Abstract
Schistosomiasis is a neglected tropical disease affecting over 250 million people worldwide. The disease is the second most prevalent neglected tropical disease after malaria. Treatment of schistosomiasis relies on the administration of praziquantel (also known as biltricide). Reliance on a single drug poses a threat to the public health system as the parasite may become resistant as shown by some laboratory findings. The possibility of the resistance rising to clinically significant levels has motivated the scientific community to search for new drug nominees. For a long time, natural products have always been a foundation for the identification of drug leads in the pharmaceutical industry. This paper reviews the progress made in the discovery of natural anti-schistosomal agents in the field of drug discovery. We focus mainly on natural products that have been tested on the schistosome parasite and exhibited potency. We also highlight applications of advanced techniques in drug discovery, with a major focus on computer-aided drug discovery methods. Specifically, we discuss structure-based drug discovery and ligand-based drug design approaches, with an emphasis on virtual screening.
Introduction
Schistosomiasis is a water-borne parasitic neglected tropical disease (NTD) and is endemic in the poverty-stricken parts of sub-Saharan Africa. Of the world’s estimated 240 million cases of the disease in 2020, 90% of the cases were recorded in sub-Saharan Africa (Aula et al. Citation2021; Abe et al. Citation2020; Fuss et al. Citation2020). The distribution of the disease is influenced by the low-income status of the regions, characterised by poor sanitation, lack of effective health policies and generally poor living conditions (Onasanya et al. Citation2021). Africa, generally, depends on herbal medicines with over 80% of the populations, particularly in rural areas, depending on herbal medicines (Cam et al. Citation2005). It is, therefore, important to understand how these communities use traditional herbal medicines given the prevalence of schistosomiasis.
Schistosomiasis is caused by five species of schistosomes; Schistosoma mansoni, Schistosoma intercalatum, Schistosoma japonicum, Schistosoma mekongi and Schistosoma haematobium. S. haematobium causes urogenital schistosomiasis, whereas the other species cause intestinal schistosomiasis. Of the species, S.mansoni and S. haematobium are the common species in Africa (Oyeyemi et al. Citation2020). Cercariae are subsequently shed by the vector snails into freshwater and penetrate the intact skin of humans who get in contact with contaminated water (Mbereko et al. Citation2020; Caffrey et al. Citation2019). After penetrating the human skin, the maturing larvae become adults that produce eggs in about 5–7 weeks. The eggs produced are usually released into the environment through faeces or urine to complete the lifecycle. However, in some cases, the eggs remain in host tissues where they induce inflammation and then die (Neves et al. Citation2015).
Over the past few years, natural products (NPs) and compounds derived from NPs are gaining popularity as a foundation for the development of new schistosomiasis drugs (Neves et al. Citation2015). The efficiency of novel compounds against schistosomes is well-defined using various approaches like prophylactic strategies, killing the adult parasite, the cercariae and schistosomula and suppressive tactics such as hindering worm egg-laying (Tekwu et al. Citation2017). Regardless of all the studies that are underway, in the absence of a vaccine, the treatment of schistosomiasis depends solely on PZQ, which has been the drug of choice since the 1970s (Gönnert and Andrews Citation1977). The other two drugs oxamniquine and metrifonate ceased to be used clinically because of various reasons such as toxicity of metrifonate to humans and the selective effectiveness of oxamniquine on S. mansoni only (Cheuka et al. Citation2017).
The primary mechanism of PZQ remains unknown. However, the drug acts on the tegument of mature worms only. It is hypothesised that PZQ interrupts calcium ion homeostasis in the parasite, which results in an uncontrolled influx of calcium ions causing muscle contraction and paralysis (Thomas and Timson Citation2020). The drug’s easy administration, safety, tolerance, affordability and effectiveness against all five species of schistosomes have made its use advantageous (Chisango et al. Citation2019). The disadvantage of PZQ is that if a patient harbours parasites at various life stages, the patient will still show symptoms of the disease regardless of treatment (Caffrey et al. Citation2019). The reliance by the medical sector on a single drug for the treatment of schistosomiasis and the failure of existing measures to eliminate the disease has led to intensified efforts to find new anti-schistosomiasis drug leads (Mtemeli et al. Citation2021; Lombardo et al. Citation2019; Gouveia et al. Citation2018). Here, we review the progress that has been made in the development of new drugs used for treating schistosomiasis based on NPs.
The schistosomiasis drug discovery journey
Drugs have assumed critical roles in the prevention and treatment of several diseases, including schistosomiasis. The historical framework of the use of drugs in treating infections and alleviating symptoms goes back to ancient times (Lesser Citation2021). At present, traditional target-based and phenotype-based screening assays are frequently employed in drug discovery; predominantly for NTDs (Moreira-Filho et al. Citation2021).
The conventional process used in the discovery and development of an effective drug is expensive and time-consuming. The process usually consists of six main steps viz; disease selections, target hypothesis, lead identification, lead optimisation, preclinical trial and clinical trial (Kumar Citation2017). These steps were devised to ensure that drugs delivered to patients are safe and efficacious.
NPs have attracted interest for therapeutic use for a long time. In fact, 64% of all marketed drugs originate from NPs (Ferreira et al. Citation2018). Some of the NP-derived drugs that are trademark of today’s pharmaceutical care include quinine, theophylline, penicillin G, morphine, paclitaxel, digoxin, vincristine, doxorubicin and cyclosporine etc (Cragg and Newman Citation2013; Shelar and Shirote Citation2011).
NPs are considered to be a significant foundation for drug discovery because they have diverse chemical components and biomedical activities (Süntar Citation2020). Also, NPs are exceptional in that they are often rich in stereogenic centres and cover portions of chemical space that is usually not occupied by a greater part of synthetic drugs and medications (Marxer et al. Citation2012).
The investigation of medicinal plants as an innovative strategy for the tentative control of schistosomiasis is one of the sustainable and encouraging research leads. Since schistosomiasis is one of the NTDs, the drug discovery channel is, by all means, underfunded (Lombardo et al. Citation2019). However, the academic research side has explored a great number of bioactive ingredients from plants with schistosomicidal properties; specifically, those used in traditional herbal medicine (Bergquist et al. Citation2017; Tung Citation2014). Some of the plants with anti-schistosomal activities that have been studied are discussed below.
Zingiber officinale
The anti-schistosomal activity of Z. officinale has been evaluated against S. mansoni and the potency of the plant has been demonstrated (Mostafa and Eid Citation2011; Sanderson et al. Citation2002). Successful in vitro studies of the anti-schistosomal activity of Z. officinale was done although the researchers observed no significant difference in treated and untreated mice for the in vivo experiments (Sanderson et al. Citation2002). Other studies revealed anti-schistosomal effects of Z. officinale with regards to the histopathological changes observed in the small intestines of infected mice (El-Sameih et al. Citation2017). A dramatic decrease in the number of granulomas and retracted granulomal development resulting in the majority of eggs being trapped within intestinal compartments not inducing an inflammatory response was observed (Aly and Mantawy Citation2013). Additionally, Z.officinale also facilitated the restoration of the appearance of normal hepatocytes and normal hepatic strand organisation by scavenging free radicals with its strong antioxidant effect (Abd El Wahab et al. Citation2021). The treatment of S. mansoni-infected mice with aqueous ginger extract loaded on chitosan nanoparticles led to an apparent decrease in elevated liver peroxidation and improved liver function that reflects the antioxidant defence system (El-derbawy1 et al. Citation2019). At a maximum nontoxic dose of the extract, the cytotoxicity assay showed that treated Vero cells did not display any morphological differences when compared to the control, at the value of 250 µl/ml (El-Nour et al. Citation2021). Another study observed the significant synergistic effect of ginger-derived nanoparticles when used in combination with PZQ or mefloquine (Abd El Wahab et al. Citation2021).
Anonidium mannii
The presence of alkaloids, phenols, polyphenols, saponins, tannins and steroids in A. manni has been demonstrated (Ngangoue et al. Citation2020). Two compounds from the plant, aristolactam A-II and piperolactam D, had good activity (IC50 of 10–20 µM) on adult S. mansoni in vitro and demonstrated effective inhibition of the recently discovered, Nicotinamide Adenine Dinucleotide catabolizing enzyme in S. mansoni (SmNACE) (ToussiMatchi et al. Citation2020). Schistosomes are unable to produce Nicotinamide Adenine Dinucleotide (NAD) – a vital cellular metabolite. The parasites salvage NAD by using vitamins from the host. Interruption of the parasite’s NAD salvage pathway adversely impacts both the mature and immature worms’ metabolism, reproduction and survival (Schultz et al. Citation2020). Cytotoxicity tests were performed on Huh7 and A549 cells at the concentration of 100 µM and the cell viability of Huh7 cells was found to be ≈15% and 80% for A549 cells (ToussiMatchi et al. Citation2020).
Rauwolfia vomitoria
Ethanolic crude extracts of R. vomitoria stem bark and root have been shown to have moderate anti-schistosomal properties against the cercariae and adult worms of S. mansoni. In a study by Tekwu et al. (Citation2017), the stem bark and root displayed half-maximal inhibitory concentration (IC50) values of 77.5 and 112 µg/mL, respectively. Their results suggested that the stem bark is comparatively more toxic than the roots. Using this criterion, all the IC50 values that were defined in their study were much greater than 20 µg/mL. This indicates that both plant parts are not intensely cytotoxic and can be used in the treatment of schistosomiasis. HepG2 and Chang liver cells were assessed using the 3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyl-2H-tetrazolium bromide (MTT) assay and the extracts were found to be safe at IC50 > 20 µg/mL (Tekwu et al. Citation2017).
Cucurbita pepo
Studies have shown that Cucurbita pepo seed oil is effective against S. mansoni schistosomula, juveniles and adults in vitro (Ammar et al. Citation2020; Beshay et al. Citation2019). The mortality rate of the worms, the effect on motility, morphological integument changes and C.pepo seed oil-induced DNA instability are indications that the seed oil is a promising anti-schistosomal product (Ammar et al. Citation2020). In older studies, the administration of the seed oil to treat S. mansoni-infected mice led to significant reductions in liver and intestinal egg burden, with a noteworthy increase in the percentage of dead eggs in the oogram pattern. The seed oil also led to an improved granuloma size, structure and fibrosis (Beshay et al. Citation2019).
Pulsatilla chinensis
It has been shown that Hederacolchiside A1 (HSA) found in the plant P. chinensis has anti-schistosomal properties (Kang et al. Citation2018). In tests done on mice infected with S. japonicum, female worm recovery and egg load in treated mice were reduced. The schistosomicidal activity of HSA against both juvenile and adult S. japonicum with a dose–response relationship was observed (Kang et al. Citation2018).
Artemisia annua
Since the 1980s, artemisinins, a family of sesquiterpene trioxane lactones, A. annua (sweet wormwood) derivatives, have shown to be anti-schistosomal agents (Neves et al. Citation2015). Artemisinins appear to be more effective on juvenile worms than adult worms (Liu et al. Citation2014). This may be due to artemisinins’ mechanism of action on the schistosomes as the artemisinins react with the iron ions of haeme. The iron ions of haeme, are derived from haemoglobin digestion. Reactive oxygen species and chelates are produced in the process (Xiao et al. Citation2001). Several clinical studies have commenced, repositioning artemisinins as schistosomicidal drugs (Panic et al. Citation2014; Liu et al. Citation2014).
In addition to the few studies discussed above, many other studies have been carried out on plants with schistosomicidal properties. Table presents a summary of other plants that have shown potency against schistosomes.
Table 1. In vivo studies of selected traditionally employed plant species against S. mansoni
Current drug nominees for schistosomiasis
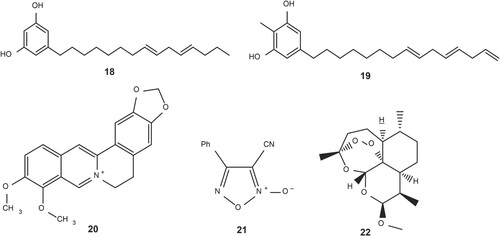
Only a limited number of plant species traditionally used against S. mansoni have undergone scientific screening. Primarily, in vitro assessments were made on these plants and the most significant results were obtained from the Abrus precatorius L. subsp. Africanus verdc root extract which, however, still needs to be further assessed in vivo and randomised clinical trials. The most progressive agents that have been tried for potential use in the treatment of schistosomiasis to date are the quinine analogues and artemisinin-based antimalarial drugs (Duarte Galhardo de Albuquerque et al. Citation2020). The trials were motivated mostly by their proven usefulness in treating malaria. The effective clinical trials by these agents give assurance for the use of NPs in this infectious disease (Cheuka et al. Citation2017). Some chemical structures of anti-schistosomal bioactive molecules are cardoldiene (18), 2-methyl cardoldiene (19) (both derived from Anacardium occidentale), berberine (20) (derived from plants in the Berberidaceae family in the genus Berberis), furoxan (21), and artemether (22) (derived from Artemisia annua) shown in (Fig ).
Figure 1. Structures of various bioactive molecules against schistosomiasis (Ghosh et al. Citation2019).
Nanoparticles and nanocarriers
Some researchers have discovered that nanoparticles such as nanoselenium and nanogold have therapeutic properties against intestinal disorders induced by S. mansoni (Adekiya et al. Citation2020 Dkhil et al. Citation2019). In these studies, the nanoparticles caused a reduction in the intensity of oxidative stress, changes in body weight, histological damage and the number of goblet cells in the jejunum. It was proposed that the therapeutic effects of nanoparticles are associated with their antioxidant activities (Dkhil et al. Citation2019). It was also revealed that the particular physical structure of nanoparticles, such as having large surface areas, permits the nanoparticles to interact with microorganisms (Ghosh et al. Citation2019). Their size gains them access to the cells. In another study that investigated the in vitro cytotoxicity and schistosomicidal activity of oleic acid-loaded nanocapsules, a 100% concentration-dependent mortality was observed from 200 µg/mL in 4 h of incubation and 50 µg/mL in 24 h (Nunes et al. Citation2021). Curcumin loaded gold-nanoparticles were tested on S. mansoni in vivo and displayed a synergistic anti-schistosomal effect when combined with PZQ. A 97.4% reduction of worm burden in the 3rd week was observed (Mokbel et al. Citation2020). The studies showed that the nanocapsules and curcumin loaded gold-nanoparticles led to severe damage to the tegument dorsal surface, making it a promising new treatment alternative.
Marine organisms
The potency of 13 macroalgae extracts from the Gracilaria, Dictyota and Laurencia genera was tested against S. mansoni in vitro (Stein et al. Citation2015). The extracts of Dictyota dichotoma, Dictyota menstrualis, Dictyota mertensii, Plocamium brasiliense, Spyridia hypnoides, Gracilaria ornate, Chondria littoralis and Laurencia dendroidea led to a100% mortality of adult worms at a concentration of 500 g/ml after 2 h. When the concentration was lowered to 100 g/ml and 100% mortality was observed after 24-72 h. In another trial, 45 crude extracts obtained from 37 species of Brazilian macroalgae were screened for schistosomicidal and molluscicidal activity in vitro on S. mansoni. Of the 37 species, 21 displayed schistosomicidal activity. Worm reproduction was notably inhibited by most of the species, as observed by egg counting (Stein et al. Citation2021). Given that there is a dearth of literature on the potential of marine organisms in schistosomiasis drug discovery, these few studies may generate increased interest to characterise more seaweeds.
Progress made in anti-schistosomal lead compound optimisation and development
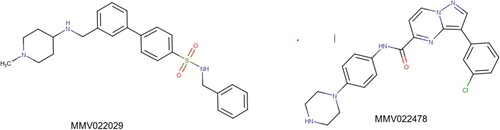
Academic and pharmaceutical research groups working on drug discovery have made significant progress in identifying a range of novel leads, which still stand as the foundation for anti-schistosomal drug discovery (Bergquist et al. Citation2017). Many experiments have been done in vitro on larval, juvenile and adult S. mansoni. To date, more than 500 000 compounds have been screened in high-throughput screening projects (Hernandez et al. Citation2019; Guidi et al. Citation2017; Neves et al. Citation2016; Kuhn et al. Citation2010). One of the latest studies reported an in vitro and in vivo screening of the Medicines for Malaria Venture (MMV) Pathogen Box containing 400 compounds. The study revealed that MMV022029 and MMV022478 had the highest worm burden reduction and granted a series of new potent scaffolds and pharmacophores that could be used to design and develop appropriate alternative(s) to PZQ (Pasche et al. Citation2019). The pharmacophores’ structures are shown in Figure .
Some of the leads are currently used in structure–activity relationships in various ongoing experiments and quantitative structure–activity relationships (QSAR) (Melo-Filho et al. Citation2016). Despite the urgent need to find new schistosomiasis drugs, there is no single drug candidate that is close to reaching the market at present (Lombardo et al. Citation2019). Of the 402 new chemical entities that were registered between 2000 and 2013, none targeted schistosomiasis nor NTDs (Ferreira et al. Citation2015).
Application of advanced techniques in schistosomiasis drugs discovery
Currently, drug design and development are driven by the modernisation and knowledge of a combination of experimental and computational approaches (Neves et al. Citation2016b). The integration of molecular and computer-aided drug design (CADD) in drug development alongside organic synthesis has led to increased availability of biological, structural and chemical data (Ferreira et al. Citation2018; Herrera Acevedo et al. Citation2017; Neves et al. Citation2015). Many molecular targets from the Schistosoma species have been evaluated (Mafud et al. Citation2016). The disclosure of the genomes of the three species of schistosomes that are usually implicated in infection has been a breakthrough in the field as it led to increased comprehension of the molecular machinery involved in the parasite–host relationship and, therefore, in the disease pathophysiology (Ferreira et al. Citation2018). Various proteins are emerging as cutting edge potential drug targets. Examples of the proteins are; G-protein-coupled receptors, kinases, ion channels, reductases, acetylases and proteases. Presently, 238 schistosomes’ protein structures are registered in the Protein Data Bank and the majority of the proteins were obtained through X-ray crystallography. The existence of large virtual libraries of small molecules has assisted medicinal chemists in virtually screening possible drug candidates for NTDs. Libraries such as the ZINC, GDB-17, Chemspider, ChemMine, Drug Bank and PubChem contain small molecules ranging between 5000 and 100 billion and are readily available online (Lin et al. Citation2020; Banegas-Luna et al. Citation2018). This information has brought schistosomiasis drug discovery into the molecular age and opened various new possibilities in the field. Currently, schistosomiasis drug discovery programs are carried out through the implementation of unprecedented molecular modelling coupled with drug repositioning research, structure-based drug design and target-focused compound screening (Ferreira et al. Citation2018). Computational techniques used in schistosomiasis’ drug discovery are generally categorised under structure-based drug design (SBDD) and ligand-based drug design (LBDD) (Wilson and Lill Citation2011).
SBDD is the design and optimisation of a chemical structure to identify a compound suitable for clinical testing as a drug lead (Batool et al. Citation2019). SBDD is based on knowledge of the drug’s three-dimensional structure and how the shape of the drug and charge cause it to interact with its biological target. In SBDD, large databases or libraries of 3D structures of small molecules can virtually be screened on a 3D structure of schistosomiasis protein (Batool et al. Citation2019). Molecular docking is a process commonly used in screening good drug-like compounds. Through the application of specialised docking programs, the process searches the possible conformation or poses of molecules inside the binding pocket of drug targets and scores them according to their stability energy-wise (Xu et al. Citation2018). LBDD is an approach commonly used in default of the receptor 3D structure. LBDD is rooted in data of established molecules that bind to the biological target of interest. 3D QSAR and pharmacophore modelling are the most essential and extensively used tools in LBDD. They can offer predictive models well-suited for lead identification and optimisation.
One refined application that can speed up the discovery of drugs with schistosomicidal activity is virtual screening (VS). VS is the use of computational filters in a database of chemical structures to predict the bioactivity of a compound concerning a specific target. The major benefit obtained from VS is the reduced time and resources required for an in vitro screen of a chemical library of known compounds (Nunes et al. Citation2016).
Efforts have been made in using computational methods to develop new schistosomicidal agents or drugs. In this section (Table ), we summarise the general categories of CADD methods used in the discovery of novel schistosomicidal drugs.
Table 2. The general categories of CADD methods used in the discovery of novel schistosomicidal drugs.
Most CADD efforts in schistosomiasis drug discovery have focused on the design and synthesis of schistosomicidal agents, followed by VS. Although the chemical space of current NPs databases is huge enough to attract VS projects, there are very few studies that have been done to advance the discovery of schistosomicidal agents. In a 2017 study (Akachukwu et al. Citation2017), African originated plant metabolites were evaluated for ‘drug-likeness’ and docked toward four selected validated Schistosoma drug targets; Glutathione S-transferase, Thioredoxinglutathione reductase, Histone deacetylase and S. mansoni. Out of a total of 27 bioactive compounds with anti-Schistosoma history, one of the compounds emerged as the most interesting candidate by both being drug-like and inhibiting the activities of the studied enzyme targets at the micromole arrangement.
For the past decade, African NP medicinal chemists and database developers have progressed much in the development of small compound NPs databases and libraries. Currently, there are more than ten NP libraries across the African continent, with 20000 chemical entries in total (Sorokina and Steinbeck Citation2020). Most of the libraries are not yet available online except for the Northern African NPs Database (NANPDB) (Ntie-Kang et al. Citation2017), Integrated Ethiopian traditional herbal medicine and phytochemicals database (ETM-DB) (Bultum et al. Citation2019) and South African natural compound database (SANCDB) (Hatherley et al. Citation2015). The development of libraries allows medicinal chemists and drug developers to conduct in-depth in silico studies that can advance the discovery of schistosomicidal drugs.
Structure-based virtual screening
Structure-based virtual screening (SBVS) explores information about a 3D structure of targets that are either determined experimentally by, for example, X-ray crystallography and nuclear magnetic resonance or are computationally predicted through homology modelling to select suitable ligands (Neves et al. Citation2015).
In one of the most recent studies, an ecto-enzyme called SmNACE in S. mansoni was discovered. SmNACE has an encouraging topology because it is one of the uncommon recognised and characterised targets on the tegument of adult schistosomes that causes serious clinical problems and could be considered a prospective target for drug nominees (ToussiMatchi et al. Citation2020). The enzyme purine nucleoside phosphorylase (PNP), one of the explored targets, is a crucial element in the purine recovery biochemical route (Pereira et al. Citation2020). Given that Schistosoma worms do not have a de novo purine biosynthesis pathway, they solely rely on the salvage pathway to fulfil their requirement for purines, as they are essential for the biosynthesis of nucleic acids (Torini et al. Citation2018). Based on these findings, PNP could be considered a pharmacological target for developing new schistosomicidal agents (Ferreira et al. Citation2018). Drug discovery studies that use diverse methods for drug screening are more likely to find new lead compounds (Tavares et al. Citation2016).
Drug repurposing
Drug repurposing/repositioning of approved drugs has been one of the starting points in identifying new schistosomiasis drug leads. Various drugs that have been FDA approved were assessed for the schistosomicidal potency (Gouveia et al. Citation2018). The initial and most extensively investigated drug class are the semisynthetic artemisinins, together with dihydroartemisinin, artemether, and artesunate, which are efficient against the juvenile schistosomes but less so against mature worms (Caffery et al. Citation2019; Gouveia et al. Citation2018).
Other drugs that have been investigated and exhibited potency include mefenamic acid which was shown to be effective against S. mansoni (Lago et al. Citation2019); promethazine on S.mansoni (Roquini et al. Citation2019); spironolactone on S. mansoni (Guerra et al. Citation2019); single oral fixed-dose of praziquantel-miltefosine-nano combination on S. mansoni (Eissa et al. Citation2020); miltefosine on S. mansoni (El-Faham et al. Citation2017); perhexiline maleate (Guidi et al. Citation2016); clofazimine and doramectin have also displayed schistosomicidal activity (Gemma et al. Citation2019). In these studies, it is evident that repurposing approaches are useful as starting points in the discovery of novel drugs against Schistosoma.
Major hindrances in drug development and prospects
As the term implies, NTDs are neglected by the pharmaceutical production sector since the diseases affect mainly the poorest societies (Kefalidou et al. Citation2016; Moon et al. Citation2012). In these countries, the majority of people can barely afford a balanced diet, accommodation, clothes and formal health care. Thus, many resort to alternative traditional medicines (Length Citation2017). Firstly, major pharmaceutical companies’ investment in the therapeutic areas is not financially appealing because of poor financial returns. It is also known that the drug discovery process is costly and with a high failure rate (Weng et al. Citation2018) thus, drug discovery against parasitic diseases, in general, has not been inspired by profit-making motives (Cheuka et al. Citation2017). As a result, pharmaceutical companies rather develop drugs that target chronic diseases, ensuring long-term sales (Kefalidou et al. Citation2016). Further, challenges also include ensuring the right to use of sufficient plant resources, licensed property rights concerns and the complications of NP chemistry with related incompetence of working with NPs (Cheuka et al. Citation2017). The number of well-validated molecular drug targets for tropical diseases is very negligible, partly because the detailed biology of many of the pathogens is yet to be understood (De Rycker et al. Citation2018). In the light of these hindrances, the application of in silico methods in the discovery of novel schistosomiasis drugs is also slackened.
VS has arisen in drug discovery as a robust computational method of screening huge libraries of small molecules for new hits with preferred properties that would then be experimentally tried (Neves et al. Citation2018). Computational simulations used in VS strategies are very proficient in the identification of pharmacologically dynamic compounds or novel indications for drugs already used to treat other diseases. The use of this technology may speed up the pace at which schistosomiasis drug discovery is moving. Testing the candidate compounds in silico and selecting those with a high chance of binding to the target in the parasite is vital, as it lowers the costs of drug discovery (Pereira et al. Citation2020). Political leaders and governments need to identify schistosomiasis as a public health problem that needs to be given greater consideration in terms of funding and setting up effective monitoring and surveillance systems (Abe et al. Citation2020). More investigation of the therapeutic impact of a wide range of natural antioxidants and herbal extracts against these diseases may prove to be of great assistance for the poorest among the human population living in the tropical nations of Asia, Africa and North and South America where schistosomiasis is endemic (Ghosh et al. Citation2019). It is vital to employ CADD methods to minimise the costs involved in the discovery of new schistosomiasis drugs, considering that the most affected regions are low-income countries (Zorn et al. Citation2021).
Future directions in CADD based on novel anti-schistosomal compounds
While CADD has made significant contributions to the development of drugs that are in clinical use or undergoing clinical trials, additional and new strategies are needed to ensure the hit compounds that target schistosome proteins are further developed into lead compounds. Molecular dynamics can be used in upscaling drug discovery, for example, through energy calculations of a binding ligand (hit compound) considering free energy perturbations (Mortier et al. Citation2015). The availability of supercomputing facilities can help facilitate enhanced CADD through parallel processing, and advanced programs, algorithms, and tools which enable lead identification (Macalino et al. Citation2015).
Artificial intelligence (AI) is another approach that has varied applications in drug discovery. These include prediction of protein folding, protein–protein interaction, evaluation of ADMET properties, virtual screening, QSAR, and de novo drug design (Wang et al. Citation2019). Generating and labeling applicable chemical, biological, and physiological data for queries related to efficacy and safety is complicated when applying AI in drug discovery (Selvaraj et al.,Citation2021). However, with proper understanding of which data to generate, and for which purpose, the discovery of anti-schistosomal drugs can be advanced using AI. This would be through measuring and capturing of appropriate biological endpoints in vivo and application of computational algorithms regarding the hit compounds ‘efficacy and safety (Bender and Cortes-Ciriano Citation2019). Hence, AI and machine learning tools promise to enable dynamic applications in the future pharma and biomedical industries. With developments in diverse fields, a drift towards an automated and more accurate approach in anti-schistosomal drug discovery, with the aid of AI can be expected (Selvaraj et al. Citation2021). Furthermore, identification of anti-schistosomal properties of approved drugs that are already used to treat other diseases can be done using CAAD (Prieto-Martínez et al. Citation2019). New applications of existing drugs may be found through repositioning them from their approved indications using information obtained through genomics, thus laying a crucial foundation for drug repurposing (Kiriiri et al. Citation2020).
Conclusions
The recent advances in CADD represent a new era in the discovery of anti-schistosomal drugs. While many plants have been tested against schistosomiasis and proved to be potent, none of them are close to reaching the market as new drugs. We propose using in silico methods to accelerate this process, as they are more accurate and more efficient than the conventional approaches. Considering that the schistosomiasis drug discovery process is already underfunded, the use of CADD methods will lead to the identification of hit and lead compounds at a lesser cost.
Conflicts of interest
No potential conflict of interest was reported by the authors.
Author contribution statement
Mtemeli F. L. – Conceived and designed the article and interpreted the relevant literature; drafted the article.
Ndlovu J. – Interpreted the relevant literature; revised the paper critically for important intellectual content.
Mugumbate G. – Interpreted the relevant literature; revised the paper critically for important intellectual content.
Makwikwi T. - Interpreted the relevant literature; revised the paper critically for important intellectual content.
Shoko R. - Conceived and designed the article and interpreted the relevant literature, revised the paper critically for important intellectual content; supervised the work.
All authors approved the final version to be published and agreed to be accountable for all aspects of the work.
Acknowledgements
The authors are indebted to Jonathan Bvunzawabaya, whose contribution improved the quality of this paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Additional information
Funding
References
- Abd El Wahab WM, El-Badry AA, Mahmoud SS, El-Badry YA, El-Badry MA, Hamdy DA. 2021. Ginger (Zingiber officinale)-derived nanoparticles in Schistosoma mansoni infected mice: hepatoprotective and enhancer of etiological treatment. PLoS Negl Trop Dis. 15(5):e0009423.
- Abe EM, Tambo E, Xue J, Xu J, Ekpo UF, Rollinson D, Yang K, Li SZ, Zhou XN. 2020. Approaches in scaling up schistosomiasis intervention towards transmission elimination in Africa: leveraging from the Chinese experience and lessons. Acta Trop. 208(July 2018):105379–105379. doi:10.1016/j.actatropica.2020.105379.
- Adekiya TA, Kondiah PP, Choonara YE, Kumar P, Pillay V. 2020. A review of nanotechnology for targeted anti-schistosomal therapy. Frontiers in Bioengineering and BiotechUhnology. 8:2296–4185.
- Akachukwu I, Olubiyi OO, Kosisochukwu A, John MC, Justina NN. 2017. Structure-Based study of Natural products with anti-Schistosoma activity. Curr Comput-Aided Drug Des. 13(2):91–100. doi:10.2174/1573409913666170119114859.
- Aleixo de Carvalho LSG, de Moraes RB, Silva Pinto J, de Faria Pinto PL, Pereira P, dos S O, Da Silva Filho AA. 2015. Schistosomicidal activity and docking of Schistosoma mansoni ATPDase 1 with licoflavone B isolated from glycyrrhizainflata (fabaceae). Exp Parasitol. 159:207–214. doi:10.1016/j.exppara.2015.09.015.
- Aly HF, Mantawy MM. 2013. Efficiency of ginger (Zingiber officinale) against Schistosoma mansoni infection during host-parasite association. Parasitol Int. 62(4):380–389.
- Ammar AI, Afifi AF, Essa A, Galal-Khallaf A, Mokhtar MM, Shehab-Eldeen S, Rady AA. 2020. Cucurbita pepo seed Oil induces microsatellite instability and tegumental damage to Schistosoma mansoni immature and adult worms In vitro. Infect Drug Resist. 13:3469–3484.
- Aula OP, McManus DP, Jones MK, Gordon CA. 2021. Schistosomiasis with a focus on Africa. Tropical Medicine and Infectious Disease. 6(3):109–109.
- Banegas-Luna A-J, Cerón-Carrasco JP, Pérez-Sánchez H. 2018. A review of ligand-based virtual screening web tools and screening algorithms in large molecular databases in the age of big data. Future Med Chem. 10(22):2641–2658. doi:10.4155/fmc-2018-0076.
- Batool M, Ahmad B, Choi S. 2019. A structure-based drug discovery paradigm. Int J Mol Sci. 20(11):2783–2783. doi:10.3390/ijms20112783.
- Bender A, Cortes-Ciriano I. 2019. Artificial intelligence in drug discovery: what is realistic, what are illusions? part 2: a discussion of chemical and biological data. Drug Discovery Today Elseiver. 26(2):511–524. doi:10.1016/j.drudis.2020.11.037.
- Bergquist R, Utzinger J, Keiser J. 2017. Controlling schistosomiasis with praziquantel: How much longer without a viable alternative? Infect Dis Poverty. 6(1):1–10. doi:10.1186/s40249-017-0286-2.
- Beshay EVN, Rady AA, Afifi AF, Mohamed AH. 2019. Schistosomicidal, antifibrotic and antioxidant effects of Cucurbita pepo L. seed oil and praziquantel combined treatment for Schistosoma mansoni infection in a mouse model. J Helminthol. 93(3):286–294. doi:10.1017/S0022149X18000317.
- Bultum LE, Woyessa AM, Lee D. 2019. ETM-DB: Integrated Ethiopian traditional herbal medicine and phytochemicals database. BMC Complement Altern Med. 19(1):212–212. doi:10.1186/s12906-019-2634-1.
- Caffrey CR, El-Sakkary N, Mäder P, Krieg R, Becker K, Schlitzer M, Drewry DH, Vennerstrom JL, Grevelding CG. 2019. Drug discovery and development for schistosomiasis. Neglected Tropical Diseases: Drug Discovery and Development. 12:187–225. doi:10.1002/9783527808656.ch8.
- Cheuka PM, Mayoka G, Mutai P, Chibale K. 2017. The role of natural products in drug discovery and development against neglected tropical diseases. Molecules. 22(1):58–58. doi:10.3390/molecules22010058.
- Chisango TJ, Ndlovu B, Vengesai A, Nhidza AF, Sibanda EP, Zhou D, … Mduluza T. 2019. Benefits of annual chemotherapeutic control of schistosomiasis on the development of protective immunity. BMC Infect Dis. 19(1):1–9.
- Cock IE, Selesho MI, Van Vuuren SF. 2018. A review of the traditional use of southern African medicinal plants for the treatment of selected parasite infections affecting humans. J Ethnopharmacol. 220:250–264.
- Cragg GM, Newman DJ. 2013. Natural products: a continuing source of novel drug leads. Biochimica et Biophysica Acta (BBA)-General Subjects. 1830(6):3670–3695.
- De Rycker M, Baragaña B, Duce SL, Gilbert IH. 2018. Challenges and recent progress in drug discovery for tropical diseases. Nature. 559(7715):498–506. doi:10.1038/s41586-018-0327-4.
- Dkhil MA, Khalil MF, Diab MS, Bauomy AA, Santourlidis S, Al-Shaebi EM, Al-Quraishy S. 2019. Evaluation of nanoselenium and nanogold activities against murine intestinal schistosomiasis. Saudi J Biol Sci. 26(7):1468–1472.
- Duarte Galhardo de Albuquerque RD, Mahomoodally MF, Lobine D, Suroowan S, Rengasamy KR. 2020. Botanical products in the treatment and control of schistosomiasis: recent studies and distribution of active plant resources according to affected regions. Biology (Basel). 9(8):223–223. doi:10.3390/biology9080223.
- Durojaye OA, Nwanguma BC, Joshua PE, Ogidigo JO, Cosmas S, Asomadu RO, Obeta JN. 2019. Evaluation of 6-gingerol and its modified analogues as therapeutic candidates against Schistosoma mansoni phosphofructokinase. VacciMonitor. 28(1):38–47.
- Eissa MM, El-Azzouni MZ, El-Khordagui LK, Abdel Bary A, El-Moslemany RM, Abdel Salam SA. 2020. Single oral fixed-dose praziquantel-miltefosine nanocombination for effective control of experimental schistosomiasis mansoni. Parasites and Vectors. 13(1):1–12. doi:10.1186/s13071-020-04346-1.
- El-derbawy1 MM, El WAEMS, Kholy1 IRB, S HS. 2019. A study of the potential therapeutic effect of ginger (Zingiber officinale) loaded nanoparticles on murine schistosomiasis mansoni. J Egypt Soc Parasitol. 49(1):123–128.
- El-Faham MH, Eissa MM, Igetei JE, Amer EI, Liddell S, El-Azzouni MZ, Doenhoff MJ. 2017. Treatment of Schistosoma mansoni with miltefosine in vitro enhances serological recognition of defined worm surface antigens. PLoS Negl Trop Dis. 11(8):1–26. doi:10.1371/journal.pntd.0005853.
- El-Nour A, Mohamed F, Fadladdin Y. 2021. Anti-schistosomal activity of Zingiber officinale, piper nigrum, and coriandrum sativum aqueous plant extracts on hamsters infected with Schistosoma mansoni. J Parasitol Res. 2021.
- El-Sameih MA, Hassan FA, Abed GH, Omar HEDM. 2017. Role Of ginger in the Treatment of Schistosoma mansoni infection a histopathological studies. Alexandria Journal of Veterinary Sciences. 52(1):198–204.
- Elujoba AA, Odeleye OM, Ogunyemi CM. 2005. Review traditional medicine development for medical and dental primary health care Delivery system in the orthodox medicine, as currently made available today in Nigeria (as in most African countries), so long as every nook and corner of our rural popu. African Ethnomedicines Network. 2:46–61.
- Eweas AF, Allam G, Abuelsaad AS, ALGhamdi AH, Maghrabi IA. 2013. Design, synthesis, anti-schistosomal activity and molecular docking of novel 8-hydroxyquinoline-5-sufonyl 1, 4-diazepine derivatives. Bioorg Chem. 46:17–25.
- Fadladdin YAJ. 2021. Antischistosomal activity of Origanum majorana, Ziziphus spina-christi, and salvia fruticosa plant extracts on hamster infected with Schistosoma haematobium. BioMed Res Int. 2021.
- Ferreira LG, Oliva G, Andricopulo AD. 2018. From medicinal Chemistry to human health: Current approaches to drug discovery for Cancer and Neglected Tropical diseases. Anais da Academia Brasileira de Ciensias. 90:645–661.
- Fuss A, Mazigo HD, Mueller A. 2020. Malacological survey to identify transmission sites for intestinal schistosomiasis on ijinga island, mwanza, north-western Tanzania. Acta Trop. 203(April 2019):105289–105289. doi:10.1016/j.actatropica.2019.105289.
- Gemma S, Federico S, Brogi S, Brindisi M, Butini S, Campiani G. 2019. Dealing with schistosomiasis: Current drug discovery strategies. In Annual Reports in Medicinal Chemistry (1st ed., Vol. 53). Elsevier Inc. https://doi.org/10.1016/bs.armc.2019.06.002.
- Ghosh S, Das AK, Sarkar P, Sil PC. 2019. Oxidative stress in schistosomiasis, echinococcosis, and trypanosomiasis: a therapeutic approach. In: Discovery and development of therapeutics from Natural products against Neglected Tropical diseases. Kolkata: Elsevier; p. 219–239.
- Gönnert R, Andrews P. 1977. Praziquantel, a new broad-spectrum anti-schistosomal agent. ZeitschriftFür Parasitenkunde. 52(2):129–150. doi:10.1007/BF00389899.
- Gouveia MJ, Brindley PJ, Gärtner F, Costa JM, Vale N. 2018. Drug repurposing for schistosomiasis: combinations of drugs or biomolecules. Pharmaceuticals. 11(1):15–15.
- Gruessner BM, Cornet-Vernet L, Desrosiers MR, Lutgen P, Towler MJ, Weathers PJ. 2019. It is not just artemisinin: Artemisia sp. for treating diseases including malaria and schistosomiasis. Phytochem Rev. 18(6):1509–1527.
- Guerra RA, Silva MP, Silva TC, Salvadori MC, Teixeira FS, De Oliveira RN, Rocha JA, Pinto PLS, De Moraes J. 2019. In vitro and in vivo studies of spironolactone as an anti-schistosomal drug capable of clinical repurposing. Antimicrob Agents Chemother. 63(3):1–11. doi:10.1128/AAC.01722-18.
- Guidi A, Lalli C, Gimmelli R, Nizi E, Andreini M, Gennari N, Saccoccia F, Harper S, Bresciani A, Ruberti G. 2017. Discovery by organism-based high-throughput screening of new multi-stage compounds affecting Schistosoma mansoni viability, egg formation and production. PLoS Negl Trop Dis. 11(10):e0005994–e0005994. doi:10.1371/journal.pntd.0005994.
- Guidi A, Lalli C, Perlas E, Bolasco G, Nibbio M, Monteagudo E, Bresciani A, Ruberti G. 2016. Discovery and Characterisation of novel anti-schistosomal properties of the anti-anginal drug, perhexiline and Its impact on Schistosoma mansoni male and female reproductive systems. PLoS Negl Trop Dis. 10(8):1–22. doi:10.1371/journal.pntd.0004928.
- Hatherley R, Brown DK, Musyoka TM, Penkler DL, Faya N, Lobb KA, Tastan Bishop Ö. 2015. SANCDB: A South African natural compound database. J Cheminform. 7(1):29–29. doi:10.1186/s13321-015-0080-8.
- Hernandez HW, Soeung M, Zorn KM, Ashoura N, Mottin M, Andrade CH, Caffrey CR, de Siqueira-Neto JL, Ekins S. 2019. High-Throughput and Computational repurposing for Neglected diseases. Pharm Res. 36(2):27–27. doi:10.1007/s11095-018-2558-3.
- Herrera Acevedo C, Scotti L, Feitosa Alves M, Formiga Melo Diniz MDF, Scotti MT. 2017. Computer-aided drug design using sesquiterpene lactones as sources of new structures with potential activity against infectious neglected diseases. Molecules. 22(1):79–79.
- Kang N, Shen W, Gao H, Feng Y, Zhu W, Yang S, Liu Y, Xu Q, Yu D. 2018. Anti-schistosomal properties of hederacolchiside a1 isolated from Pulsatilla chinensis. Molecules. 23(6):1–16. doi:10.3390/molecules23061431.
- Kefalidou AA, Nations U, Affairs S. 2016. Brief for GSDR – 2016 Update Sustainable drug development for Neglected Tropical Diseases. 1–6.
- Kiriiri GK, Njogu PM, Mwangi AN. 2020. Exploring different approaches to improve the success of drug discovery and development projects: a review. Futur J Pharm Sci. 6:27–27. doi:10.1186/s43094-020-00047-9.
- Kuhn I, Kellenberger E, Said-Hassane F, Villa P, Rognan D, Lobstein A, Haiech J, Hibert M, Schuber F, Muller-Steffner H. 2010. Identification by high-throughput screening of inhibitors of Schistosoma mansoni NAD+ catabolizing enzyme. Bioorg Med Chem. 18(22):7900–7910. doi:10.1016/j.bmc.2010.09.041.
- Kumar D. 2017. Software and web resources for computer-aided molecular software and web resources for computer-aided molecular modelling and drug discovery. March 2016. https://doi.org/10.1201/b19853-3.
- Lago EM, Silva MP, Queiroz TG, Mazloum SF, Rodrigues VC, Carnaúba PU, Pinto PL, Rocha JA, Ferreira LLG, Andricopulo AD, de Moraes J. 2019. Phenotypic screening of nonsteroidal anti-inflammatory drugs identified mefenamic acid as a drug for the treatment of schistosomiasis. EBioMedicine. 43:370–379. doi:10.1016/j.ebiom.2019.04.029.
- Length F. 2017. Determination of bioactive constituents of Rauwolfia vomitoria afzel(asofeyeje) roots using gas chromatography-mass spectrometry (GC-MS) and Fourier transform infrared spectrometry (FT-IR). African Journal of Pharmacy and Pharmacology. 11(2):25–31. doi:10.5897/AJPP2016.4712.
- Lesser B. 2021. The Origin and Mechanism of Consuming and Administering Medicine. Dual diagnosis. orghttps://dualdiagnosis.org/drug-addiction/evolution-administering-consuming-drugs/.
- Lin X, Li X, Lin X. 2020. A review on applications of Computational methods in drug screening and design. Molecules. 25(6):1375–1375. doi:10.3390/molecules25061375.
- Liu Y-X, Wu W, Liang Y-J, Jie Z-L, Wang H, Wang W, Huang Y-X. 2014. New uses for old drugs: The tale of artemisinin derivatives in the elimination of schistosomiasis japonica in China. Molecules. 19:15058–15074.
- Lombardo FC, Pasche V, Panic G, Endriss Y, Keiser J. 2019. Life cycle maintenance and drug-sensitivity assays for early drug discovery in Schistosoma mansoni. Nat Protoc. 14(2):461–481.
- Macalino SJY, Gosu V, Hong S, Choi S. 2015. Role of computer-aided drug design in modern drug discovery. Arch Pharmacal Res. 38(9):1686–1701.
- Mafud AC, Silva MP, Monteiro DC, Oliveira MF, Resende JG, Coelho ML, … de Moraes J. 2016. Structural parameters, molecular properties, and biological evaluation of some terpenes targeting Schistosoma mansoni parasite. Chem-Biol Interact. 244:129–139.
- Marxer M, Ingram K, Keiser J. 2012. Development of an in vitro drug screening assay using Schistosoma haematobium schistosomula. Parasit Vectors. 5(1):1–8.
- Mbereko A, Chimbari MJ, Manyangadze T, Mukaratirwa S. 2020. Knowledge and perceptions of schistosomiasis, a water-borne disease, in two semi-arid rural areas of South Africa (ndumo) and Zimbabwe (ntalale). Food and Waterborne Parasitology. 21:e00091–e00091.
- Melo-Filho CC, Dantas RF, Braga RC, Neves BJ, Senger MR, Valente WCG, Rezende-Neto JM, Chaves WT, Muratov EN, Paveley RA, et al. 2016. QSAR-Driven Discovery of novel chemical scaffolds active against Schistosoma mansoni. Journal of Chemical Information and Modelling. 56(7):1357–1372. doi:10.1021/acs.jcim.6b00055.
- Mokbel KEDM, Baiuomy IR, Sabry AEHA, Mohammed MM, El-Dardiry MA. 2020. In vivo assessment of the antischistosomal activity of curcumin loaded nanoparticles versus praziquantel in the treatment of Schistosoma mansoni. Sci Rep. 10(1):1–9.
- Mølgaard P, Nielsen SB, Rasmussen DE, Drummond RB, Makaza N, Andreassen J. 2001. Anthelmintic screening of Zimbabwean plants traditionally used against schistosomiasis. J Ethnopharmacol. 74(3):257–264.
- Moon S, Bermudez J, ‘t Hoen E. 2012. Innovation and access to medicines for neglected populations: could a treaty address a broken pharmaceutical R&D system? PLoS Med. 9(5):e1001218–e1001218. doi:10.1371/journal.pmed.1001218.
- Moreira-Filho JT, Silva AC, Dantas RF, Gomes BF, Neto LRS, Brandao-Neto J, … Andrade CH. 2021. Schistosomiasis drug discovery in the Era of automation and Artificial intelligence. Front Immunol. 12:642383–642383.
- Mortier J, Rakers C, Bermudez M, Murgueitio MS, Riniker S, Wolber G. 2015. The impact of molecular dynamics on drug design applications for the characterization of ligand-macromolecule complexes. Drug Discov Today. 20:20686–20702.
- Mostafa OMS, Eid RA. 2011. Anti-schistosomal activity of ginger (Zingiber officinale) against Schistosoma mansoni harboured in C57 mice. 395–403. https://doi.org/10.1007/s00436-011-2267-x.
- Mtemeli FL, Walter I, Tinago T, Shoko R. 2021. An assessment of the molluscicidal potential of Cucurbita maxima seed extracts on biomphalaria pfeifferi and bulinus globosus snails. All Life. 14(1):244–255. DOI: 10.1080/26895293.2021.1901788.
- Ndamba J, Nyazema N, Makaza N, Anderson C, Kaondera KC. 1994. Traditional herbal remedies used for the treatment of urinary schistosomiasis in Zimbabwe. J Ethnopharmacol. 42(2):125–132.
- Neves BJ, Andrade CH, Cravo PVL. 2015. Natural products as leads in schistosome drug discovery. Molecules. 20(2):1872–1903. doi:10.3390/molecules20021872.
- Neves BJ, Braga RC, Melo-Filho CC, Moreira-Filho JT, Muratov EN, Andrade CH. 2018. QSAR-based virtual screening: Advances and applications in drug discovery. Front Pharmacol. 9(NOV):1–7. doi:10.3389/fphar.2018.01275.
- Neves BJ, Dantas RF, Senger MR, Melo-Filho CC, Valente WCG, de Almeida ACM, Rezende-Neto JM, Lima EFC, Paveley R, Furnham N, et al. 2016a. Discovery of New anti-schistosomal hits by integration of QSAR-based virtual screening and high content screening. J Med Chem. 59(15):7075–7088. doi:10.1021/acs.jmedchem.5b02038.
- Neves BJ, Muratov E, Machado RB, Andrade CH, Cravo PV. 2016b. Modern approaches to accelerate the discovery of new anti-schistosomal drugs. Expert Opin Drug Discovery. 11(6):557–567. doi:10.1080/17460441.2016.1178230.
- Ngangoue MO, Ngameni B, Ambassa P, Chi GF, Wamba BEN, Ombito JO, … Ngadjui BT. 2020. A phenanthridine-6(5H)-one derivative and a lanostane-type triterpene with antibacterial properties from Anonidium mannii (oliv). engl. & diels (annonaceae). Nat Prod Res. 0(0):1–10. doi:10.1080/14786419.2020.1758094.
- Ntie-Kang F, Telukunta KK, Döring K, Simoben CV, Moumbock AAF, Malange YI, Njume LE, Yong JN, Sippl W, Günther S. 2017. NANPDB: A resource for Natural products from Northern African sources. J Nat Prod. 80(7): 2067–2076. doi:10.1021/acs.jnatprod.7b00283.
- Nunes R, Campos P, Pinto R, Mioduski J, Santos R, Justus B, Padilha de Paula F, Klein T, Boscardin P, Corrêa S, et al. 2021. The promising antischistosomal activity of oleic acid-loaded polymeric nanocapsules for oral administration. Journal of Drug Delivery Science and Technology. 63:102429–102429. doi:10.1016/j.jddst.2021.102429.
- Nunes RR, Costa MdS, Santos BdR, Fonseca ALd, Ferreira LS, Chagas RCR, Silva AMd, Varotti FdP, Taranto AG. 2016. Successful application of virtual screening and molecular dynamics simulations against antimalarial molecular targets. Memórias Do Instituto Oswaldo Cruz. 111(12):721–730. doi:10.1590/0074-02760160207.
- Onasanya A, Bengtson M, Oladepo O, Engelen JV, Diehl JC, Author C. 2021. Rethinking the top-down approach to schistosomiasis control and elimination in sub-Saharan Africa. 9(February), 1–12. doi:10.3389/fpubh.2021.622809.
- Otarigho B. 2019. Structural, functional and docking analysis against Schistosoma mansoni dihydroorotate dehydrogenase for potential chemotherapeutic drugs. F1000Res. 8:651–651. doi:10.12688/f1000research.18904.1.
- Oyeyemi OT, de Jesus Jeremias W, Grenfell RFQ. 2020. Schistosomiasis in Nigeria: gleaning from the past to improve current efforts towards control. One Health. 11:10013–100183. doi:10.1016/j.onehlt.2020.100183.
- Panic G, Duthaler U, Speich B, Keiser J. 2014. Repurposing drugs for the treatment and control of helminth infections. Int J Parasitol 4:185–200.
- Pasche V, Laleu B, Keiser J. 2019. Early antischistosomal leads identified from in vitro and in vivo screening of the Medicines for Malaria Venture pathogen Box. ACS Infect Dis. 5(1):102–110. doi:10.1021/acsinfecdis.8b00220.
- Pereira C, Saye M, Reigada C, Silber A, Labadie G, Miranda M, & Valera-Vera E. 2020. Computational approaches for drug discovery against trypanosomatid-caused diseases. Parasitology. 147(6):611–633. doi:10.1017/S0031182020000207.
- Prieto-Martínez FD, López-López E, Juárez-Mercado KE, Medina-Franco JL. 2019. Computational drug design methods—current and future perspectives. In: In silico drug design. Veracruz: Academic Press; p. 19–44.
- Roquini DB, Cogo RM, Mengarda AC, Mazloum SF, Morais CS, Xavier RP, Salvadori MC, Teixeira FS, Ferreira LE, Pinto PL, et al. 2019. Promethazine exhibits antiparasitic properties In vitro and reduces worm burden, Egg production, hepatomegaly, and splenomegaly in a schistosomiasis animal model daniel. American Society for Microbiology. 63(12):1–10.
- Sanderson L, Bartlett A, Whitfield P. 2002. In vitro and in vivo studies on the bioactivity of a ginger (Zingiber officinale) extract towards adult schistosomes and their egg production. J Helminthol. 76(3):241–247. doi:10.1079/JOH2002116.
- Schultz MD, Dadali T, Jacques SA, Muller-Steffner H, Foote JB, Sorci L, … Lund FE. 2020. Inhibition of the NAD salvage pathway in schistosomes impairs metabolism, reproduction, and parasite survival. PLoS Pathog. 16(5):e1008539–e1008539.
- Selvaraj C, Chandra I, Kumar Singh S. 2021. Artifcial intelligence and machine learning approaches for drug design: challenges and opportunities for the pharmaceutical industries. Mol Diversity. doi:10.1007/s11030-021-10326-z.
- Shelar DB, Shirote PJ. 2011. Natural product in drug discovery: back to future. Biomedical & Pharmacology Journal. 4(1):141–141.
- Singh R, Pandey PN. 2015. Molecular docking and molecular dynamics study on SmHDAC1 to identify potential lead compounds against schistosomiasis. Mol Biol Rep. 42(3):689–698.
- Sorokina M, Steinbeck C. 2020. Review on natural products databases: where to find data in 2020. J Cheminform. 12(1):1–51.
- Sparg SG, Van Staden J, Jäger AK. 2000. Efficiency of traditionally used South African plants against schistosomiasis. J Ethnopharmacol. 73(1-2):209–214.
- Stein EM, Machado LP, Roffato HK, Miyasato PA, Nakano E, Colepicolo P, Andreguetti DX. 2015. Antischistosomal activity from Brazilian marine algae. Revista Brasileira de Farmacognosia. 25:663–667.
- Stein EM, Tajú SG, Miyasato PA, de Freitas RP, Tallarico LDF, Dos Santos GS, … Nakano E. 2021. The prospective use of Brazilian marine macroalgae in schistosomiasis control. Mar Drugs. 19(5):234–234.
- Süntar I. 2020. Importance of ethnopharmacological studies in drug discovery: role of medicinal plants. Phytochem Rev. 19(5):1199–1209. doi:10.1007/s11101-019-09629-9.
- Tavares NC, de Aguiar PHN, Gava SG, Oliveira G, Mourão MM. 2016. Schistosomiasis: setting routes for drug discovery. Special Topics in Drug Discovery. Chapter 5:116–24. doi:10.5772/65386.
- Tekwu EM, Bosompem KM, Anyan WK, Appiah-Opong R, Owusu KBA, Tettey MD, … Nyarko AK. 2017. In vitro assessment of Anthelmintic activities of Rauwolfia vomitoria (apocynaceae) stem bark and roots against parasitic stages of Schistosoma mansoni and cytotoxic study. J Parasitol Res. 2017:2090–0023. doi:10.1155/2017/2583969.
- Thomas CM, Timson DJ. 2020. The mechanism of action of praziquantel: can new drugs exploit similar mechanisms? Curr Med Chem. 27(5):676–696.
- Torini JR, Romanello L, Batista FAH, Serrão VHB, Faheem M, Zeraik AE, … Pereira HDM. 2018. The molecular structure of Schistosoma mansoni PNP isoform 2 provides insights into the nucleoside selectivity of PNPs. PloS one. 13(9):e0203532–e0203532.
- ToussiMatchi JL, TchamoNoungoue D, Kuhn I, Boissier J, Tchouankeu JC, Nothisen M, Wagner A. 2020. Manniindole, an indole derivative from the roots of Anonidium mannii and combined antischistosomal and enzymatic activities. Nat Prod Res. 0(0):1–9. doi:10.1080/14786419.2020.1824227.
- Tung C. 2014. Public Databases of Plant Natural Products for Computational Drug Discovery. 191–196.
- Wang L, Ding J, Pan L, Cao D, Jiang H, Ding X. 2019. Artificial intelligence facilitates drug design in the big data era. Chemom Intell Lab Syst. 194:article 103850–103850.
- Weng HB, Chen HX, Wang MW. 2018. Innovation in neglected tropical disease drug discovery and development. Infect Dis Poverty. 7(1):1–9.
- Wilson GL, Lill MA. 2011. Integrating structure-based and ligand-based approaches for computational drug design. Future Med. Chem. 3(6):735–750.
- Xiao S, Chollet J, Utzinger J, Matile H, Mei J, Tanner M. 2001. Artemether administered together with haemin damages schistosomes in vitro. Trans R Soc Trop Med Hyg 95:67–71.
- Xu X, Huang M, Zou X. 2018. Docking-based inverse virtual screening: methods, applications, and challenges. Biophys Rep. 4:1–16. doi:10.1007/s41048-017-0045-8.
- Yousif F, Hifnawy MS, Soliman G, Boulos L, Labib T, Mahmoud S, … El-Menshawi BS. 2007. Large-scale in vitro. screening of Egyptian native and cultivated plants for schistosomicidal activity. Pharm Biol. 45(6):501–510.
- Zorn KM, Sun S, Mcconnon CL, Ma K, Chen EK, Foil DH, Lane TR, Liu LJ, El-Sakkary N, Skinner DE, et al. 2021. A machine learning strategy for drug discovery identifies anti-schistosomal small molecules. ACS Infect Dis. 7(2):406–420. doi:10.1021/acsinfecdis.0c00754.