Abstract
Parkinson’s disease (PD) is a neurodegenerative disorder caused due to the degeneration of dopamine-releasing neurons. Mucuna pruriens (MP) and Sesame oil (SO) are well-known for their neuroprotective and antioxidative properties but to date, no experimental study proves their synergistic effects in PD. The protective and therapeutic potential of MP extract containing 40% levodopa along with SO were evaluated, individually and together, on a multi-low dose (0.006 mg/g) of a rotenone-induced zebrafish model of PD. Neuroprotective roles of MP extract and SO on the activities of enzymatic and non-enzymatic antioxidants such as Catalase, GST, AChE, BChE, adenosine deaminase, and content of Glutathione and Malondialdehyde were examined. Also, the dopamine, TNF-α, and IL-1β levels were estimated via the ELISA method. Histopathological studies were performed for the confirmation of neuronal damage. Swiss Target Prediction software was used to predict the target protein receptors for MP and SO and protein interaction networks were used to decipher their role in molecular pathways such as synaptic plasticity, dopamine synthesis, etc. through Cytoscape and bingo plugin. The combination of MP extract and SO was observed to ameliorate oxidative stress and significantly increase the cholinergic enzyme activity and dopamine level and thereby helps in preventing neuronal cell damage.
Introduction
PD is the second most pervasive neurodegenerative disorder, which is estimated to affect 1% of the population over the age of 60 years worldwide (González-Casacuberta et al. Citationn.d.). The major established hallmarks for PD are depletion of the neurotransmitter dopamine in the striatum and preferential degeneration of dopaminergic neurons in the substantia nigra pars compacta and are, therefore, responsible for impairments in the motor, neuropsychological and cognitive systems (Garrabou et al. Citation2019). Evidence suggests that oxidative stress caused by an overproduction of Reactive oxygen species is involved in the etiology of PD. Oxidative stress reflects a disturbance in the balance between the production of free radical species and an antioxidant defense system of the human body. Reactive oxygen species that are mostly deleterious to the cells include hydroxyl radical, hydrogen peroxide, superoxide radical, and nitric oxide, whereas the antioxidant defense system comprises catalase, uric acid, glutathione, glutathione S-transferase, and SOD. In addition to this, 8-OhdG and MDA are the indicators of DNA damage and lipid peroxidation respectively. The inability of the body’s antioxidant defense system in protecting against the generation of reactive oxygen species and hence damages components of the cell, including proteins, DNA, and lipids, finally leading to cell death, which has been observed as associated with the onset of PD (Wei et al. Citation2018).
To date, PD remains an incurable disease. Not only its etiology is obscure but the ailment-adjusting medications are also inaccessible. The symptomatic management of PD is usually achieved through medicinal products including levodopa, dopamine agonists, monoamine oxidase B inhibitors, catecholamine-O methyl transferase inhibitors, and antimuscarinics. Although levodopa (L-dopa) is considered the gold standard drug for the management of PD symptoms, its prolonged use is associated with severe motor complications, such as dyskinesias, loss of appetite, dizziness, lowered blood pressure, vomiting, lightheadedness, etc. MP is a natural source of levodopa and causes the least symptomatic effects and can work efficiently in PD due to its free-radical disease management activity (Johnson et al. Citation2018; Rai et al. Citation2015). Several reports have suggested that it exhibits numerous pharmacological properties such as anti-microbial activities, analgesic, anti-epileptic, anti-neoplastic, and anti-inflammatory (Shasmitha Citation2015). MP has been reported to be rich in bioactive compounds like flavonoids, tannins, phenolics compounds, and alkaloids (Yadav et al. Citation2016).
Different dietary oils are currently getting significant consideration over the world for their potential medicinal advantages in neurological disorders. SO is one of the most widely-used medicinal products in ayurveda. It has been known to maintain healthy systems in the body involving the digestive tract, bones, the nervous system, muscles, and the reproductive system of both sexes. It is also recognized for its neuroprotective, antioxidant, immune-modulatory, anti-inflammatory, and anti-stress properties (Duke Citation2002). SO contains a good quantity of sesamol, phenol, sesamin, and sesamolin and comparatively fewer amounts of tocopherol which provides oxidative stability. SO in comparison to other dietary oils such as groundnut, canola, and sunflower oil depicted better protection against increased blood pressure, hyperlipidemia, and LPO by increasing enzymatic and non-enzymatic antioxidants (Anilakumar et al. Citation2010).
In the current study, the neuroprotective efficiencies of MP extract and SO were tested on zebrafish – one of the promising animal models utilized to investigate neurological disorders. It has previously been used to mimic events of the disease model. This non-mammalian species model has offered numerous opportunities for the investigation and study of brain mechanisms (Vaz et al. Citation2018). It is an emerging model where the choice of zebrafish has proven to be accurate because both humans and zebrafish share similarities in their psychological behavior. The present study, for the first time, elucidates the efficacy of administering MP extract containing 40% levodopa and SO, intraperitoneally, on a multi-low dose model of rotenone in zebrafish.
Materials and methods
Mucuna pruriens seed authentication
Authentication of MP seeds was done by the National Institute of Science Communication and Information Resources vide Ref. No. – NISCAR/RHMD/Consult/2018/3265-66. The identification was done using macroscopic studies, followed by detailed scrutiny of relevant literature and matching the sample with an authentic sample.
Mucuna pruriens seed extraction and estimation of levodopa content by HPLC
MP seeds were grounded into powder to the size of 2 mm. The powder was extracted using 3.0 liters solvent mixture (w/v) of primary alcohol (40%) and glacial acetic acid (0.33%) for 3 hours by constantly stirring at 45°C and it was again extracted twice with 2.0 liters solvent mixture (w/v) of 40% primary alcohol mixed and 0.35% of glacial acetic acid under similar conditions. The yield was 16.3 g. Later, the levodopa content was estimated in MP extract by carrying out HPLC analysis using a C-18 column, 0.1 M potassium dihydrogen phosphate (pH 3 with H3PO4) as mobile phase and the detection was done at λmax: 283nm, where flow rate was maintained as 1 mL/min (Patent grant number:430/DEL/2009) (Author Citationn.d.).
GC-MS analysis
GC-MS analysis of MP extract was performed at the Centile Biotech Pvt LTD labs, Uttarakhand, India, displayed in supplementary Table 1.
Dietary sesame seed oil
Authentic Sesame seed was provided by CSIR, New Delhi, India. Oil was extracted from the sesame seeds according to the method of Fazli et al. Citation2005. In brief, the sesame seeds were ground in a disintegrator. The ground seeds were extracted with n-hexane for 48 h and then filtered. This process was repeated three times using fresh solvent each time to extract most of the oils from the ground seeds. Free fatty acids were determined by Gas–Liquid Chromatography (GLC) as per the protocol of (Fazli et al. Citation2005) and sesamin and sesamol content were analyzed according to a method of Budowski et al. Citation1950 (Data has already been reported in our earlier study Ahmad et al. Citation2012) (Fazli et al. Citation2005; Budowski et al. Citation1950; Ahmad et al. Citation2012).
Fourier-transform infrared spectroscopy (FTIR) studies for MP extract and SO
The FTIR studies were carried out to find the possible interaction between MP extract and SO. The interaction was determined by comparing the peaks of different functional groups obtained in the FTIR spectra of pure MP extract with the peaks of FTIR spectra of sesame oil available in the literature.
Procurement of zebrafish
All animal studies have been done as per IACE (Institutional Animal Ethical Committee).
1000 Adult wild zebrafishes, weighing 0.3–0.5 g, were procured from a licensed vendor and acclimatized for 2 months under standard laboratory conditions i.e. the temperature was 25° Celsius and an exposure of 12 hours of light/dark cycles was maintained. 100 fishes per group were placed in separate tanks before the start of an experiment. Normal fish food from Toya company (comprising the following ingredients: Fish derivatives, soya bean meal, rice, and wheat flour) was fed to them twice a day.
Testing the toxicity of MP extract and SO alone and in combination
Fishes were segregated into 19 groups consisting of 30 fishes in each group.
MP extract alone: first six groups received five different concentrations of MP extract; 0.002 mg/g, 0.006 mg/m, 0.01 mg/g, 0.014 mg/g, 0.018 mg/g and 0.022 mg/g
SO alone: next six groups received six different percentages of SO; 0.1% w/v, 0.3% w/v, 0.5% w/v, 0.7% w/v, 0.9% w/v and 1.1% w/v.
MP extract and SO in combination: the last six groups received six different ratios of MP extract and SO in combination; 1:1 (1part MP extract (0.00 2 mg/g) + 1part SO (0.1%w/v), 1:1.5 (1part MP extract(0.002 mg/g) +1.5 part SO (0.3% w/v), 1:2 (1part MP extract (0.006 mg/g) + 2parts SO (0.5% w/v), 2:2.5 (2 parts MP extract (0.006 mg/g) + 2.5 parts SO (0.7% w/v), 2:3 (2 parts MP extract (0.01mg/g) + 3parts SO (0.9% w/v), 3:3 (3parts MP extract (0.014 mg/g) + 3parts SO (1.1% w/v).
All the eighteen groups were compared with control which received normal saline (0.9% solution)
Fishes received the dose intraperitoneally, once a week for 28 days. Fishes were monitored daily and sacrificed from each group at the gap of 7 days for four weeks.
Standardization of dose was based on the following parameters:
Mortality rate: It represents the number of casualties in a specified number of fishes in each group.
Biochemical estimation: Lipid peroxidation estimation, Total Glutathione content analysis, AChE, GST, and Catalase test (discussed in 2.11 sections).
Biochemical data in tabular form is displayed in supplementary Table 2.
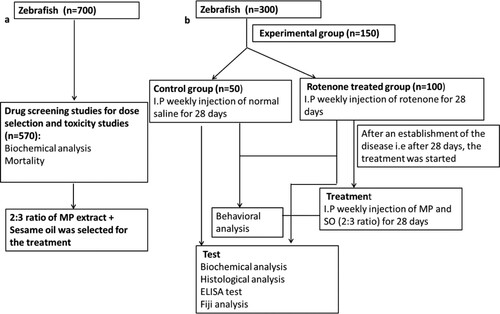
Toxin model development and treatment design
Through this study, a multi-low dose model of Parkinson’s disease was established for the first time in Zebrafish where rotenone was administered intraperitoneally. Zebrafishes were segregated into two groups consisting of 50 fishes in the control group and 100 fishes in the rotenone intoxicated group (Scheme 1):
Control (n = 50): Fishes were given 0.006 mg/g normal saline once a week for 28 days through the intraperitoneal route.
Diseased group (n = 100): The fishes were administered with 0.006 mg/g of rotenone intraperitoneally, once a week, for 28 days.
After the establishment of the disease on the 28th day, the treatment was started with MP extract and SO in combination. The procedure lasted for four weeks where the rotenone-intoxicated group was administered with a 2:3 ratio of MP extract + SO in combination.
Treatment group (n = 50): Fishes received 2:3 ratio of MP extract and SO (2parts MP extract (0.01 mg/g) + 3parts SO (0.9% w/v), via intraperitoneal route once in a week for 28 days.
The fishes were daily monitored for their behavioral changes which were analyzed meticulously. Eventually, the fish were sacrificed periodically in a gap of 7 days i.e. on the 7th, 14th, 21st, and 28th day.
Tissue preparation
Once the fishes were treated, examined, and analyzed, they were sacrificed ethically using ice-cold water treatment. The brain tissue was kept in 0.1 mL of Phosphate saline buffer (PBS), pH 7.4. It was first homogenized and then centrifuged at 10,500 g for twenty minutes at 4°C to obtain Post Mitochondrial Supernatant for biochemical estimations. For histopathological analysis, brain tissues were stored in formalin at room temperature.
Behavioral assessment
Dark and light test
The box was made of non-toxic glass and measured 30 × 22 × 22 cm (length x width x height). It was divided into two equal chambers i.e. dark and light chambers. There was a separator wall erected between the chambers with a circular opening of 4 cm diameter which allowed the fish to move between the dark and light partitions. A live camera was positioned above the glass box, which was connected to a laptop to monitor the subjects from a distance. The total duration of the test was 10 minutes and started immediately after the fish was placed into the light chamber. After the completion of the task, the subjects were placed back in their original place. Other unusual behavioral patterns of the subjects were also observed (Blank et al. Citation2009).
Tank diving test
A set of rules for the tank diving test was customized according to Egan et al. Five hours after the administration of the drug; subjects were transferred to a glass tank measuring 60 × 30 × 46 cm. As the fish was placed into the tank, the camera recording was begun and the subject was observed from a distance. The glass tank was divided into three sections – bottom, middle, and upper. The fishes could explore the tank freely for 10 minutes. The test was based on three parameters: the time spent in the respective area (bottom, middle, and upper). Also, the subjects’ shifting frequency in all three sections was recorded separately (Egan et al. Citation2009).
Biochemical estimations
Estimation of malondialdhyde (MDA)
Lipid peroxidation assay was performed using the protocol of Wright et al. (Citation1981). The test mixture contained 0.5 mL of the brain tissue sample, 0.5 mL of TBA (0.67%), and 0.5 mL of TCA (10%). The absorbance was recorded at 532nm (Wright et al. Citation1981).
Estimation of catalase activity (CAT)
The catalase assay was performed according to the method described by Claiborne (Citation1985). The test mixture of 3 mL contained 1.99 mL of phosphate buffer (0.05M, pH 7), 1 mL of H2O2 (20mM), and 10 µl of the brain tissue sample. The activity was measured in terms of n mole H2O2 consumed/min/mg protein by recording the absorbance at 240 nm (Claiborne Citation1985).
Estimation of reduced glutathione (GSH) level
The GSH content in serum was estimated by following the protocol as described by Jollow et al. (Citation1974) where 0.5 mL of brain tissue sample was combined with 0.5 mL of sulphosalicylic acid (4%). The test tubes containing the sample were kept at 40° C for 1 hour and later centrifuged at 1,200xg (4000rpm) for 15 minutes at 4° C. Finally, the test mixture of 1.5 mL contained 0.2 mL of filtered aliquot, 0.2 mL of 10 mM DTNB (5,5'-dithiobis-(2-nitrobenzoic acid) and 1.1 mL of 0.1 M phosphate buffer (pH 7.4)). The optical density of yellow color developed was read immediately at 412 nm (Jollow et al. Citation1974).
Estimation of GST level
The GST content in PMS was measured by the protocol set by Habig et al. (Citation1974). The test mixture contained100 µl of the brain tissue, 1.6 5mL of phosphate buffer (0.1M, pH-6.5), and 0.2mL of 1mm reduced glutathione. To the test mixture, 50µl of freshly prepared CDNB (2.025 mg in 5.7 mL of absolute alcohol, vortex, and 10 mL distilled water) was added. The absorbance was recorded at 340 nm (Habig et al. Citation1974).
Determination of cholinergic enzyme activity
Determination of acetylcholinesterase and butyrylcholinesterase activity
AChE activity was estimated by Ellman et al. (Citation1961) protocol. For activity measurement, the 1.56 mL of reaction volume contained 1.3 mL sodium phosphate buffer, 10 µl ATC, 0.05 mL DTNB and 0.2 mL of supernatant. Finally, the absorbance was recorded at 412 nm. Similarly, butyrylcholinesterase activity was determined by following the protocol of a commercially available assay kit (Ben Biochemical Enterprise). According to this assay, the Cholinesterase enzyme catalyzes the hydrolysis of butyrylthiocholine into butyrate and thiocholine. Here, 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) reacts with thiocholine and therefore, forming a colored compound. The absorbance was recorded at 405nm (Ellman et al. Citation1961).
Assessment of brain dopamine, TNF-α, IL-1β level by ELISA
To determine the brain dopamine, TNF-α, IL-1β level, fish were euthanized by submerging in ice water (0–4° C) for 1 minute, followed by cessation of opercular (i.e. gill) movement. The whole fish brain was extracted to measure the dopamine level via ELISA (enzyme-linked immunosorbent assay, Merck & Sigma) by following the kit-based protocol. The sample was prepared by pooling 4 whole-brain sections of zebrafish from the individual group.
Assessment of adenosine deaminase activity (ADA)
ADA activity was evaluated spectrophotometrically (colorimetric assay) by following the protocol reported by Weisman et al. Citation1988. The brain tissue sample was added to the reaction mixture containing 50 mM sodium acetate buffer (pH 5.0) and 50 mM sodium phosphate buffer (pH 7.0) and the reaction was initiated by the addition of adenosine as substrate. The phenol-nitroprusside reagent was used to remove non-enzymatic hydrolysis of substrates. The absorbance was recorded at 635nm (Weisman et al. Citation1988).
Statistical analysis
The resulting data are expressed as Mean ± Standard deviation. All the statistical analysis was done using the Origin 9.0 software. Comparisons between the mean of control and treated animals were carried out by one-way analysis of variance (ANOVA) followed by Tukey test with P<.05(***), P<.01(#) and P<.0001(*) as the limits of significance.
Histopathological analysis
Zebrafishes were sacrificed by cold water treatment and their brain tissue was removed and preserved in buffered formalin (10%) for histopathological studies. A minimum of four sections (5 µm thick) was hacked on a microtome. The sections were stained with Hematoxylin (H) and Eosin (E).
Fiji software analysis of histopathological studies
The results of histopathological studies were validated by using Fiji Software to determine the neuronal cell count, and the total area (Horai et al. Citation2017).
Bioinformatics analysis for the study of the mechanism of action
Swiss Target Prediction software was used to predict the target protein receptors for natural levodopa (MP extract constituent (present in large quantity) and sesamol (SO major constituent)) (Gfeller et al. Citation2014). The consolidated list of receptors is displayed in Supplementary Tables 3 & 4. A total of 102 and 105 protein receptors were predicted for levodopa and sesamol respectively. The list of proposed target receptors was submitted in Cytoscapev3.7 and a refined master network was created (Saito et al. Citation2012). The network obtained through Cytoscape was studied to understand the possible mechanism of action of levodopa contained in MP extract and sesamol constituent of SO in PD management.
Results and discussion
Estimation of levodopa content by HPLC
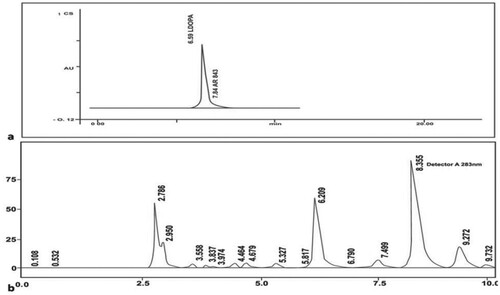
The levodopa in the pure MP extract was confirmed by the peak with a retention time of 6.2 minutes and the corresponding area under the curve was found to be 549771. From HPLC, the levodopa content in the pure extract was found to be 41.5 mg per 100 mg of the crude extract. Thus, levodopa content is 41.5 mg. (Figure (a, b)).
FTIR spectroscopy of MP extract and SO
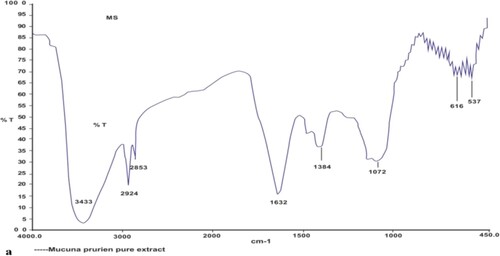
The FTIR spectra of pure MP extract were obtained and its fingerprint region was compared to the fingerprint regions of SO obtained from the literature to find out any possible interaction. Fingerprint regions of SO available in the literature; 1504 (Phenyl Out-of-plane CH bending), 3007 (CH Saturated –CH), 3401 (OH of phenolic O-H), 1397 (Methyl symmetric bending), 1039 (=C–O-C) Symmetric stretching, 944 (=C–O) Most characteristic for methylenedioxy (927), 835 (-CH, CH of two adjacent hydrogen at 3, 4 positions), 714 (-C–O-C) weak bonds, was found to be different from the peaks obtained in FTIR spectra of MP extract (3433 (-OH stretch), 2924 (Aliphatic-CH stretch),1632 (Carbonyl C = O ester), 1384 (O-H binding), 1072 (C–O stretch), 616,537) (Figure ). Hence, it was predicted that there is no interaction between both the medicinal herbs, and therefore the combination of MP extract and SO was taken for evaluation of therapeutic efficacy (Mirghani et al. Citation2003).
Testing the efficacy and toxicity of MP extract and SO alone and in combination
Preliminary studies were performed to find out the dose-dependent chronic toxicity of MP extract and SO for the standardization of the dose for the treatment of multi low dose rotenone model of PD in zebrafish.
Biochemical estimations
The activities of the antioxidant enzyme were found to be increased with an increase in the dose of MP extract from 0.002 mg/g to 0.018 mg/g in the zebrafish model with 0% mortality. On the contrary, the group administered with 0.022 mg/g of MP extract dose showed 20% mortality along with a decline in the level and activities of the antioxidant enzyme also with a substantial increase in the level of MDA. Similarly, in SO treated groups, it was observed that till 0.7% w/v dose the activities of antioxidant enzymes were increased with a simultaneous decrease in the level of MDA but from 0.9% w/v onwards the activities of the enzymes recorded were the same with 0% mortality.
At the same time, 0% mortality was recorded in all the six groups receiving six different ratios of MP extract and SO; (1:1), (1:1.5), (1:2), (2:2.5), (2:3), and (3:3). It was observed that with an increase in the ratio of MP extract and SO till 2:3, the activities of antioxidant enzymes have increased and the level of GSH content and MDA (significance of lipid peroxidation) were within their normal range. But the drastic effects on oxidative stress parameters were observed with 3:3 ratio in zebrafish brain tissue when compared to the control brain. High levels of MDA and decreased levels of antioxidant enzymes were recorded in the 3:3 ratio of MP extract and SO treated brain (Figures and ).
Figure 3. Graphical representation of effects of different doses of MP extract, SO and MP extract + SO at 28th day on (a) catalase (b) acetylcholinesterase (c) glutathione s transferase. Values are mean ± SD 30 fishes in each group, *p<.0001 compared to control, Groups (1–19, Group1: control); P<.05(***), P<.01(#) and P<.0001(*).
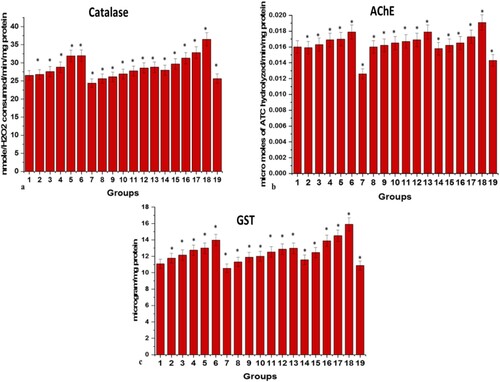
Figure 4. Graphical representation of effects of different doses of MP extract, SO and MP extract + SO at 28th day on (a) glutathione content and (b) MDA level. Values are mean ± SD 30 fishes in each group, *p<.0001 compared to control, (1–19, Group1: control); P<.05(***), P<.01(#) and P<.0001(*).
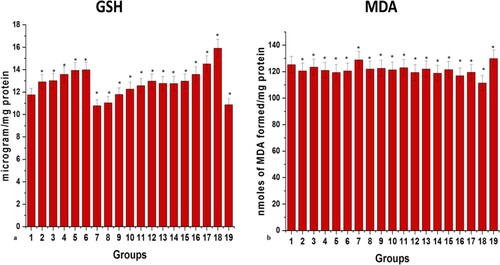
Based on mortality rate and biochemical analysis, the 2:3 ratio of MP extract and SO was considered to be the best dose for the treatment of the rotenone model of PD.
Effect of 2:3 ratio of MP extract and SO on rotenone model of PD
Behavior analysis
Behavior is an important parameter for the indication of neurodegenerative diseases and the zebrafish exhibits the best behavior for the study of neurological disorders. Normal fish prefer to swim in a group, prefer dark regions, stay in their native area, remain away from predators and always avoid exploring the foreign area. In the present study, we have reported the behavior of control, diseased and 2:3(MP extract + SO) treated groups.
Phenotypic changes
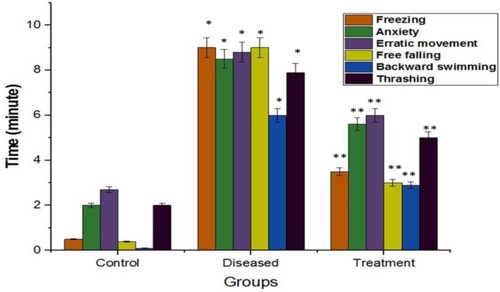
Fishes were observed daily for changes in their behavioral parameters from the 7th to the 56th day. The behavioral analysis includes a meticulous study of the subjects when kept under controlled conditions. A glass tank of 5L capacity was taken and filled with water. The test ran for 10 minutes and started after the fish was placed into separate tanks individually from each group respectively. It was observed that with every dose of rotenone the anxiety level, erratic movement, and level of thrashing have been increased. At the same time freezing (latency), backward swimming, and free-falling was also observed in their behavior during rotenone administration. Based on these behavioral parameters, mild cognition, and rigidity in movement can be predicted in rotenone administered fish. A significant improvement was observed in these behavioral parameters when the diseased group was administered with 2:3 (MP extract + SO) (Figure ).
In the tank diving test (Figure (a))
It was observed that control fishes preferred to stay and swim in the bottom region. While, diseased fishes were observed to spend lesser time at the bottom compared to the control group and swim in every region i.e. upper, middle, and bottom. As the disease progressed, the fishes were found to spend maximum time fearlessly in the upper region of the tank with minimal movement. A significant difference was observed in the number of transitions between the bottom and upper region of the tank between the controlled and diseased fish. However, the diseased group, after receiving the treatment with 2:3 (MP extract + SO), individually, had shown less anxiety and spent more time in the bottom area compared to the diseased group.
Figure 6. Graphical representation of the time spent in the water surface in tank diving test (a) and Dark region in dark and light test (b) within a maximum of 10 min. Values are mean ± SD 6fishes in each group, *p<.0001 compared to control and **p<.0001 compared to diseased; P<.05(***), P<.01(#) and P<.0001(*).
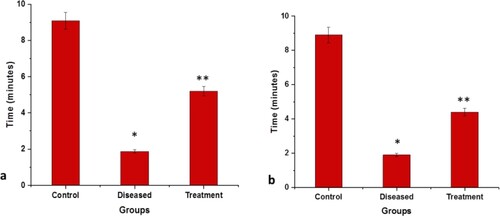
In the dark and light regions (Figure (b))
The control group had spent more time in the darker area compared to the light area. On the other hand, memory impairment was noticed in the diseased group. With the disease progression, the time duration between the dark and light areas decreases as the number of transitions between the dark to the light region was reduced drastically. However, the diseased group, after receiving the treatment, had shown better results by regaining interest and cognition. The 2:3 (MP extract and SO) treatment had reduced the time difference between dark and light transitions. The time spent in the light region had increased.
Assessment of oxidative stress & cholinergic enzyme activity
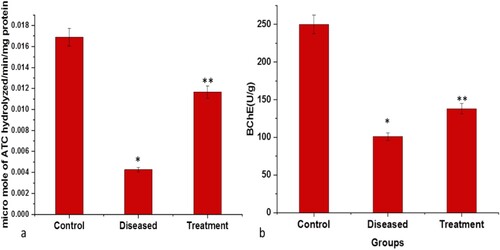
Administration of rotenone via intraperitoneal route caused severe neuronal damage as indicated by changes in the levels of oxidative stress parameters. A substantial decrease in the activities of catalase and cholinergic enzymes like AChE and BChE and an increase in the level of MDA/TABARS (Malondialdehyde, Thiobarbituric acid reactive substances) were observed in the rotenone-induced diseased group. In neurodegenerative disorders, under oxidative stress, the level of AChE and BChE activity declines which further reduces the levels of choline and dopamine neurotransmitters as the ratio of Dopamine/ACh gets altered. The level of GSH content was significantly depleted in the diseased group. Similarly, a significant decline was observed in GST activities in the diseased group as compared to the control group, which strongly recommends a significant level of neuronal damage. However, a substantial reduction in MDA level and a significant increase in the level of GSH and GST were observed in fishes treated with both rotenone and 2:3 (MP extract + SO). Also, the activities of antioxidant and cholinergic enzymes like catalase, AChE and BChE were found to be rose after the treatment with 2:3 (MP extract + SO) (Figures and ).
Figure 7. Graphical representation of significant changes on the: (a) Catalase activity (nmole/H2O2 consumed/min/mg protein) (b) GSH level (microgram/mg protein) (c) GST activity (microgram/mg protein) (d) MDA level (n moles of MDA formed/min/mg protein; between control, diseased and treatment). Values are mean ± SD 6fishes in each group, *p<.0001 compared to control and **p<.0001 compared to diseased; P<.05(***), P<.01(#) and P<.0001(*).
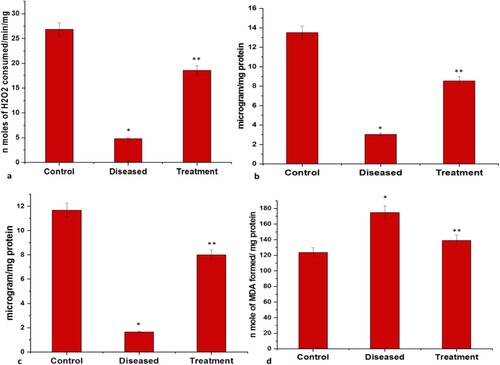
Figure 8. Graphical representation of significant changes on the: (a) AChE activity (micromole of ATC hydrolyzed/min/mg protein) (b) BChE activity (U/g); between control, diseased and treatment. Values are mean ± SD 6fishes in each group, *p<.0001 compared to control and **p<.0001 compared to diseased, P<.05(***), P<.01(#) and P<.0001(*).
Assessment of brain dopamine, TNF-α, IL-1β level by ELISA
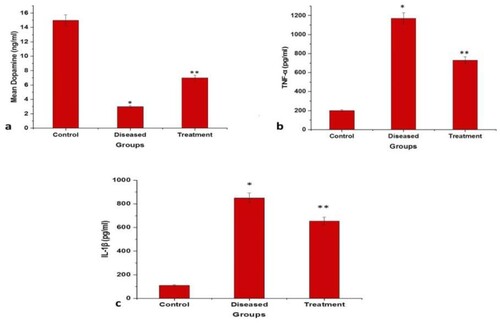
Locomotor activity is correlated to the dopamine level in the brain. The obtained results showed a significantly decreased level of dopamine (p<.0001) in the rotenone administered group (diseased group) when compared to control which justifies the results of locomotor impairment observed during the behavioral analysis. However, an increase in dopamine level was recorded when the diseased group was treated with 2:3 (MP extract + SO). Therefore, it is assumed that 2:3 (MP extract + SO) could protect dopaminergic neuron from rotenone destruction and maintain dopamine levels appropriately (Figure (a)). Also, the pro-inflammatory cytokines (TNF-α, IL-1β) levels were determined by ELISA (Enzyme-linked immunosorbent assay), the production of TNF-α and IL-1β was observed to be markedly increased following the rotenone administration, whereas treatment with 2:3 (MP extract + SO) considerably attenuated the rotenone-induced secretion of TNF-α and IL-1β (Figure (b, c)).
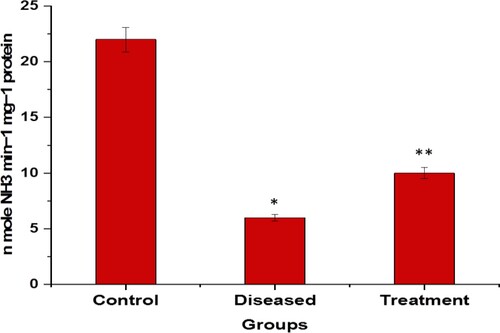
Assessment of adenosine deaminase level (ADA)
Rotenone produced a significant decrease in ADA level compared to the control value. The fishes treated with rotenone and 2:3 (MP extract + SO), ADA level were observed to be increased compared to the diseased only group. The units are expressed in n mole NH3 min−1 mg−1 protein (Figure ).
Histopathological analysis of brain sections of zebrafish of PD model
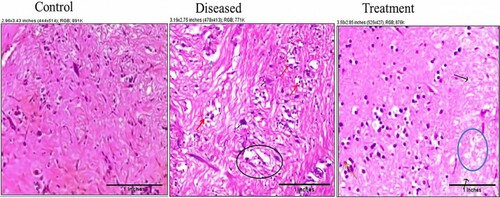
The results obtained from the histological sections of toxin-treated brain tissue were in support of biochemical studies. Rotenone exposure induced severe glial edema and focal features of demyelination in the hypothalamus region of the brain. The forebrain cortex showed focal evidence of astroglial degeneration, demyelination, and necrosis. A mild perivascular lymphoid aggregate was also seen. No such defects were observed in the control group. In the control group, the normal granular layer and molecular layers were within their normal limits. Treatment with 2:3 (MP extract + SO) has reduced the inflammation and, there was a reduction in focal necrosis of parts in the brain tissue. Demyelination was absent. The ganglionic cells have shown features of mild degeneration (Figure ).
Figure 11. Photomicrographs of histopathological changes in brain tissue sections (sacrificed on the 28th day) of control (a): showing astroglial cells and molecular cells within normal limit; diseased brain (b): showing glial nodules (red arrow), necrosis (black circle), apoptotic neurons (black arrow), astroglial degeneration (green arrow) and inflammation were also present; 2:3 (MP extract and SO) treated brain (c): showing reduced inflammation and focal necrosis (blue circle). The ganglionic cells show features of degenerations (black arrows) and the molecular layer shows features of glial edema (red arrows). Demyelination was not observed.
Fiji software analysis of histopathological studies
The results of histopathological studies were validated by using Fiji software to determine the neuronal cell count and the total area. It was observed that the total area and neuronal cell count of the diseased brain were reduced significantly. The average size of the diseased brain, when compared to the control fish brain was less. Similarly, the neuronal cell count of the diseased brain was reduced to 1051 from 4975. However, after the treatment with 2:3 (MP extract + SO), the condition of the brain was found to be significantly improved (Figure and Table ).
Figure 12. Analysis of photomicrographs by Fiji software where, (a) control brain (b) diseased brain (c) Treatment, red portion represents the cell count.
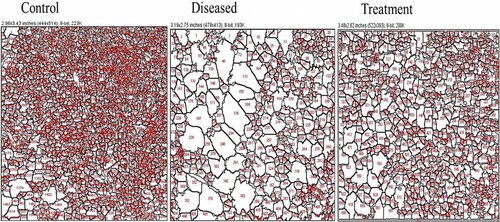
Table 1. Analysis of histopathological sections of brain tissue (Cell count, Total area and Percent area).
Bioinformatics analysis for the study of the mechanism of action
Receptor identification and network analysis
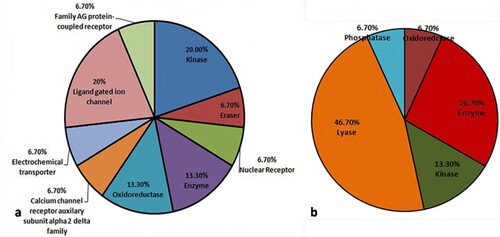
Swiss target prediction tool was used to predict all the possible receptors for levodopa and sesamol. The consolidated list of levodopa and sesamol is presented in supplementary table 3 and table 4. A total of 102 and 105 receptors were predicted for levodopa and sesamol, respectively. The receptors for levodopa belonged to Kinase, Eraser, Enzyme, Nuclear receptor, Oxidoreductase, Calcium channel auxiliary subunit alpha2 delta, Electrochemical transporter, Ligand-gated ion channel, and A G protein-coupled receptor families (Figure (a)). Similarly, receptors for sesamol were observed to fall in Oxidoreductase, Kinase, Lyase, Enzyme and Phosphatase protein families (Figure (b)).
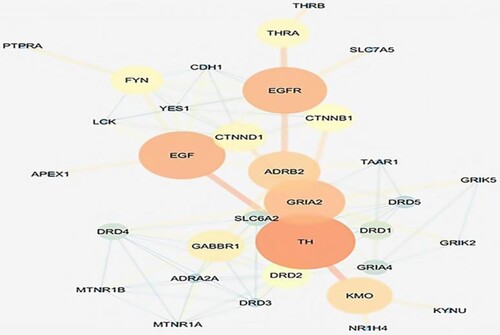
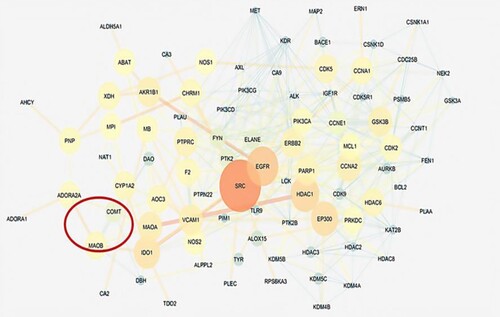
Later these protein receptors were taken forward to cytoscapev3.7 for the construction of a protein–protein interaction network for levodopa and sesamol. The obtained master network for levodopa had 4084 nodes and 6771 edges, while the network for sesamol had 4954 nodes and 11237 edges. The network statistics were calculated; levodopa network had (0.109) correlation coefficient, (0.394) betweenness centrality, and (0.619) closeness centrality, while sesamol network had (0.150) correlation coefficient, (0.387) betweenness centrality, and (0.390) closeness centrality respectively. The master network was composed of query proteins and other neighboring proteins which interact with each other at a biological level. The mechanism of action of levodopa and sesamol was predicted around the Hub nodes which can control or modulate the obtained master network. Hub nodes in a protein network are those proteins that have the highest number of connections to other nodes and hold the central position in a network. The hub nodes (controlling nodes) in the levodopa network were EGFR, THRA (Thyroid hormone receptor alpha), EGF, CTNND1, FYN (Proto-oncogene tyrosine-protein kinase), TH (Tyrosine hydroxylase), etc. (Figure ), while hub modes for sesamol network were SRC (proto-oncogene c-Src), EGFR, HDAC1 (Histone Deacetylase 1), GSK3B (Glycogen synthase kinase 3 beta), CDK2 (Cyclin-Dependent Kinase 2), MAOA (Monoamine oxidase A) gene, etc. (Figure ). The list of levodopa and sesamol Hub nodes associated with biological bioprocesses is displayed in supplementary table 5 and table 6.
Predicted receptors are the proteins targeted by the levodopa and sesamol in PD management and help decipher a probable mode of action. Levodopa and sesamol can easily fit into the active sites of the receptor proteins and modulate their activities. A protein interaction network was generated around these receptors to identify their role in a specific process or pathway. A hypothesis (mechanism of action) for both the compounds designed around their predicted receptors is given below:
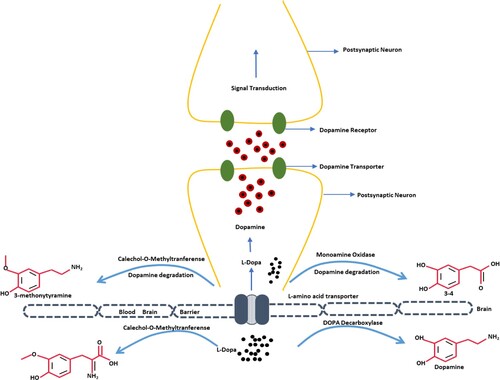
It is proposed that the network of the mechanism of action may have started from ADORA2A and terminated at TH, which is a dopamine-producing enzyme. ADORA2A is a highly expressed receptor in the striatum locale of the brain, it is when stimulated from outside, adenosine is up-regulated in the body leading to its binding to ADORA2A and resulting in increased permeability of the blood–brain barrier causing an influx of neurotransmitters. Subunit of dopamine receptor when interacting with ADORA2A forms a functional heteromeric complex and inhibits adenylyl cyclase. Activation of adenosine A2A receptor disturbs the balance between dopamine and acetylcholine. The dynamic degeneration of neurons of striatum and substantia nigra happening in PD brings about the pathological features and results in involuntary muscular movements called a hypertonic-hypokinetic syndrome. ADORA2A when interacts with DRD2, it modulates the dopamine levels in the brain (Rieck et al. Citation2015; Stockwell et al. Citation2017). Further DRD2 interfaces with SLC6A3 (dopamine transporter, solute carrier family 6) which determines the extracellular concentration of dopamine. SLC6A3 terminates the action of dopamine by its high-affinity sodium-dependent reuptake into presynaptic terminals. Non-activation of SLC6A3 causes the reduced uptake of dopamine and it forms aggregates in the synaptic cleft (Dos Santos et al. Citation2019). DAT when interacts with DRD2 causes dysregulation of DAT which results in the slow uptake of extracellular dopamine from the synaptic cleft to the neurons (Magistrelli et al. Citation2021). MAOB and DDC interaction with DRD2 increases the degeneration of the dopaminergic system in PD (Arenas et al. Citation2015). On the other hand gene interactions between CREB (cAMP response element-binding DAT when interacts with DRD2 causes dysregulation of DAT which results in the slow uptake of extracellular dopamine from the synaptic cleft to the neurons protein), AKT1 (AKT Serine/Threonine Kinase 1), PLA2G6 (Phospholipase A2 Group VI), DDIT3 (DNA Damage Inducible Transcript 3), TFEB (Transcription Factor EB), TFAM (Mitochondrial transcription factor A), MAPT (microtubule-associated protein tau), FKBP4 (FKBP ProlylIsomerase 4), YWHAZ (Tryptophan 5-Monooxygenase Activation Protein Zeta) and BAD all together leads to an increase in neuron degeneration, mitochondrial dysfunction and cytoskeleton dysfunction, which leads to tremors in PD (Naz and Siddique Citation2020). EGFR and EGF are required for neurogenesis, neuron survival, and maintenance. The formation of Lewy bodies causes the release of inflammatory cytokines from the microglial cells which are responsible to activate JNK signaling and eventually results in impairment of PARK14 related Ca2+ signaling and deterioration of dopaminergic neurons. α-Syn binds directly to tau and tubulin and inactivates the interaction between tau and tubulin which obstructs the physiological function of tau protein, further α-Syn brings kinases and enhances the hyperphosphorylation of tau protein(Von Wrangel et al. Citation2015). Due to mutation in SNCA, TH (dopamine-producing enzyme) gets inhibited which is responsible for the synthesis of dopamine hence dopamine levels decrease and leading to synaptic plasticity (Orr et al. Citation2017; Masato et al. Citation2019).
Proposed mechanism of action of MP extract containing 40% levodopa and SO
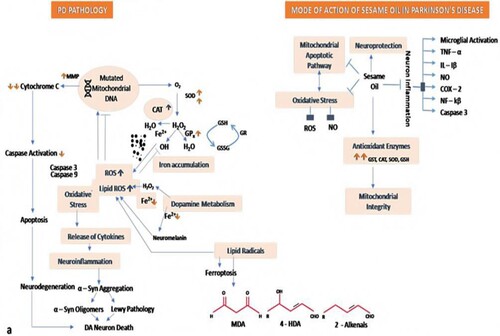
Dopamine has low bioavailability and is incapable of crossing the blood–brain barrier. Therefore, its precursor, levodopa is used clinically which can be easily transported to the Central Nervous System (CNS). In CNS levodopa converts into dopamine via DOPA decarboxylase, however, only a small fraction of its administered dose enters the brain which is sufficient enough to restore the dopaminergic neurotransmission. While the conversion of levodopa into dopamine is the basic pharmacological effect of levodopa, it also possesses direct neuromodulatory properties. The bioavailability of levodopa can be increased by inhibiting the dopamine degrading enzymes like COMT (Catechol-O-methyltransferase) and MAOB (Monoamine oxidase B; dopamine degrading enzymes) (Figure ). SO has already been reported to possess antioxidant activities which help in protecting the neurons by elevating the enzymatic antioxidant biomarkers. Due to its antioxidant potential, it inhibits the formation of oxygen-free radical species along with the aggregation of protein adducts. Interestingly, through Swiss target prediction software; it was observed that SO targets the COMT and MAOB protein receptors (Figure ). Sesame oil can also reduce the levels of ROS, NO, and LDH to maintain the oxidant–antioxidant balance by increasing the levels of GSH, GST, and catalase. It is also capable of maintaining mitochondrial integrity, thus inhibiting the mitochondrial apoptotic pathway (Figure ). Hence, this might be the reason that levodopa contained in MP extract and SO performed synergistically when given in combination rather than alone.
Discussion
In the present study, to evaluate the neuroprotective effects of MP extract and SO on dopaminergic neurons, rotenone was used, which is one of the most recommended toxin for developing PD model (Masato et al. Citation2019; Cannon et al. Citation2009; Zhang et al., Citation2017). The nigrostriatal damage due to intraperitoneal administration of rotenone is associated with inflammatory response and oxidative stress probably by generating free radicals (Siddiqui et al. Citation2013; Verma and Nehru Citation2009). Thus, ROS-scavenging antioxidants may play an important role in the treatment of PD and therefore, help in combating against oxidative stress-caused progressive neuron damage. Moreover, MP and SO are well known for their potent anti-inflammatory and antioxidant properties and considerably prevented all the alterations induced by intraperitoneal administration of rotenone. This neuroprotective behavior is consistent with previous published reports (Ahmad et al. Citation2012; Park et al. Citation2010; Hussian and Manyam Citation1997; Rai et al. Citation2017; Kasture et al. Citation2009). The behavioral effects are reported to be directly linked with degree of neuronal dysfunction. We report here a significant decline in toxin induced anxiety and motor coordination dysbalance following exposure to MP and SO in combination. Our results correlate well with the previous reported studies, where motor deficits in Parkinsonian zebrafish have been attenuated by Centella asiatica, Danshensu and Ellagic acid and Curcumin (Khatri and Juvekar Citation2016; Chong et al. Citation2013; Khotimah et al. Citation2015). Brain cells are susceptible to oxidative damage due to stress leading to the production of ROS. This damage is also caused due to a comparatively low antioxidant defense system, which can be efficiently protected by the use of several antioxidants. ROS stress to dopaminergic neurons in the substantia nigra pars compacta is believed to be one of the primary causes of neuron damage in PD. Oxidative stress disturbs the antioxidant defense system and therefore, promotes lipid peroxidation leading to brain cell death. The reduced level of MDA in the brain was observed after an exposure of herbal drug combination. Also, there was a simultaneous rise in the enzymatic and non-enzymatic antioxidants. Glutathione reductase converts the oxidized form of (GSSH) to the reduced form (GSH), thereby acting as an important cellular antioxidant enzyme. It helps in preventing the damage of various cellular components caused by ROS (superoxide radical, hydroxyl radical and singlet oxygen). In the brain, it is particularly involved in peroxide disposal and helps in maintaining the integrity of brain cells (Rai et al. Citation2017; Yadav et al. Citation2017). Hydrogen peroxide (H2O2) is a damaging by-product of various metabolic processes which gets converted into H2O and O2 via the catalase enzyme. The loss of the activity of this enzyme leads to many deleterious effects due to the accumulation of highly toxic metabolites and H2O2 (Chen et al. Citation2015). MDA is one of the end products of the lipid peroxidation process indicating oxidative damage. The increased level of TBARS was observed in rotenone-treated brain tissue, along with suggested lipid peroxidation, and massive tissue damage (Ayala et al. Citation2014). There are numerous studies about neuroprotective effect of MP extract and SO individually on lipid peroxidation and enzymatic and non-enzymatic antioxidants. In agreement with these studies, we also observed that MP extract and SO significantly decreased the MDA level along with increased in the activity of antioxidant enzymes in striatum portion following rotenone induction. The brain dopamine plays a vital role in neurodegenration, which undergoes oxidation to form toxic electrophilic species and quinone, thereby; causing PD. Antioxidants administration inhibits the reduction in brain dopamine levels (Lee et al. Citation2020). Histopathological studies and Fiji software have supported the fact of neuronal damage due to oxidative stress with reduced cell number and prominent altered cell integrity. Restoration of striatal dopamine content and antioxidants defense after an administration of MP extract and SO demonstrated the neuroprotective behavior of the herbal drug combination. Acetylcholinesterase is an enzyme that catalyzes the breakdown of acetylcholine and some of the other choline esters that act as neurotransmitters but in PD, the disruption in DA/AChE balance results in excessive release of acetylcholine in the striatum. A significant correlation exists between cholinergic deficit and cognitive impairment in PD. Therefore, the cholinergic system was evaluated by measuring the AChE and BChE activity (Perez-Lloret and Barrantes Citation2016; Singh et al. Citation2020). The results obtained from our study suggest that 2:3 (MP extract + sesame oil), has significantly increased the AChE activity in the striatum, reflecting the increased level of acetylcholine availability at the synaptic terminal, leading to the improvement of cognitive functions (learning and memory process). Proinflammatory cytokines, including IL-1β and TNF, have been described as involved in promoting neurodegeneration via stimulating neuroinflammatory process and by the formation of ROS following neuronal cell death. Studies in the past have shown that the MP extract has the ability to reduce the neuroinflammatory level and therefore, helped in managing the PD. Our results are in agreement with published reports (Ahmad et al. Citation2012; Rai et al. Citation2017). To elucidate signaling pathways affected by herbal drug combination, in-silico studies were performed. A considerable number of researchers have used networking studies to identify new targets to support their wet-laboratory results (Mishra and Katare Citation2017). Protein–protein interaction studies determined the overall signaling pathway for the treatment of PD through MP extract and SO in combination. Reports have demonstrated the role of ADORA2A, TH, COMPT, MAOB, EGFR, etc. in PD. These studies are in support of the major hub nodes obtained via Cytoscape (Rieck et al. Citation2015; Stockwell et al. Citation2017; Dogra et al. Citation2020). Therefore, from the receptor identification and Protein–protein interaction studies, it is hypothesized that the cell signaling might have started from ADORA2A and ends with the depletion of TH. These neuroprotective effects of MP extract and SO may be attributed partially to their antioxidant anti-inflammatory potential. Hence, these findings suggest that the naturally occurring MP extract and SO combination could represent an alternative for the treatment of PD. Further studies are required to elucidate the neuroprotective effects of this herbal drug combination at molecular level.
Conclusions
Through Swiss target prediction software and Cytoscapev3.7, it is proposed that the network of the mechanism of action may have started from ADORA2A and terminated at TH, which is a dopamine-producing enzyme. SO also found to target COMT and MAOB (dopamine degrading enzymes). Therefore, it is also proposed that SO might have inhibited the dopamine degrading enzymes, thus helping levodopa contained in MP extract to increase the transport of L-DOPA converted dopamine to the brain. Hence, both the herbal compounds work synergistically in PD management. 2:3 ratio MP extract and SO treatment was observed to exhibit neuroprotectiveactivity as displayed by enhanced levels and activities of enzymatic and non-enzymatic antioxidants (glutathione reductase, catalase, AChE activity, BChE, adenosine deaminase, and glutathione peroxidase) and a significantly decreased lipid peroxidation. The herbal drug combination had significantly helped in maintaining the Ach/dopamine neurotransmitter levels and hence further clinical studies are required.
Acknowledgments
We acknowledge Dr. Ashok Chauhan, Founder, Amity University, for providing us infrastructure and facilities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in figshare.com at 10.6084/m9.figshare.15069936.
Additional information
Funding
References
- Ahmad S, Khan MB, Hoda MN, Bhatia K, Haque R, Fazili IS, Jamal A, Khan JS, Katare DP. 2012. Neuroprotective effect of sesame seed oil in 6-hydroxydopamine induced neurotoxicity in mice model: cellular, biochemical and neurochemical evidence. Neurochem Res. 37(3):516–526.
- Anilakumar KR, Pal A, Khanum F, Bawa AS. 2010. Nutritional, medicinal and industrial uses of sesame (Sesamumindicum L.) seeds-an overview. Agric Conspec Sci. 75(4):159–168.
- Arenas E, Denham M, Villaescusa JC. 2015. How to make a midbrain dopaminergic neuron. Development. 142(11):1918–1936.
- Author. n.d. An improved process for the production of Mucunapruriens seeds extract. Patent grant number:430/DEL/2009.
- Ayala A, Muñoz MF, Argüelles S. 2014. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longevity. 2014:1–31.
- Blank M, Guerim LD, Cordeiro RF, Vianna MR. 2009. A one-trial inhibitory avoidance task to zebrafish: rapid acquisition of an NMDA-dependent long-term memory. Neurobiol Learn Mem. 92(4):529–534.
- Budowski P, O'Connor RT, Field ET. 1950. Sesame oil. IV. Determination of free and bound sesamol. J Am Oil Chem Soc. 27(8):307–310.
- Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, Greenamyre JT. 2009. A highly reproducible rotenone model of Parkinson's disease. Neurobiol Dis. 34(2):279–290.
- Chen Y, Zhang DQ, Liao Z, Wang B, Gong S, Wang C, Zhang MZ, Wang GH, Cai H, Liao FF, Xu JP. 2015. Anti-oxidant polydatin (piceid) protects against substantianigral motor degeneration in multiple rodent models of Parkinson’s disease. Mol Neurodegener. 10(1):1–14.
- Chong CM, Zhou ZY, Razmovski-Naumovski V, Cui GZ, Zhang LQ, Sa F, Hoi PM, Chan K, Lee SM. 2013. Danshensu protects against 6-hydroxydopamine-induced damage of PC12 cells in vitro and dopaminergic neurons in zebrafish. Neurosci Lett. 543:121–125.
- Claiborne AL. 1985. Catalase activity. CRC handbook of methods for oxygen radical research. CRC press, 1:283–284.
- Dogra N, Mani RJ, Katare DP. 2020. Protein interaction studies for Understanding the tremor pathway in Parkinson’s disease. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders). 19(10):780–790.
- Dos Santos EU, Sampaio TF, Tenório dos Santos AD, Bezerra Leite FC, da Silva RC, Crovella S, Asano AG, Asano NM, de Souza PR. 2019. The influence of SLC 6A3 and DRD 2 polymorphisms on levodopa-therapy in patients with sporadic Parkinson's disease. J Pharm Pharmacol. 71(2):206–212.
- Duke JA. 2002. Handbook of medicinal herbs. CRC press.
- Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, Elkhayat SI, Bartels BK, Tien AK, Tien DH, Mohnot S. 2009. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 205(1):38–44.
- Ellman GL, Courtney KD, Andres Jr V, Featherstone RM. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 7(2):88–95.
- Fazli IS, Abdin MZ, Jamal A, Ahmad S. 2005. Interactive effect of sulphur and nitrogen on lipid accumulation, acetyl-CoA concentration and acetyl-CoA carboxylase activity in developing seeds of oilseed crops (brassica campestris L. and eruca sativa mill. Plant Sci. 168(1):29–36.
- Garrabou G, Gonzalez-Casacuberta I, Juarez-Flores D, Moren C. 2019. Bioenergetics and autophagic imbalance in patients-derived cell models of Parkinson disease supports systemic dysfunction in neurodegeneration. Front Neurosci. 13:894.
- Gfeller D, Grosdidier A, Wirth M, Daina A, Michielin O, Zoete V. 2014. Swisstargetprediction: a web server for target prediction of bioactive small molecules. Nucleic Acids Res. 42(W1):W32–W38.
- González-Casacuberta I, Juárez-Flores DL, Morén C, Garrabou G. n.d. Bioenergetics and autophagic imbalance Parkinson ‘s disease Foundation. [accessed 2019 Sept 19]. https://www.parkinson.org/Understanding-Parkinsons/Statistics.
- Habig WH, Pabst MJ, Fleischner G, Gatmaitan Z, Arias IM, Jakoby WB. 1974. The identity of glutathione S-transferase B with ligandin, a major binding protein of liver. Proc Natl Acad Sci USA. 71(10):3879–3882.
- Horai Y, Kakimoto T, Takemoto K, Tanaka M. 2017. Quantitative analysis of histopathological findings using image processing software. J Toxicol Pathol. 30(4):351–358.
- Hussian G, Manyam BV. 1997. Mucuna pruriens proves more effective than L-DOPA in Parkinson's disease animal model. Phytotherapy Research: An International Journal Devoted to Medical and Scientific Research on Plants and Plant Products. 11(6):419–423.
- Johnson SL, Park HY, DaSilva NA, Vattem DA, Ma H, Seeram NP. 2018. Levodopa-reduced Mucunapruriens seed extract shows neuroprotective effects against Parkinson’s disease in murine microglia and human neuroblastoma cells, Caenorhabditiselegans, and Drosophila melanogaster. Nutrients. 10(9):1139.
- Jollow DJ, Mitchell JR, Zampaglione NA, Gillette JR. 1974. Bromobenzene-induced liver necrosis. protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 11(3):151–169.
- Kasture S, Pontis S, Pinna A, Schintu N, Spina L, Longoni R, Simola N, Ballero M, Morelli M. 2009. Assessment of symptomatic and neuroprotective efficacy of Mucuna pruriens seed extract in rodent model of Parkinson’s disease. Neurotox Res. 15(2):111–122.
- Khatri DK, Juvekar AR. 2016. Abrogation of locomotor impairment in a rotenone-induced Drosophila melanogaster and zebrafish model of Parkinson's disease by ellagic acid and curcumin. Int J Nutr, Pharmacol, Neurol Dis. 6(2):90.
- Khotimah H, Ali M, Sumitro SB, Widodo MA. 2015. Decreasing α-synuclein aggregation by methanolic extract of Centella asiatica in zebrafish Parkinson's model. Asian Pac J Trop Biomed. 5(11):948–954.
- Lee KH, Cha M, Lee BH. 2020. Neuroprotective effect of antioxidants in the brain. Int J Mol Sci. 21(19):7152.
- Magistrelli L, Ferrari M, Furgiuele A, Milner AV, Contaldi E, Comi C, Cosentino M, Marino F. 2021. Polymorphisms of dopamine receptor genes and Parkinson’s disease: clinical relevance and future perspectives. Int J Mol Sci. 22(7):3781.
- Masato A, Plotegher N, Boassa D, Bubacco L. 2019. Impaired dopamine metabolism in Parkinson’s disease pathogenesis. Mol Neurodegener. 14(1):1–21.
- Mirghani ME, Man YC, Jinap S, Baharin BS, Bakar J. 2003. Application of FTIR spectroscopy in determining sesamol in sesame seed oil. J Am Oil Chem Soc. 80(1):1–4.
- Mishra S, Katare DP. 2017. Synergistic combination for chemoprevention of hepatocellular carcinoma: an in silico and in vitro approach. Basic Clin Pharmacol Toxicol. 120(6):532–540.
- Naz F, Siddique YH. 2020. Role of genes and treatments for Parkinson’s disease. Open Biol J. 8(1).
- Orr ME, Sullivan AC, Frost B. 2017. A brief overview of tauopathy: causes, consequences, and therapeutic strategies. Trends Pharmacol Sci. 38(7):637–648.
- Park SH, Ryu SN, Bu Y, Kim H, Simon JE, Kim KS. 2010. Antioxidant components as potential neuroprotective agents in sesame (Sesamum indicum L.). Food Rev Int. 26(2):103–121.
- Perez-Lloret S, Barrantes FJ. 2016. Deficits in cholinergic neurotransmission and their clinical correlates in Parkinson’s disease. npj Parkinson's Disease. 2:16001.
- Rai SN, Birla H, Singh SS, Zahra W, Patil RR, Jadhav JP, Gedda MR, Singh SP. 2015. Mucunapruriens protects against MPTP intoxicated neuroinflammation in Parkinson’s disease through NF-κB/pAKT signaling pathways. Front Aging Neurosci. 19(9):421.
- Rai SN, Birla H, Singh SS, Zahra W, Patil RR, Jadhav JP, Gedda MR, Singh SP. 2017a. Mucuna pruriens protects against MPTP intoxicated neuroinflammation in Parkinson’s disease through NF-κB/pAKT signaling pathways. Front Aging Neurosci. 9:421.
- Rai SN, Birla H, Zahra W, Singh S.S, Singh S.P. 2017. Immunomodulation of Parkinson’s disease using Mucuna pruriens (Mp). J Chem Neuroanat. 85:27–35.
- Rieck M, Schumacher-Schuh AF, Callegari-Jacques SM, Altmann V, Schneider Medeiros M, Rieder CR, Hutz MH. 2015. Is there a role for ADORA2A polymorphisms in levodopa-induced dyskinesia in Parkinson's disease patients? Pharmacogenomics. 16(6):573–582.
- Saito R, Smoot ME, Ono K, Ruscheinski J, Wang PL, Lotia S, Pico AR, Bader GD, Ideker T. 2012. A travel guide to Cytoscape plugins. Nat Methods. 9(11):1069.
- Shasmitha R. 2015. Health benefits of SesamumIndicum: A short review. Asian J Pharm Clin Res. 8(6):1–3.
- Siddiqui MA, Ahmad J, Farshori NN, Saquib Q, Jahan S, Kashyap MP, Ahamed M, Musarrat J, Al-Khedhairy AA. 2013. Rotenone-induced oxidative stress and apoptosis in human liver HepG2 cells. Mol Cell Biochem. 384(1):59–69.
- Singh SS, Rai SN, Birla H, Zahra W, Rathore AS, Dilnashin H, Singh R, Singh SP. 2020. Neuroprotective effect of chlorogenic acid on mitochondrial dysfunction-mediated apoptotic death of DA neurons in a Parkinsonian mouse model. Oxid Med Cell Longevity. 2020:1–14.
- Stockwell J, Jakova E, Cayabyab FS. 2017. Adenosine A1 and A2A receptors in the brain: current research and their role in neurodegeneration. Molecules. 22(4):676.
- Vaz RL, Outeiro TF, Ferreira JJ. 2018. Zebrafish as an animal model for drug discovery in Parkinson’s disease and other movement disorders: a systematic review. Front Neurol. 9(347).
- Verma R, Nehru B. 2009. Effect of centrophenoxine against rotenone-induced oxidative stress in an animal model of Parkinson's disease. Neurochem Int. 55(6):369–375.
- Von Wrangel C, Schwabe K, John N, Krauss JK, Alam M. 2015. The rotenone-induced rat model of Parkinson's disease: behavioral and electrophysiological findings. Behav Brain Res. 279:52–61.
- Wei Z, Li X, Li X, Liu Q, Cheng Y. 2018. Oxidative stress in Parkinson's disease: a systematic review and meta-analysis. Front Mol Neurosci. 5(11):236.
- Weisman MI, Caiolfa VR, Parola AH. 1988. Adenosine deaminase-complexing protein from bovine kidney. isolation of two distinct subunits. J Biol Chem. 263(11):5266–5270.
- Wright JR, Colby HD, Miles PR. 1981. Cytosolic factors which affect microsomal lipid peroxidation in lung and liver. Arch Biochem Biophys. 206(2):296–304.
- Yadav SK, Rai SN, Singh SP. 2016. Mucunapruriens shows neuroprotective effect by inhibiting apoptotic pathways of dopaminergic neurons in the paraquat mouse model of parkinsonism. Eur. J. Pharmaceut. Med. Res. 3:441–451.
- Yadav SK, Rai SN, Singh SP. 2017. Mucunapruriens reduces inducible nitric oxide synthase expression in Parkinsonian mice model. J Chem Neuroanat. 80:1–10.
- Zhang X, Du L, Zhang W, Yang Y, Zhou Q, Du G. 2017. Therapeutic effects of baicalein on rotenone-induced Parkinson’s disease through protecting mitochondrial function and biogenesis. Sci Rep. 7(1):1–4.