?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The repellency of solvent extracts of Calpurnia aurea leaves was evaluated on the maize weevils and the red flour beetles. Nine-cm Whatman number 1 filter paper that is partitioned into treatment, neutral and non-treatment portions was used for the experiment. Each solvent extract treatment was applied at 2.5, 5 and 10% rates. Insects were released in the neutral portion, while the untreated part served as a control. A choice bioassay experimental method that is laid down in a completely randomized design within three replications was used. 5 and 10% rates of the polar solvent extracts of Calpurnia aurea leaves induced significantly (p < 0.05) higher percentage weevils and beetles repellency (≥50.00%) at 2 days after treatment than non-polar and partial polar solvent extract treatments and the untreated check. 10% dosage of Calpurnia aurea leaves’ polar solvent extract treatments caused 100% weevils and beetles repellency three days after treatment. Consequently, the polar solvent extracts of the tested plant were potent and could be used at 5 and 10% rates for the maize weevils and the red flour beetles’ management under farmers’ maize storage conditions. Nevertheless, their impact on cost-effectiveness, natural enemies and human beings needs additional study.
Introduction
Maize is the third most vital cereal crop in the world next to rice and wheat in terms of both consumption and area-grown (Kornher Citation2018). It is also the most extensively cultivated staple crop in Sub-Saharan Africa (SSA), accounting for a larger area coverage of the farmland each year (Jacob Citation2018; Santpoort Citation2020). It could play a crucial role in the transformation of Sub-Saharan Africa’s agriculture, reducing poverty and improvement of food security (Erenstein and Kassie Citation2018).
Maize is also the most widely grown staple crop by poor farmers in Ethiopia. It has been cultivated for different purposes, including for human and animal consumption, for generating income, for construction and other uses. But, insect pests both in the field and storage have been affecting the yield, quality, quantity and market value of maize (Demissie et al. Citation2008; Hiruy Citation2018; Fufa et al. Citation2021). In addition, the storage insect pests are the most harmful of pests, as their loss is none recompensed (Chomchalow Citation2003; Hiruy and Getu Citation2018a). Not only in Ethiopia but also in all tropical worlds, storage insect pests are important problems that pose both quantitative and qualitative loss of food grains, including maize (Chebet et al. Citation2013). Of these storage pests, Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) and Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae), respectively are revealed to be the most damaging primary and secondary pests that have been causing considerable loss of maize grains (Srivastava and Subramanian Citation2016; Hiruy Citation2018; Hiruy and Getu Citation2020). Management of these insect pests has heavily relied on the use of synthetic insecticides and fumigants (Jahromi et al. Citation2012; Goudoungou et al. Citation2018; Laizer et al. Citation2019; Mubayiwa et al. Citation2021) to which negative attributes such as environmental pollution, health risk to human and non-target organisms, among others have been associated for years (Cherry et al. Citation2005; Gu et al. Citation2008; Cosimi et al. Citation2009; Ofuya and Longe Citation2009; Sousa et al. Citation2009; Harish et al. Citation2013; Goudoungou et al. Citation2018; Mubayiwa et al. Citation2021). These impacts of synthetic pesticides have been commencing the exploration of bio-rational pest management options such as botanicals (Karunaratne and Karunaratne Citation2015; Hiruy Citation2018; Hiruy and Getu Citation2020).
Thus, protection of stored food grains such as maize from insect pests by designing and implementing affordable, effective, safe and environmentally sound management options such as repellent botanicals is critical and timely to ensure a constant supply of food and feed for resource-poor farmers of Ethiopia at stable prices year round. Isolation of homegrown effective botanicals is also indicated to serve as a cheap, safe, eco-sound and sustainable management alternative to synthetic insecticides for stored grains protection from insect pests (Hiruy Citation2018; Hiruy and Getu Citation2020).
Accordingly, an investigation of phytochemical analysis of Calpurnia aurea revealed that the leaves and roots are a chief source of terpenoids, saponins, tannins, flavonoids, steroids, glycosides and alkaloids (Nega et al. Citation2016; Kemal et al. Citation2020). This plant belongs to the subfamily Papilionoideae of the family Fabaceae. It is a small 3–4 m tall, multi-stemmed tree, which is widely grown in highland areas of Ethiopia (Birhanu and Asale Citation2015).
C. aurea is usually used in traditional medicine to treat different disorders and parasitic infections of animals and humans nationally. It is used to treat stomach disorders, amebic dysentery and eye diseases (Hiruy Citation2018). Its potency as a toxicant in the control of storage pests has also been reported (Blum and Bekele Citation2002; Hiruy Citation2018; Hruy and Getu Citation2018b). Nonetheless, information on the repellent potency of C. aurea in managing storage insect pests, in general, and weevils and beetles, in particular, is lacking nationally, and yet to be investigated. Moreover, the extract quantity and quality, the velocity of the extraction, the repellency, toxicity and anti-feedant activities as well as the biosafety of plant extracts were reported to be greatly influenced by the type and polarity of the solvent, and the plant parts used for extraction (Rafińska et al. Citation2019; Wakeel et al. Citation2019; Zhang et al. Citation2019). Extraction of toxicant and repellent constituents from botanicals was also shown to be affected by different factors, including solvent polarity and concentration, as well as temperature and time. Based on chemical composition, solvents of different polarities were used for extracting various plant phytochemicals, as no single solvent might be reliable to extract all secondary plant metabolites with repellent and toxicant effects (Nawaz et al. Citation2020). Consequently, the identification of an effective solvent to test the efficacy of botanicals against stored grain insect pests is considered to be very vital. Therefore, this study was initiated to evaluate the repellency of solvent extracts of Calpurnia aurea leaves on the maize weevils and the red flour beetles under laboratory conditions in Ethiopia.
Materials and methods
The study area and period
The study was conducted in the Insect Science Insectary of Addis Ababa University of Zoological Sciences Department of Life Sciences Faculty of Collage of Natural Sciences of Ethiopia between 1 October 2016 and 30 June 2017.
Collection, identification and preparation of the plant materials
The plant leaves (Figure ) were collected from the Hadiya Zone of Southern Ethiopia (Figure ) and identified as Calpurnia aurea species at the Life Sciences Faculty Herbarium of Addis Ababa University. In three weeks, known weight the fresh leaves of C. aurea were dried under shade in a well-ventilated room and powdered into fine texture via pestle and mortar. Then the powder was sieved to remove larger material and to get a more fine texture for solvent extraction (Figure ) (Tekie Citation1999; Jembere Citation2002; Hiruy and Getu Citation2020).
Mass culture of the test insects
Adults of the maize weevils and the red flour beetles obtained from farmers’ storages (Figure ) were cultured at 27 ± 3°C and 55–70% RH in Insect Science Insectary of Addis Ababa University (Jembere et al. Citation1995; Zewde and Jembere Citation2010; Hiruy Citation2018). Shone variety of maize grains was used in culture, as it is the most commonly grown variety in the area and is considered to be susceptible to insect infestation. Before use in the raring experiment, the grains were kept in a deep freezer at −20 ± 2°C for two weeks to disinfect them from egg larvae, pupa and adults of any insect (Gemechu et al. Citation2013; Hiruy Citation2018). Besides, the grains were mechanically damaged in a subtitle manner for culturing the red flour beetles, as beetles cannot feed on sound grains. Fifteen pairs of the adult test insects then were placed in 12 one-liter glass jars containing 200 g of maize grains (Zewde and Jembere Citation2010; Hiruy Citation2018; Hiruy and Getu Citation2020). The jars were then covered with nylon mesh and held in a place with rubber bands to allow ventilation and prevent the escape of the experimental insects. After an oviposition period of 13 days, all the parental insects were removed from the grain through the sieve. The jars were then kept until the emergence of F1 progeny under the laboratory condition mentioned above. Up on emergence after 30 days, three to seven days old unsexed adults of the F1 progenies were sieved and used in the experiment.
Experimental design and repellency bioassay
The experiment was laid down in a completely randomized design (CRD) in three replications in a factorial arrangement. A choice bioassay method was used to conduct the repellency test (Talukder and Howse Citation1995; Jahromi et al. Citation2012; Hiruy and Getu Citation2020). 2.5%, 5.0% and 10.0% solutions of solvent extracts were prepared by diluting the dry fine powder of the test plant in different solvents with different polarity (N-hexane (partial polar), chloroform (non-polar) and ethanol, acetone, methanol, distilled water (polar). Nine-cm Whatman number 1 filter paper that is divided into treatment and non-treatment (control) portions (3.5 cm each), and the central neutral portion (2 cm) was used in the experiment (Figure ) (Hiruy and Getu Citation2020). The partitioned filter paper discs were placed inside the Petri dishes. Then, in each non-treatment and treatment portion of the Petri dishes, three grams of the maize grains (slightly damaged for the red flour beetles and sound for the maize weevils) were placed, leaving the central neutral portion free. Using a pipette, the grains in one-half of the Petri dishes (treatment portion) were treated with 1 ml solutions of the respective plant solvent extracts, leaving the other side (untreated) as the control side (Figure ). Three to seven days old ten unsexed experimental insects were released at the central portion of each Petri dish, after air-drying the treated halves for 30 min to allow complete evaporation of the solvents. Subsequently, the Petri dishes were covered with nylon mesh and held in a place with rubber bands and kept at 25–30°C, temperature and 65–70%, relative humidity (Jembere et al. Citation1995; Zewde and Jembere Citation2010). The number of insects found on the control (Nc) and treated (Nt) parts of the Petri dishes were counted at an interval of 12 h for three days. Percentage repellency (PR) was computed by the following formula (Thein et al. Citation2013; Loko et al. Citation2017; Estelle et al. Citation2018; Hiruy and Getu Citation2020; Gitahi et al. Citation2021).
where Nc was the number of insects in the untreated area after the exposure interval and Nt was the number of insects in the treated area after the exposure interval. The mean percent repellency of each treatment was apportioned to the repellent classes ranging from 0 to V; Class 0 (PR ≤ 0.1%), Class I (PR = 0.1–20%), Class II (PR = 20.1–40%), Class III (40.1–60%), Class IV (60.1–80%) and Class V (80.1–100%) (Loko et al. Citation2017; Estelle et al. Citation2018).
Figure 3. Partial view of the Petri dish chamber used for repellency test of solvent extracts of leaf powder of Calpurnia aurea on the maize weevils and the red flour beetles.

As adopted in prior studies (Nerio et al. Citation2009; Loko et al. Citation2017; Rajabpour et al. Citation2018; Estelle et al. Citation2018), the index of repellency (IR) was calculated by the following formula:
where C and T represent the percentage of insects in the untreated and treated areas of preference, respectively. The IR values are between 0 and 2 (Gusmão et al. Citation2013) with, IR = 1 indicating a similar repellency (neutral treatment), IR > 1 indicating a lower repellency (being attractant) and IR < 1 representing a greater repulsion (Nerio et al. Citation2009; Loko et al. Citation2017; Estelle et al. Citation2018; Rajabpour et al. Citation2018; Gitahi et al. Citation2021).
Data analysis
Microsoft Excel version 2013 software (Microsoft Corporation, Albuquerque, New Mexico, U.S.) system was used for compiling the data on the percent repellency of the maize weevils and the red flour beetles due to the tested botanical leaf solvent extract treatments at different hours after treatment. Then the data were subjected to analysis of variance (ANOVA) of the Statistical Program for Social Sciences (SPSS) version 2016 for Windows (IBM, Chicago, USA). Two-way ANOVA or univariate analysis of the general linear model was employed for analysis, as there were two independent variables (solvent extract treatments and time). Significant differences between means of different treatments of different solvent extracts of Calpurnia aurea leaves applied at different rates at different times after treatment were separated using Turkey’s studentized (HSD) test at a 5% confidence interval. Correlation between the treatments of solvent extracts of C. aurea leaves applied at different rates at different hours after treatment application and the percent repellency was determined using Pearson’s correlation of the SPSS program of version 16.
Result
The repellent effects of the tested botanical treatment on the tested insect pests differed significantly with the difference in polarity of solvent extracts, dosage and exposure time after treatment. Repellency of the maize weevils and the red flour beetles was increased with an increase in dosage, polarity and exposure time after the treatment of tested botanical solvent extracts (Table , and Figures ).
Figure 4. Mean percent repellency of (a) the maize weevils and (b) the red flour beetles due to Calpurnia aurea leaves’ solvent extract treatment at a 2.5% rate. N.B. Means with different colors followed by error bars (±SE) at different days after treatment application are significantly different at p < 0.05, SE = standard error of the mean, h = hour.
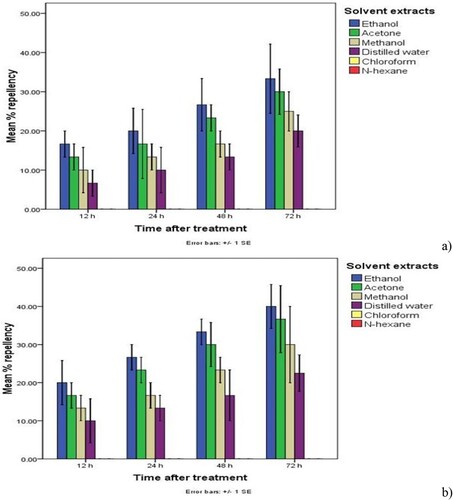
Figure 5. Mean percent repellency of (a) the maize weevils and (b) the red flour beetles due to Calpurnia aurea leaves’ solvent extract treatment at a 5% rate. N.B. Means with different colors followed by error bars (±SE) on different days after treatment application are significantly different at p < 0.05.
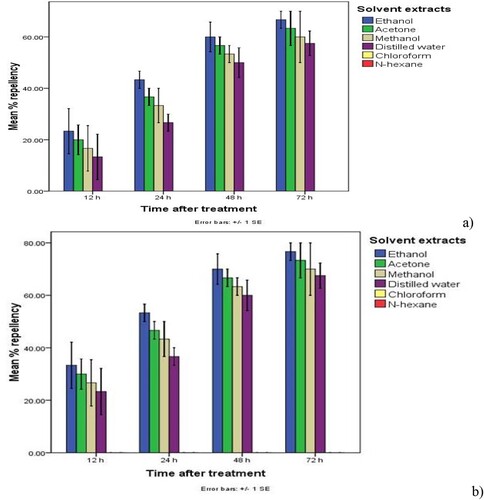
Figure 6. Mean percent repellency of (a) the maize weevils and (b) the red flour beetles due to Calpurnia aurea leaves’ solvent extract treatment at a 10% rate. N.B. Means with different colors followed by error bars (±SE) on different days after treatment application are significantly different at p < 0.05.
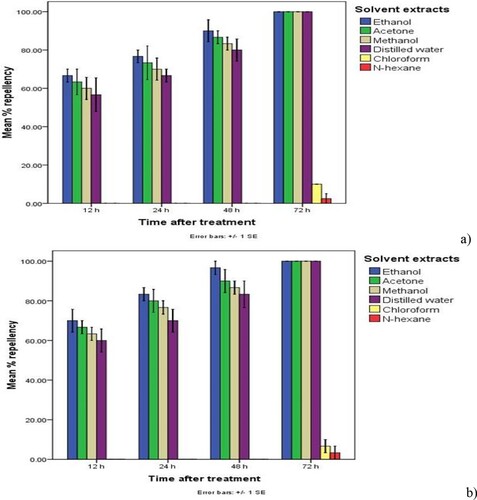
Table 1. Cumulative mean percent repellency and the repellency index of the maize weevils and red flour beetles due to Calpurnia aurea leaves’ solvent extract treatment at different rates at different times after the treatment.
Accordingly, the distilled water, methanol, ethanol and acetone (the polar solvents’ extract treatments) of C. aurea applied at all rates induced significant (p < 0.05) mean percent (≥6.67%) repellency of the maize weevils and the red flour beetles in comparison to non-polar and partial polar solvent extract treatments and the untreated check at all days (1, 2 and 3 days) after treatment. Of which ethanol extract treatments applied at higher rates (5% and 10%) caused higher (≥ 43.33%) percent repellency (class III repellency, ranging from 40.1 to 60%) of the maize weevils and the red flour beetles than the treatment applied at a lower rate (2.5%) at one day after treatment. Following ethanol extract treatments, high percent repellency of the maize weevils and the red flour beetles was caused by acetone (≥ 36.67%) (Class II) and methanol (≥ 33.33%) (Class II) extract treatments of C. aurea leaves applied at 5% and 10% rates at one day after treatment than the treatment applied at a rate of 2.5%. Water extract treatments of C. aurea leaves applied at rates of 5% and 10% also caused substantial (≥ 26.67%) (Class II) percent repellency of the maize weevils and the red flour beetles at one day after treatment than the treatment at the rate of 2.5%. These class III and II repellency suggests the induction of substantial repellency by polar solvent extract treatments of C. aurea against the test insects at 5 and 10% dosage at one date after treatment (Table and Figures ).
Nevertheless, all polar solvent extract treatments of C. aurea leaves applied at dosages 5% and 10% induced significantly (p < 0.05) higher (≥50.00%) percent repellency of the maize weevils and the red flour beetles at two days after treatment (≥ class III repellency), implying higher repellency of the tested botanical treatments on the maize weevils and the red flour beetles at 5 and 10% rates. Of which ethanol extracts caused higher (≥60.00) (Class IV) repellency, followed by acetone (≥56.67%) (Class III) and methanol (≥53.33%) (Class III), while water extract induced considerable repellency (≥50.00%) (Class III) (Table and Figures ). The index of repellency ranged from 0.12 to –0.87 for polar solvent extract treatments of C. aurea leaves, indicating possession of a great repellant activity of all polar solvent extract treatments against the test insects (Table ). Furthermore, all polar extract treatments of C aurea leaves applied at rates of 10% caused 100% (Class V) repellency of the maize weevils and the flour beetles three days after treatment (Figures ).
The correlation between the treatments of C. aurea leaves applied at different dosages, and the percent repellency of the maize weevils was highly (p < 0.01) significant and strongly negative (r is −0.604**) (Table ). The correlation between the treatments of C. aurea leaves applied at different rates, and the percent repellency of the red flour beetles was also highly (p < 0.01) significant and strongly negative (r is −0.650**) (Table ).
Table 2. Correlation among percent repellency efficacy determining parameters of Calpurnia aurea leaves’ solvent extracts on the maize weevils.
Table 3. Correlation among percent repellency efficacy determining parameters of Calpurnia aurea leaves’ solvent extracts on the red flour beetles.
Discussion
The repellency of the tested botanical treatments on weevils and beetles varied significantly with a difference in dosage and exposure time, and it was increased with an increase in dosage and exposure time after treatment. This might be due to an increase in the concentration of active metabolites and the associated volatiles of the tested botanical as the dosages of plant extract treatments are increased. Consistently, Gitahi et al. (Citation2021) revealed dose-dependent repellency activities of T. diversifolia and V. lasiopus extracts on weevils. Dose-dependent repellency of methanol extracts of the leaves of Cinnamomum tamala, Rosmarinus officinalis, and Pelaragonium graveolens on red flour beetles was also confirmed (Manonmani et al. Citation2017). Concentration and exposure time-dependent increment on the repellent effect of A. indica, T. vogelii, and L. camara powders against adult P. truncates was also reported (Chebet et al. Citation2013). Karakas (Citation2016) showed the dosage and solvent polarity-dependent mortality of weevils by leaf extracts of dill, Anethum graveolens and purple basil, Ocimum basilicum. The mortality of ticks was shown to increase with the increased exposure time after treatment and dose of C. aurea (Kemal et al. Citation2020). Concentration, solvent polarity and exposure time-dependent weevils mortally due to C. aurea leaves’ solvent extract treatments were also confirmed (Hruy and Getu Citation2018b).
Percent repellency of the maize weevils and the red flour beetles due to the tested botanical leaf extract treatments also varied with the polarity (type) of solvent used in extraction; the highest being in ethanol extracts, followed by extracts in acetone and methanol, while water extract induced considerable repellency. Broad organic compounds’ solubility properties in ethanol might be a contributor to the relatively higher repellant efficacy of the tested botanical leaf ethanol extracts. Consistently, Mulatu (Citation2020) revealed that the percent yield of the plant extracts varies from solvent to solvent due to their different polarities and extracting potential. According to him the great efficacy of ethanol extract of C. aurea leaves in antibacterial activity is attributed due to ethanol’s ability in dissolving the polar and nonpolar phytochemical constituents (i.e. broad solubility property of ethanol). Of various solvents such as water, ethanol, acetone and petroleum ether used for plant extract preparation, ethanol is revealed to be an accepted solvent for its broad solubility properties (Amoh-Amoah Citation2010). Hruy and Getu (Citation2018b) also indicated concentration, solvent polarity and exposure time-dependent weevils mortally due to C. aurea leaves’ solvent extract treatments. Jembere (Citation2002) also revealed the effectiveness of the aqueous extracts of M. ferruginea, following acetone and ethanol extracts against the maize weevils after three days post-treatment exposure.
The polar solvent extracts of the tested botanical leaves applied at all rates caused significant (p < 0.05) percent repellency of the maize weevils and the red flour beetles in comparison to non-polar and partial polar solvent extract treatments and the untreated control. This suggests the possession of more polar solvent-soluble volatile active biochemical constituents by the C. aurea leaves, which are responsible for considering the maize weevil’s and the red flour beetles’ repellency. Accordingly, Gebre-slassie and Eyasu (Citation2019) indicated that the ethanol extract of C. aurea leaves yields the highest active secondary bio-chemicals, followed by ethyl acetate than petroleum ether and chloroform extracts, and they suggested that the leaves extract of C. aurea possessed more polar compounds than non-polar. The strongest repellent effect of methanol and acetone solvent extract treatments of M. viridis and O. gratissimum on C. maculatuswas was reported, and the solvents’ methanol and acetone were suggested to be more effective in extracting bio-active compound/s of the tested plants. Previous phytochemical screening investigation from the leaf, bark, stem, and root of C. aurea through aqueous and ethanol extraction methods indicated the existence of alkaloids, terpenoids, steroids, saponins, tannins, flavonoids and phlorotannin phytochemicals (Adedapo et al. Citation2008; Nega et al. Citation2016). Recent secondary metabolites screening study on C. aurea also confirmed the existence of different major active phytochemicals; alkaloids, cardiac glycosides, flavonoids, phenols, phytosteroids, saponins, terpenoids, and tannins in its different parts, including its leaves (Mulatu Citation2020). Kemal et al. (Citation2020) also showed that C. aurea leaf methanol extracts possessed phenolic, alkaloids, steroids, flavonoids, saponins, phlobatannin, glycosides and tannins. Adedapo et al. (Citation2008) also revealed much higher antibacterial activity of the methanol extracts of the leaves of the C. aurea than the stem. The active ingredients in the leaf extract of the castor bean plant tested against ectoparasites of animals were confirmed to reside in the polar fractions and suggested as the active principals are polar in nature (Amante Citation2016). Jembere et al. (Citation2005) reported that M. ferruginea seed water extract induced high Z. subfasciatus mortality and justified it as it is due to the presence of high water-soluble chemicals in the seeds of the botanical. Methanolic, aqueous and ethanolic extracts of Euphorbia balsa mifera, Lawsonia inermis, Mitracarpus hirtus and Senna obtusifolia were also shown to have repellent potential against Sitophilus zeamais of stored sorghum (Suleiman et al. Citation2018).
As mentioned previously, various phytochemicals screening investigations revealed that the leaves, roots, seeds and stems of C. aurea are a rich source of terpenoids, saponins, tannins, flavonoids, steroids, glycosides and alkaloids by (Adedapo et al. Citation2008; Nega et al. Citation2016; Kemal et al. Citation2020; Mulatu Citation2020). The higher repellency of C. aurea leaves’ polar solvent extracts applied at rates of 5 and 10% on the test insects after two days of treatment exposure and 100% repellency at 10% dosage application at three days after treatment might probably be due to the synergism of some or all of these active volatile plant phytochemicals’ olfaction to repel away the tested pests from the treatments. This higher repellent efficacy of the polar solvent extracts of C. aurea leaves might probably be more significant than our former study on mortality, as repellency pushes away the insects from the grain and reduce contamination with remains of dead body parts, which could be retained through toxicity. Nevertheless, supplementary investigation on screening the major contributors for such repellency of the test pests due to the tested botanicals is needed. Consistently, it was revealed that plant essential oils and/or solvent extracts are composed of several secondary active metabolites, and repellency to insects is typically enhanced by the additive effects of the individual compounds (Zhang et al. Citation2013). Plants are indicated to be a chief source of bioactive metabolites, which display repellent, toxic and anti-feedant effects in a wide range of insects (Jahromi et al. Citation2012) and consequently, their extracts and products have been considered to be an alternative means of controlling insect pests (Tripathi et al. Citation2009). Tsegay et al. (Citation2018) reported the highest repellency (80%) of termites by C. aurea leaf powder treatment at 1 h after treatment application. Berhanu et al. (Citation2006) also reported C. aurea as one of the major mosquito repellent botanicals used in Ethiopia, among many ethno botanicals surveyed by him.
The higher repellency C. aurea leaves’ polar solvent extracts applied at rates of 5 and 10% on the test insects two days after treatment, and 100% repellency at 10% dosage application at three days after treatment (especially, the water extracts which are cheap and safe) in the present study might have the potential utility of this plant leaves extracts in the management of insect pests of stored maize as benign pest management alternative to synthetic insecticides under resource-poor farmers traditional storage condition in Sub-Saharan Africa, in general, and Ethiopia, in particular. Accordingly, sustainable use of botanicals with pesticidal properties is shown to be worth in the enhancement of food security, especially in areas where the use of synthetic pesticides for pest control is risky full and uneconomic (Qwarse Citation2015). Tsegay et al. (Citation2018) also reported the highest repellent (80%) potency by C. aurea leaf powder treatment at 1 h after treatment application on a termite. Berhanu et al. (Citation2006) also reported C. aurea as one of the major potent mosquito repellent botanicals used in Ethiopia, among many ethno botanicals surveyed by him. Meragiaw and Asfaw (Citation2014) also reported C. aurea as one of the potent repellent, pesticidal and antimalarial plants in Ethiopian traditional herbal medicine. Pavela and Benelli (Citation2016) also indicated C. aurea as one of the repellent plants used against mosquito vectors in Africa.
The non-existence of the co-evolutionary interaction between the tested botanical and the tested insect pests is suggested as one of the contributors to the higher repellent effect of the tested botanicals applied at rates of 5 and 10% at two days after treatment, as the test insets have been mainly restricted to the grain stores (Hiruy and Getu Citation2020). Nevertheless, the great efficacy of C. aurea leaves’ polar solvent extracts applied at rates of 5 and 10% on the maize weevils and the red flour beetles repellency two days after treatment application in the current study was against the finding of Zorloni et al. (Citation2010), in which C. aurea crude extracts didn’t induce any repellent effect on ticks. This difference in repellency efficacy might probably be due to inherent differences in plant chemistries that may be genotypic or Spatio-temporal variations caused by abiotic factors such as altitude, rainfall and soil type or different chemistries expressed in different plant parts (Sarasan et al. Citation2011; Belmain et al. Citation2012).
Conclusion
The current study provides some insight regarding the existence of the possibility of using the repellency of C. aurea solvent extract on the maize weevils and the red flour beetles’ management. Accordingly, the distilled water, methanol, ethanol and acetone (the polar solvents) extract treatments of C. aurea induced significantly (p < 0.05) higher repellency of the maize weevils and the red flour beetles in comparison to non-polar and partial polar solvent extract treatments and the untreated check at all days after treatment at 5% and 10% rates. Thus, the polar solvents’ extract treatments of C. aurea were potent for the management of the maize weevils and the red flour beetles at 5% and 10% dosages. There is also the possibility of using these polar solvents in the preparation of plant extracts for testing repellency and toxicity, screening phytochemicals and other possible bioassays against the maize weevils and the red flour beetles. Furthermore, the water extracts of the tested plants’ leaves could be used for the management of the maize weevils and the red flour beetles under traditional storage conditions in Ethiopia at 5% and 10% dosages, as their preparation is cheap and easy, safe and environmentally sound. Thus, the water extracts at the aforementioned rates can be recommended for managing the maize weevils and the red flour beetles in stored maize grain under farmers’ storage conditions in Ethiopia and elsewhere with similar environmental conditions. Nonetheless, the crude extracts’ effect on human beings, natural enemies and cost-effectiveness under traditional storage conditions need additional study before wide implementation of the outcomes of this study.
Authors’ statement
This manuscript describes original work that has not been published elsewhere and is not under consideration by any other journal.
Authors’ contributions
BH was responsible for planning the research proposal as well as searching literature reviews and writing up the paper. EG contributed to the endorsement of the research strategy, groundwork, and standardization of the manuscript. Both authors had equal responsibility for the preparation and approval of the final manuscript for publication.
Ethical statement
This study does not involve any human or animal testing.
Acknowledgements
The authors sincerely thank Arba Minch University and the Zoology Department of Addis Ababa University for providing them with financial support to conduct the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available in OSF repository [https://osf.io/] at https://osf.io/5xtcs.
Additional information
Funding
References
- Adedapo AA, Jimoh FO, Koduru S, Afolayan AJ, Masika PJ. 2008. Antibacterial and antioxidant properties of the methanol extracts of the leaves and stems of Calpurnia aurea. BMC Complement Altern Med. 8(1):1–8. DOI: 10.1186/1472-6882-8-53.
- Amante M. 2016. In vitro louscidal and acaricidal activities of alkaloid of Calpurnia aurea and fractions of Ricinus communis extracts against Linognathus ovillus and Amblyomma variegatum [M. Sc. Thesis]. College of Veterinary Medicine and Agriculture, Department of Pathology and Parasitology, Addis Ababa University.
- Amoh-Amoah B. 2010. Efficacy of ethanolic extract of Thevetia peruviana (Pers.) and K. Schum. (Milk Bush) root in the control of major insect pests of cowpea [M. Sc. Thesis]. Kwame Nkrumah University.
- Belmain SR, Amoah BA, Nyirenda SP, Kamanula JF, Stevenson PC. 2012. Highly variable insect control efficacy of Tephrosia vogelii chemotypes. J Agric Food Chem. 60(40):10055–10063. DOI: 10.1021/jf3032217.
- Berhanu A, Asfaw Z, Kelbessa E. 2006. Ethnobotany of plants used as insecticides, repellents and antimalarial agents in Jabitehnan District, West Gojjam. Ethiopian J Sci. 29(1):87–92. DOI: 10.4314/sinet.v29i1.18263.
- Birhanu A, Asale AG. 2015. Larvicidal activity of solvent extractions from some selected indigenous plants against the Mediterranean fruit fly larvae Ceratitis Capitata identified from coffee berry (Diptera: Tephritidae) in Jimma Zone, southwestern Ethiopia. J Appl Sci Agric. 10(6):78–85.
- Blum A, Bekele A. 2002. Storing grains as a survival strategy of small farmers in Ethiopia. J Int Agric Extension Edu. 9(1):77–83.
- Chebet F, Deng AL, Ogendo JO, Kamau AW, Bett PK. 2013. Bioactivity of selected plant powders against Prostephanus truncatus (Coleoptera: Bostrichidae) in stored maize grains. Plant Prot Sci. 49(1):34–43. DOI:10.17221/56/2011-PPS.
- Cherry AJ, Banito A, Djegui D, Lomers C. 2005. Suppression of the stem borer Sesamia calamistis (Lepidoptera; Noctuidae) in maize following seed dressing, topical application and stem injection with African isolates of Beauveria bassiana. Int J Pest Manag. 50(1):67–73. doi:10.1080/09670870310001637426.
- Chomchalow N. 2003. Protection of stored products with special reference to Thailand. Assumption Univ J Technol. 7(1):31–47.
- Cosimi S, Rossi E, Cioni PL, Canale A. 2009. Bioactivity and qualitative analysis of some essential oils from Mediterranean plants against stored-product pests: evaluation of repellency against Sitophilus zeamais Motschulsky, Cryptolestes ferrugineus (Stephens) and Tenebrio molitor (L.). J Stored Prod Res. 45(2):125–132. DOI:10.1016/J.JSPR.2008.10.002.
- Demissie G, Tefera T, Tadesse A. 2008. Efficacy of Silicosec, filter cake and wood ash against the maize weevil, Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) on three maize genotypes. J Stored Prod Res. 44(3):227–231.
- Erenstein O, Kassie G. 2018. Seeding Eastern Africa’s maize revolution in the post-structural adjustment era: a review and comparative analysis of the formal maize seed sector. Int Food Agribusiness Manage Rev. 21:39–52. DOI: 10.22434/IFAMR2016.0086.
- Estelle LYL, Christelle GA, Joelle T, Azize O, Alexandre D, Manuele T. 2018. Management of Dinoderus porcellus L. (Coleoptera: Bostrichidae) infesting yam chips using varietal resistance and botanical powders of three medicinal plants. Afr J Agric Res. 13(40):2118–2133. doi:10.5897/AJAR2018.13418.
- Fufa N, Zeleke T, Melese D, Daba T. 2021. Assessing storage insect pests and post-harvest loss of maize in major producing areas of Ethiopia. Int J Agric Sci Food Technol. 7(1):193–198. DOI: 10.17352/2455-815X.000106.
- Gebre-slassie HB, Eyasu A. 2019. Phytochemical Screening of the Leaves of Calpurnia aurea (Ait.) Benth extract. Int J Clin Chem Lab Med (IJCCLM). 5(4):18–24. doi:10.20431/2455-7153.0504004.
- Gemechu F, Santiago DR, Sori W. 2013. Laboratory evaluation of cotton (Gossypium hirsutum) and Ethiopian Mustard (Brassica cariata) seed oils as grain protectants against maize weevil, Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). Afr J Agric Res. 8(32):4374–4379. DOI: 10.5897/AJAR2013.6932.
- Gitahi SM, Piero MN, Mburu DN, Machocho AK. 2021. Repellent Effects of selected organic leaf extracts of Tithonia diversifolia (Hemsl.) A. Gray and Vernonia lasiopus (O. Hoffman) against Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). Scientific World J. 2021:1–13. doi:10.1155/2021/2718629.
- Goudoungou JW, Nukenine EN, Suh C, Gangué T, Ndjonka D. 2018. Effectiveness of binary combinations of Plectranthus glandulosus leaf powder and Hymenocardia acida wood ash against Sitophilus zeamais (Coleoptera: Curculionidae). Agric Food Sec. 7(1):1–12. doi:10.1186/s40066-018-0179-z.
- Gu H, Edwards OR, Hardy AT, Fitt GP. 2008. Host plant resistance in grain crops and prospects for invertebrate pest management in Australia: an overview. Aust J Exp Agric. 48(12):1543–1548. doi:10.1071/EA08027.
- Gusmão NM, de Oliveira JV, do AF Navarro DM, Dutra KA, da Silva WA, Wanderley MJ. 2013. Contact and fumigant toxicity and repellency of Eucalyptus citriodora Hook, Eucalyptus staigeriana F, Cymbopogon winterianus Jowitt and Foeniculum vulgare Mill essential oils in the management of Callosobruchus maculatus (Fabr) (Coleoptera: Chrysomelidae, Bruchinae). J Stored Prod Res. 54(2013):41–47.
- Harish G, Nataraja MV, Ajay BC, Holajjer P, Savaliya SD, Gedia MV. 2013. Comparative efficacy of storage bags, storability and damage potential of bruchid beetle. J Food Sci Technol. 51(12):4047–4053. DOI: 10.1007/s13197-013-0964-4.
- Hiruy B. 2018. Status, species composition and management of stored maize grain insect Pests in Southern Ethiopia [A PhD thesis]. Addis Ababa University of Ethiopia, Addis Ababa.
- Hiruy B, Getu E. 2018a. Insect pests associated to stored maize and their bio rational management options in Sub Sahara Africa. Int J Academic Res Dev. 3(1):741–748.
- Hiruy B, Getu E. 2018b. Efficacy of solvent extracts of Calpurnia aurea (Ait.) Benth and Milletia ferruginea (Hochest) Baker leaves against maize weevils, Sitophilus zeamais (Motsch.) of stored maize in Ethiopia. J Stored Prod Postharvest Res. 9(3):27–35. DOI: 10.5897/JSPPR2018.0259.
- Hiruy B, Getu E. 2020. Militia feruginaea solvent extracts on maize weevils and red flour beetles repellency; an implication to use them in storage pest management in Ethiopia. Cogent Food Agric. 6(1):1860562. doi:10.1080/23311932.2020.1860562.
- Jacob LD. 2018. Evaluation of post-harvest maize treatment and the effectiveness of essential oils of local plants against Sitophilus zeamais and fungal pests in stored maize [A PhD thesis]. University of Ngaoundere. https://www.researchgate.net/ publication/ 326655570.
- Jahromi MG, Pourmirza AA, Safaralizadeh MH. 2012. Repellent effect of sirinol (garlic emulsion) against Lasioderma serricorne (Coleoptera: Anobiidae) and Tribolium castaneum (Coleoptera: Tenebrionidae) by three laboratory methods. Afr J Biotechnol. 11(2):280–288. DOI: 10.5897/AJB11.2548.
- Jembere B. 2002. Evaluation of the toxicity potential of Millettia ferruginea (Hochst) Baker against Sitophilus zeamais (Motsch). Int J Pest Manag. 48(1):29–32. doi:10.1080/09670870110065253.
- Jembere B, Getahun D, Negash M, Seyoum E. 2005, August. Toxicity of Birbira (Milletia ferruginea) seed crude extracts to some insect pests as compared to other botanical and synthetic insecticides. In Proceedings of the 11th NAPRECA Symposium on Natural Products and Drug Delivery. p. 9–12.
- Jembere B, Obeng-Ofori D, Hassanali A. 1995. Products derived from the leaves of Ocimum kilimandsharicum (Labiate) as post-harvest grain protectants against the infestation of three major stored product insect pests. Bull Entomol Res. 85(3):361–367. DOI: 10.1017/S0007485300036099.
- Karakas M. 2016. Toxic, repellent and antifeedant effects of two aromatic plant extracts on the wheat granary weevil, Sitophilus granarius L. (Coleoptera: Curculionidae). Int J Entomol Res. 1(6):24–28.
- Karunaratne UKPR, Karunaratne MMSC. 2015. Evaluation of methanol, ethanol and acetone extracts of four plant species as repellents against Callosobruchus maculatus (Fab.). Vidyodaya J Sci. 17:1–8. https://dr.lib.sjp.ac.lk/handle/ 123456789/1870.
- Kemal J, Alemu S, Tsegaye B, Tamerat N. 2020. Study on ruminant tick infestation, phytochemical analysis and in vitro acaricidal effect of Calpurnia aurea and Otostegia integrifolia extracts on Amblyomma variegatum. Ethiop Vet J. 24(1):34–51. doi:10.4314/evj.v24i1.3.
- Kornher L. 2018. Maize markets in Eastern and Southern Africa (ESA) in the context of climate change. The State of Agricultural Commodity Markets (SOCO) Background Paper. p. 58 (2018).
- Laizer HC, Chacha MN, Ndakidemi PA. 2019. Farmers’ knowledge, perceptions and practices in managing weeds and insect pests of common bean in Northern Tanzania. Sustainability. 11(15):4076. doi:10.3390/su11154076.
- Loko LY, Alagbe O, Dannon EA, Datinon B, Orobiyi A, Thomas-Odjo A, Tamò M. 2017. Repellent effect and insecticidal activities of Bridelia ferruginea, Blighia sapida, and Khaya senegalensis leaves powders and extracts against Dinoderus porcellus in infested dried yam chips. Psyche (Stuttg). 2017:1–18. doi:10.1155/2017/5468202.
- Manonmani P, Usha rani R, Ramar M. 2017. Repellent activity of some selected aromatic plant extracts against rust-red flour beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Int J Curr Eng Sci Res. 4(9):38–46.
- Meragiaw M, Asfaw Z. 2014. Review of antimalarial, pesticidal and repellent plants in the Ethiopian traditional herbal medicine. Res Rev: J Herbal Sci. 3(3):21–45.
- Mubayiwa M, Mvumi BM, Stathers T, Mlambo S, Nyabako T. 2021. Field evaluation of hermetic and synthetic pesticide-based technologies in smallholder sorghum grain storage in hot and arid climates. Sci Rep. 11(1):1–13. doi:10.1038/s41598-021-83086-3.
- Mulatu G. 2020. Antibacterial activities of Calpurnia aurea against selected animal pathogenic bacterial strains. Adv Pharmacol Pharm Sci. 2020:1–9. doi:10.1155/2020/8840468.
- Nawaz H, Shad MA, Rehman N, Andaleeb H, Ullah N. 2020. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Braz J Pharm Sci. 56:1–9. doi:10.1590/s2175-97902019000417129.
- Nega A, Lemessa F, Berecha G. 2016. Distribution and importance of maize grey leaf spot Cercospora zeae-maydis (Tehon and Daniels) in south and southwest Ethiopia. J Plant Pathol Microbiol. 7(362):2. DOI: 10.4172/2157-7471.1000362.
- Nerio LS, Olivero-Verbel J, Stashenko EE. 2009. Repellent activity of essential oils from seven aromatic plants grown in Colombia against Sitophilus zeamais Motschulsky (Coleoptera). J Stored Prod Res. 45(3):212–214. DOI: 10.1016/j.jspr.2009.01.002.
- Ofuya TI, Longe OO. 2009. Investigation into fumigant effect of Eugenia aromatica dust against Callosobrunchus maculatus (Fabricius). Int J Crop Sci. 1(1):44–49.
- Pavela R, Benelli G. 2016. Ethnobotanical knowledge on botanical repellents employed in the African region against mosquito vectors–a review. Exp Parasitol. 167:103–108. doi:10.1016/j.exppara.2016.05.010.
- Qwarse M. 2015. Assessment of bioactivity of selected plants against pests and microbes from agro-pastoral communities in Mbulu district [M.Sc. thesis]. Open University of Tanzania.
- Rafińska K, Pomastowski P, Rudnicka J, Krakowska A, Maruśka A, Narkute M, Buszewski B. 2019. Effect of solvent and extraction technique on composition and biological activity of Lepidium sativum extracts. Food Chem. 289:16–25. DOI:10.1016/j.foodchem.2019.03.025.
- Rajabpour A, Mashhadi ARA, Ghorbani MR. 2018. Acaricidal and repellent properties of some plant extracts against poultry red mite, Dermanyssus gallinae (Mesostigmata: Dermanyssidae). Persian J Acarology. 7(1).
- Santpoort R. 2020. The drivers of maize area expansion in sub-Saharan Africa. How policies to boost maize production overlook the interests of smallholder farmers. Land (Basel). 9(3):68. doi:10.3390/land9030068.
- Sarasan V, Kite GC, Sileshi GW, Stevenson PC. 2011. The application of phytochemistry and in vitro tools to the sustainable utilization of medicinal and pesticidal plants for income generation and poverty alleviation. Plant Cell Rep. 30(7):1163–1172. doi:10.1007/s00299-011-1047-5.
- Sousa A, Faroni L, Pimentel M, Guedes R. 2009. Developmental and population growth rates of phosphine-resistant and susceptible populations of stored product insect pests. J Stored Prod Res. 45(4):241–246. DOI: 10.1016/j.jspr.2009.04.003.
- Srivastava C, Subramanian S. 2016. Storage insect pests and their damage symptoms: an overview. Indian J Entomol. 78(special):53–58. DOI: 10.5958/0974-8172.2016.00025.0.
- Suleiman M, Rugumamu CP, Ibrahim ND. 2018. Repellency potential of some botanicals against the maize weevil, Sitophilus zeamais (Motschulsky, 1855) (Coleoptera: Curculionidae) in stored sorghum. Polish J Entomol. 87(1):85–99. doi:10.2478/pjen-2018-0007.
- Talukder FA, Howse PE. 1995. Evaluation of Aphanamixis polystachya as a source of repellents, antifeedants, toxicants and protectants storage against Tribolium castaneum (Herbst). J Stored Prod Res. 31(1):55–61.
- Tekie H. 1999. The effect of neem (Azadirachta indica A. Juss) seed powder and extract on the maize stem borer, Chilo partellus (Swinhoe), and its potential in pest management [A PhD thesis]. Addis Ababa University.
- Thein WM, Javier PA, Ceballo FA. 2013. Insecticidal activity of crude plant extracts against Sitophilus spp. (Coleoptera: Curculionidae) and Callosobruchus chinensis (L.) (Coleoptera: Bruchidae). Philippine Agric Sci. 96(2):154–162.
- Tripathi KA, Upadhyay S, Bhuiyan M, Bhattacharya PR. 2009. A review on prospects of essential oils as biopesticide in insect-pest management. J Pharm Phytotherapy. 1(5):52–63. doi:10.5897/JPP.9000003.
- Tsegay BA, Weldeyes AT, Cardelús CL. 2018. Traditional use of botanicals in reducing post-harvest loss at crop stacking stage in Ethiopia: a case of Farta district. Indian J Traditional Knowl. 17(3):534–541. https://nopr.niscair.res.in/handle/123456789/44579.
- Wakeel A, Jan SA, Ullah I, Shinwari ZK, Xu M. 2019. Solvent polarity mediates phytochemical yield and antioxidant capacity of Isatis tinctoria. Peer J. 7:e7857. DOI: 10.7717/peerj.7857.
- Zewde DK, Jembere B. 2010. Evaluation of orange peel Citrus sinensis (L) as a source of repellent, toxicant and protectant against Zabrotes subfasciatus (Coleoptera: bruchidae). Momona Ethiopian J Sci. 2(1):61–75. DOI: 10.4314/mejs.v2i1.49652.
- Zhang H, Birch J, Pei J, Mohamed Ahmed IA, Yang H, Dias G, Bekhit AED. 2019. Identification of six phytochemical compounds from Asparagus officinalis L. root cultivars from New Zealand and China using UAE-SPE-UPLC-MS/MS: effects of extracts on H2O2-induced oxidative stress. Nutrients. 11(1):1–1710.3390/nu11010107.
- Zhang QH, Schneidmiller RG, Hoover DR. 2013. Essential oils and their compositions as spatial repellents for pestiferous social wasps. Pest Manage Sci. 69(4):542–552. doi:10.1002/ps.3411.
- Zorloni A, Penzhorn BL, Eloff JN. 2010. Extracts of Calpurnia aurea leaves from southern Ethiopia attract and immobilize or kill ticks. Vet Parasitol. 168(1–2):160–164.