTS by DPPH vs. time. For Cymbopogon citratus, the inhibition rate remained stable through the analysis reaching 93%, however, the peak of the inhibition rate for Hypericum perforatum was reached at 0 h with a 75% inhibition rate. Finally, for BTS the inhibition rate remained between 10% and 12% through the analysis period.
Determination of total phenolics
As we can see in Table , the results of total phenols content show that, for Hypericum perforatum a concentration of 264 mg GAE/100 g was obtained, in comparison with Cimbopogon citratus which showed a concentration lower than 160 mg GAE/100 g.
Table 2. Total phenolic contents of Cymbopogon citratus and Hypericum perforatum extracts.
HPLC
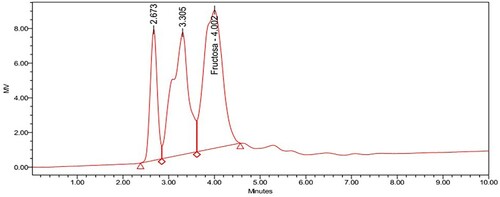
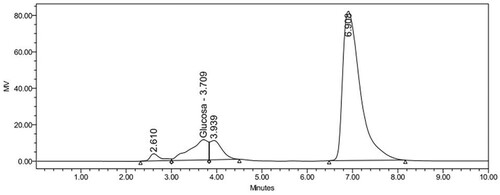
As it can be observed in Figure , for Hypericum perforatum both glucose and fructose concentrations are below equipment quantification limit; and for Cymbopogon citratus 0.558 g/L of glucose was obtained (Figure ), the amount of fructose present is irrelevant due to its low concentration. Both extracts were diluted and supplemented in concentrations specific to the defined seminal fractions, and acceptable vitality was observed for 72 consecutive hours.
Motility
The motility percentages are shown in Table . It was observed that, for all the treatments, there is a significant difference (P < 0.05), where in the analyses performed at 24 h, the highest motility averages were T1 (55.0 ± 4.5), T2 (52.5 ± 4.2) and T7 (51.7 ± 2.6). For times 48 and 72 h, the most significant averages were for T1 (43.3 ± 8.2), the other treatments had declines up to 0.7% of motility.
Table 3. Seminal motility averages for all times and extender types.
SQS
Table shows the different treatments with three concentrations each, measured by SQS. No significant difference (P > 0.05) is shown for time 24 h, however, T1 (83.3 ± 4.3), T2 (83.1 ± 5.5) and T6 (83 ± 6.1) showed the highest percentages of viability. On the other hand, at 48 h the percentage with the highest viability was at T2 (85.3 ± 2.4) showing a significant difference (P < 0.05) compared to all other treatments. Finally, for the 72 h, no significant difference (P > 0.05) was observed in any of the treatments. The treatments with the highest percentage of viability were T2 (85.1 ± 5.5), T5 (85.3 ± 2.7) and T6 (85.1 ± 3.3).
Table 4. Seminal viability averages for all times and extender types.
Seminal morphology
The main abnormalities that can be found in seminal doses were analyzed, due to the side effects that these can cause, among these abnormalities being damaged acrosomes and detached heads. The dilutions made with both natural extracts were compared with respect to BTS.
Table shows the results of the analysis on primary sperm morphology in interaction with natural extracts and BTS. The percentage of normal sperm (N) at 24 h did not show significant differences (P > 0.05) compared to the treatments at 72 h where a significant difference (P < 0.05) was observed between the highest (T6: 87.6 ± 2.4) and the lowest percentage (T1; 72.9 ± 5.7). For the treatments related to damaged acrosomes (DA) and detached heads (DH) at 24 h, no significant difference (P > 0.05) was observed, compared to 72 h where for AD a significant difference (P < 0.05) was observed between T1 (18.2 ± 7.7) and T9 (5.8 ± 1).
Table 5. Average of primary morphologies of seminal doses preserved with different extenders and their specific concentrations at 24 and 72 h.
Discussion
Boar spermatozoids are highly susceptible to membrane damage during its preservation, decreasing its viability and fertilization rate since its lipidic and protein structure, at the moment of being altered start up lipid peroxidation, triggering cellular apoptosis thus releasing ROS (Johnson et al. Citation2000). The incorporation of antioxidant agents at seminal doses has been proposed as a new alternative to the preservation of boar sperm (Flores et al. Citation2018). This, due to the polyphenols present in the leaves, as well as in the fruits of the plant, which act as antioxidants, their action is attributed to the combination of aromatic rings and the hydroxy groups, that ensemble their chemical structures, neutralizing the free radicals from the lipids, consequently decreasing ROS, as well as sperm death (Martínez-Flórez et al. Citation2002; Kumar and Pandey Citation2013; Spinaci et al. Citation2018).
In the present study, two different natural extracts were selected because there are multiple reports in the literature about their antioxidant properties. Investigations by Nambiar and Matela (Citation2015) report that for Cymbopogon citratus an 85% inhibition rate was obtained at 60 µg ml−1. By the other hand, there are reports where it’s been observed that extracts obtained through water vapor distillation present up to a 72% inhibition rate (Hartatie et al. Citation2018). The inhibition rate obtained in this investigation was of 98% at 72 h for a concentration of 25 mg ml−1, this is because it was synthetized from an infusion of a commercial tea, compared to the previously mentioned.
However, in past investigations by Oboh et al. (Citation2010), it’s been reported a thermal dependance in the synthesis of Cymbopogon citratus with respect to its antioxidant properties in hot water (100 °C), in which, it showed a higher capacity in the elimination of free radicals in comparison with cold or at room temperature synthesis; this thermo dependence is owed to the presence of bioactive molecules in this plant such as ketones, alcohols, phenols, terpenes, flavonoids, saponins, steroids, tannins, alkaloids, geraniols, terpenoids, polyphenols, esters, aldehydes and fatty acids (Oladeji et al. Citation2019). The determination of said extract for this work showed a total phenolic compounds content of 160 mg GAE/100 g.
In the case of Hypericum perforatum, Fathi et al. reported in 2013 that the antioxidant agents present in the plant accumulate mostly in the leaves and the flowers, reaching up to a 96% inhibition rate for a concentration of 200 µg ml−1. However, for methanolic extracts showed inhibition rates, between 6% and 90% approximately for concentrations between 100 and 250 µg ml−1 (Erdogan and Kartal Citation2015), compared to the obtained in this investigation, which decreased to a 68% inhibition rate at a 16 µg ml−1 at 72 h. The gap in the concentration/inhibition rate results may be because the extract was obtained through an aqueous phase without any posterior process to increase its concentration.
The antioxidant property is attributable to the flavonoids, xanthones, n-alkanes, carotenoids, amongst others, displaying a significative capacity capturing the superoxide radical, produced by the xanthine/xanthine oxidase system, being optimal for sperm interaction (Hobbs Citation1998; Silva et al. Citation2004). A 264 mg GAE/100 g of total polyphenols content was reported by Sekeroglu et al. Citation2017, where the analysis of the extract was carried on an aqueous phase and one on ethanol as a control group, obtaining a concentration of 88.93–175.41 mg GAE/g and 146. 35–182. 93 mg GAE/g respectively.
The antioxidant capacity showed by both extracts exceeded the one shown by the commercial extender during the time of the analysis, which is approximately 12% inhibition rate.
However, other factors can lead to sperm deficiencies, for example, the lack of necessary energy intake, which normally allows maintaining cellular metabolism and flagellum movement, achieving adequate motility. For this reason, the analysis of total sugars by HPLC was carried out as a secondary study for both natural extracts to determine the concentrations of glucose and fructose present, which are responsible for providing an energy contribution to the spermatozoa (De Ambrogi et al. Citation2006).
The energy molecule reacts activating hexokinase allowing said sugars to enter glycolysis through another route (fructose phosphate aldolase) generating ATP necessary for the motility to preserved sperm acting as species modulators and specific sugars for various sperm functions (Flores and Vilanova Citation2015).
For Hypericum perforatum extract, the concentrations of both glucose and fructose were below the limit of quantification, which coincides with that reported by Martínez-Solís (Citation2015) where it's mentioned that the maximum sugar content of this plant is located in the stems with 0.0004%. On the other hand, for Cymbopogon citratus extract, the fructose content was not relevant due to its low concentrations, however, for glucose a concentration of 0.558 g/l was obtained, which is slightly less than the amount reported in the technical data sheet of the commercial tea.
These values are below the concentrations usually used in the short-term extender such as BTS which reaches up to 37 g/l and below long-term extenders where there is a much lower concentration, reaching 11.5 g/l due to the conservation time required to decrease the motility of the sperm.
In reports by Ren et al. (Citation2019), aliquots of boar semen were prepared with a commercial extender which either contained IRPS (Isatis root polysaccharide) and, a control group, only with the extender. They observed motility for 7 consecutive days, obtaining a higher motility percentage in those treatments added with IRPS extract (50%). Compared to Hypericum perforatum which showed the highest percentage at 24 h with 30% that decreased to 2.5% on the third day and, to Cymbopogon citratus which showed the highest percentage with 51% that decrease to 5.8%, because that the latter contains a higher concentration of glucose. The control group reached a high percentage of 55% motility. However, in the present investigation, these extracts were not added together with commercial diluent.
As reported by Del Valle Rodríguez (Citation2017), the acceptable parameter of sperm viability in seminal doses must be greater than 70%. In the statistical analysis carried out, there was no significant difference (P > 0.05) in the percentages obtained of seminal viability with the use of BTS neither for the natural extracts for each of the three dilutions, at 24 and 72 h, obtaining a viability percentage above 70%, as well, at 48 h; even though a significant difference (P < 0.05) was observed for those treatments.
In previous reports by Funahashi and Sano (Citation2005), three different antioxidants (glutathione, cysteine and hypotaurine) were added in aliquots of boar seminal plasma where it was observed that after 14 days the samples diluted with glutathione and cysteine maintained a higher seminal viability. Separately, in a research carried out by Ros-Santaella and Pintus Citation2017, they reported the interaction of pig sperm with different concentrations of rooibos extract (Aspalathus linearis), showing that said extract improves sperm speed and provides protection of the acrosome structure by preserving the integrity of the membrane for 96 h, due to its high content of total polyphenols and antioxidant capacity.
At last, the analysis of the main sperm abnormalities in presence of different preservatives observed were damaged acrosomes and detached tails, these being responsible for fertilization. The analysis performed with three different preservatives (with its respective dilutions) showed no significant difference (P > 0.05) after 24 h in any of both parameters, displaying an 80% of normal sperms, as well as, after 72 h despite showing a significative difference (P < 0.05) in all the treatments, the damaged acrosomes percentage was below 20%, which agrees with the previously reported by Čeřovský et al. (Citation2005). Thus, by the use of natural extracts better conservation of plasmatic membrane and middle piece is achieved, due to the great antioxidant power that these extracts provide compared to BTS.
Conclusion
The antioxidant capacity of Cymbopogon citratus and Hypericum perforatum help to protect the sperm membrane, retarding cellular apoptosis, more efficiently than the observed with commercial diluents. These natural extracts showed that they can be used as preservatives of boar semen, as they maintained the seminal viability and morphology over 70% for a period of 72 h.
Author contribution statement
The authors contributed equally in preparation of the manuscript.
Acknowledgements
For the time and support they provided us during our research at the University of Guadalajara's Cofradía Ranch, we want to thank Jorge and Pedro González Campos as well as Diana Zamudio for her company and the knowledge she shared with us throughout the execution of the study. Additionally, our thanks to MVZ Antonio Méndez Lugo for sponsoring the use of the SQS equipment. And last but not least, we want to thank the CONACYT for the grant it awarded us during the period 2018–2020.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement (DAS)
The data that support the findings of this study are available in Figshare at https://doi.org/10.6084/m9.figshare.13363559.
Additional information
Funding
References
- Azam S, Hadi N, Khan NU, Hadi SM. 2003. Antioxidant and pro-oxidant properties of caffeine, theobromine and xanthine. Med. Sci. Monit. 9. n.9. ISSN 1234-1010.
- Balakrishnan B, Paramasivam S, Arulkumar A. 2014. Evaluation of the lemongrass plant (Cymbopogon citratus) extracted in different solvents for antioxidant and antibacterial activity against human pathogens. Asian Pac. J. Trop. Dis. 4(1):S134–S139. https//doi.org/10.1016/S2222-1808(14)60428-X.
- Bathgate R. 2011. Antioxidant mechanisms and their benefit on pot-thaw boar semen quality. Reprod. Dom. Anim. 46(2):23–25. https://doi.org/10.1111/j.1439-0531.2011.01826.x.
- Benedıí J, Arroyo R, Romero C, Martıín-Aragón S, Villar AM. 2004. Antioxidant properties and protective effects of a standardized extract of Hypericum perforatum on hydrogen peroxide-induced oxidative damage in PC12 cells. Life Sci. 75(10):1263–1276. https//doi:10.1016/j.lfs.2004.05.001.
- Čeřovský J, Frydrychová S, Lustyková A, Rozkot M. 2011. Changes in boar semen with a high and low level of morphologically abnormal spermatozoa. Czech. J. Anim. Sci. 50:289–299. https//doi:10.17221/4170-CJAS.
- De Ambrogi M, Ballester J, Saravia F, Caballero I, Johannisson A, Wallgren M, Andersson M, Rodríguez-Martínez H. 2006. Effect of storage in short- and long- term commercial semen extenders on the motility, plasma membrane and chromatin integrity of boar spermatozoa. Int. J. Andrology. 29:543–552. https//doi:10.1111/j.1365-2605.2006.00694.x.
- Del Valle Rodríguez A. 2017. Evaluation of semen quality in boars used in natural-service breeding. Red. Vet. 8:1–17. ISSN 1695-7504.
- Desroches NR, McNiven MA, Foote KD, Richardson GF. 2005. The effect of blueberry extracts and quercetin on capacitation status of stored boar sperm. Cell Preserv Technol. 3(3):165–168. https://doi.org/10.1089/cpt.2005.3.165.
- Erdogan O, Kartal M. 2015. LC-DAD-MS-Assisted quantification of marker compounds in Hypericum perforatum L. (St. John’s wort) and its antioxidant activity. Turk. J. Pharm. Sci. 12(3):279–286. https//doi:10.5505/TJPS.2015.47965.
- Flores C, Meléndez C, Mendoza C, Márquez Y, Vilanova LT. 2018. Efecto antioxidante de la melatonina durante la conservación de semen de cerdo. Rev. Vet. 29(1):13–17. http://doi.org/10.30972/vet.2912780.
- Flores C, Vilanova L. 2015. Metabolismo espermático. Gaceta Cien Veter. 20(1):23–32.
- Foote RH. 2002. The history of artificial insemination: selected notes and notables. J. Anim Sci. 80:1–10. ISSN 00218812.
- Funahashi H, Sano T. 2005. Select antioxidants improve the function of extended boar semen stored at 10°C. Theriogenology. 63(6):1605–1616. https://doi.org/10.1016/j.theriogenology.2004.06.016.
- Hartatie ES, Prihartini I, Widodo W, Wahyudi A. 2019. Bioactive compounds of lemongrass (Cymbopogon citratus) essential oil from different parts of the plant and distillation methods as natural antioxidant in broiler meat. IOP Conf. Series: Mat. Sci. and Eng. 532:012018. https//doi:10.1088/1757-899X/532/1/012018.
- Hobbs C. 1998. St. Johńs: The mood enhancing herb. Interweave Pr. Edition 1.
- Johnson L, Weitze K, Fiser P, Maxwell WM. 2000. Storage of boar semen. Animal Reprod. Sci. 62(1-3):143–172. https//doi:10.1016/s0378-4320(00)00157-3.
- Kumar S, Pandey AK. 2013. Chemistry and biological activities of flavonoids: An overview. Sci. World. J. 162750. https://doi.org/10.1155/2013/162750.
- Martínez-Flórez S, González-Gallego J, Culebras JM, Tuñón MJ. 2002. Flavonoids: antioxidant properties and activity. Nutr. Hosp. 17(6):271–278. ISSN 0212-1611.
- Martínez-Solís I. 2015. Fitoterapia. Elsevier Ed. 1a ed.
- Nambiar VS, Matela H. 2012. Potential functions of lemon grass (Cymbopogon citratus) in health and disease. Int. J. Pharmaceut Biol Arch. 3(5):1035–1043. ISSN 0976–3333.
- Oboh G, Adefegha SA, Ademosun AO, Unu D. 2010. Effects of hot water treatment on the phenolic phytochemicals and antioxidant activities of lemon grass (Cymbopogon citratus). EJEAFC. 9(3):503–513. ISSN:!1579-4377.
- Oladeji OS, Adelowo FE, Ayodele DT, Odelade KA. 2019. Phytochemistry and pharmacological activities of Cymbopogon citratus: A review. Sc. African. 6:e00137. https://doi.org/10.1016/j.sciaf.2019.e00137.
- Orčić DZ, Mimica-Dukić NM, Francišković MM, Petrović SS, Jovin EĐ. 2011. Antioxidant activity relationship of phenolic compounds in Hypericum perforatum L. Chemistry Central J. 5:1. https://doi:10.1186/1752-153x-5-34.
- Ren Z, Shaoyong W, Li Q, Ma L, Xiao J, Jiao J, Yang G, Pang W. 2019. Effects of isatis root polysaccharide on boar sperm quality during liquid storage and in vitro fertilization. Animal Reprod. Sci. 210:106178. https://doi:10.1016/j.anireprosci.2019.106178.
- Rodríguez-Gil JE, Estrada E. 2013. Artificial insemination in boar reproduction. Chapter 2. In: Bonet S., Casas I., Holt W., Yeste M, editor. Boar Reproduction. Heidelberg, Berlín: Springer; p. 589–607. https://doi.org/10.1007/978-3-642-35049-8_12.
- Ros-Santaella JL, Pintus E. 2017. Rooibos (Aspalathus linearis) extract enhances boar sperm velocity up to 96 h of semen storage. PLoS ONE. 12(8):e0183682. https://doi: 10.1371/journal.pone.0183682.
- Sekeroglu N, Urlu E, Kulak M, Gezici S, Dang R. 2017. Variation in total polyphenolic contents, DNA protective potential and antioxidant capacity from aqueous and ethanol extracts in different plant parts of Hypericum perforatum L. Indian J. Pharmaceut Educ Res. 51(2S):s1–s7. https://doi.org/10.5530/ijper.51.2s.43.
- Silva B, Ferreres F, Malva J, Dias A. 2005. Phytochemical and antioxidant characterization of Hypericum perforatum alcoholic extracts. Food Chem. 90:157–167. https://doi.org/10.1016/j.foodchem.2004.03.049.
- Spinaci M, Muccilli V, Bucci D, Cardullo N, Gadani B, Tringali C, Galeati G, Tamanini C. 2018. Biological effects of polyphenol-rich extract and fractions from an oenological oak-derived tannin on in vitro swine sperm capacitation and fertilizing ability. Theriogenology. 108(284):290. https://doi.org/10.1016/j.theriogenology.2017.12.015.
- Szczęśniak-Fabiańczyk B, Bochenek M, Smorąg Z, Ryszka F. 2003. Effect of antioxidants added to boar semen extender on the semen survival time and sperm chromatin structure. Reprod. Biol. 3(1):81–87. ISSN: 1642-431X.
- Tsantarliotou M, Sapanidoi V. 2018. The importance of antioxidants in sperm quality and in vitro embryo production. J. Vet. Andrology. 3:1–12. ISSN 2542-3045.
- Valdebenito N. 2007. Effect of caffeine on the motility and fertility of rainbow trout (Oncorhynchus mykiss). Inf. Tecnol. 8:2. http://doi.org/10.4067/S0718-07642007000200009.