?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Globally, about 779 million people are estimated to be at risk of developing schistosomiasis and around 250 million people are infected. There are 120 million asymptomatic people and 20 million with severe disease. Schistosomiasis causes the highest morbidity and mortality among school-aged children. The main objective of the study was to determine the prevalence and burden of schistosomiasis in the Mount Darwin and Makoni Districts. Five hundred children from both schools aged 6–13 were included in the study. Urinary schistosomiasis was diagnosed using the urinary filtration method and the presence of egg(s) under microscopy in the urine sample indicated an infection, whereas the absence of eggs was ruled as no infection. Boys were more infected than girls in both schools, with Bemberi having 14.88% males infected and 10.47% females infected and Bandanyenje having 13% males infected and 10.76% females infected with Schistosoma haematobium. The age group 10–13 years old from Bemberi primary had a higher prevalence rate of 17.72% than Bandanyenje primary with a prevalence of 12%. There is a need for the provision of an adequate supply of safe drinking water, health education campaigns and regular screening, among this age group.
Introduction
Schistosomiasis is one of the leading top ten causes of hospital admissions in Zimbabwe. The general population prevalence is 22.7% (Ministry of Health and Child Welfare Citation2012; Midzi et al. Citation2014). Urogenital schistosomiasis is one of the largely ignored tropical diseases of poverty in many tropics and subtropics, including Zimbabwe (Sady et al. Citation2013). It is associated with significant morbidity and mortality in tropical and subtropical areas. Urogenital schistosomiasis is one of the most devastating tropical diseases in the world, causing significant morbidity and mortality in developing countries such as throughout Africa, South America, the Caribbean, the Middle East and Asia (Doowuese et al. Citation2019; Mnkugwe et al. Citation2020). Human urinary schistosomiasis is caused by Schistosoma haematobium (S. haematobium) which is the most common species in Zimbabwe and is transmitted by Bulinus snails (Doowuese et al. Citation2019).
Globally, the infection rate of urogenital schistosomiasis is relatively high, especially in developing countries, including Zimbabwe. Recently, 779 million people are estimated to be at risk of infection in 78 countries and about 250 million are currently infected with schistosomiasis (Tchueme et al. Citation2017; McManus et al. Citation2018; Mnkugwe et al. Citation2020). In 2018, the research highlighted that at least 229 million people required preventive treatment (Mnkugwe et al. Citation2020).
Regarding the at-risk population, 90% of schistosome infections are found in Sub-Saharan Africa (Akindele et al. Citation2020). Urinary schistosomiasis is highly prevalent in poor communities that do not have access to safe drinking water, a clean environment, good hygiene and adequate sanitation (Hajissa et al. Citation2018). About 120 million people are symptomatic with urinary schistosomiasis with 20 million having a severe clinical disease. Around 200 000 deaths annually are attributed to schistosomiasis in Sub-Saharan Africa (Doowuese et al. Citation2019).
Most developing countries, including Zimbabwe, grossly underestimate the morbidity and mortality caused by urinary schistosomiasis (Ismail et al. Citation2016). Schistosomiasis is mostly associated with school-aged children, teenagers, women and young adults (Hajissa et al. Citation2018). Children infected with schistosomiasis may experience developmental delays, sluggishness, memory loss, cognitive impairment, poor academic performance and other symptoms that limit their potential in life (Lothe et al. Citation2018). Schistosomiasis, though seldom fatal, can result in long-term morbidity including anaemia, from bleeding from urinary and gastrointestinal systems due to worm invasion and movement. The condition also has iron deficiency as a side effect, which follows digestive problems such as diarrhoea and nutritional problems such as nutrient malabsorption (Adenowo et al. Citation2015).
The highest rate of infection of human schistosomiasis occurs in children, adolescents and young adults who suffer from the highest morbidity and mortality (Afrifa et al. Citation2017; Phillips et al. Citation2018). The percentage of infected children in the 10-13-year age group was higher than that in the 6–9 and 14–17 age groups (Hajissa et al. Citation2018). This was due to the excessive frequent contact with the river when swimming or playing and due to the excessive mobility of children at this age.
Previous studies done in Zimbabwe include the 8–10-year-old children in 157 schools which showed that most regions, where commercial farming is done, have higher levels of infection as compared to the subsistence-farming areas. Males had the highest level of infection of S. haematobium than females. The survey revealed that age prevalence showed that the 7–20-old-age group contains 91.5% of the heavy infection of S. haematobium and 83.7% of the heavy infection of S. mansoni. The availability of surface water has been cited as the major contributing factor to the distribution and prevalence of schistosomiasis (Taylor and Makura Citation1985).
With the high burden of urogenital schistosomiasis in the two districts, school-aged children in the age group 6–13 were overlooked in the previous studies. Previous studies from endemic areas have shown that the 6–13-year-old group carries a significant level of infection and highly contributes to the transmission of the disease. This study investigated the prevalence, burden and risk factors for urogenital schistosomiasis among the 6- to 13-year-olds living in the two districts in Zimbabwe. The data from this study will go a long way in assisting the Government of Zimbabwe in planning prevention strategies for the defined age group, thereby reducing the associated transmission and morbidity of urogenital schistosomiasis.
Material and methods
Study area and population
A school-based cross-sectional study was conducted from June to July 2017 in the Makoni and Mount Darwin districts. These were Bemberi Primary and Bandanyenje Primary schools in Zimbabwe. The schools and villages are surrounded by small rivers that make it conducive for the aquatic life cycle of the schistosomes. The study population comprised 500 primary school-aged children aged 6–13 years who were permanent residents of the area or would have relocated one year back.
Sample size
The sample size for each school was calculated using Dobson’s formula:
Z1-a/2 represents the value of the reference normal distribution for the desired confidence level which is Z = 1.96 for the 95% confidence interval. P denotes the expected prevalence of 0.62 (the expected prevalence of schistosomiasis observed in school-aged children (SAC) by Midzi et al. Citation2014. d denotes the absolute error or precision of 0.05 for a 95% confidence interval. The resulting sample size was 363. However, 500 participants from each school making 1000 SAC for both schools were used which surpassed the calculated sample size of 363. The sample size of 500 students took care of the anticipated sample size, allowed the better determination of accurate mean values, and avoided errors, which may arise from testing fewer possibly atypical samples.
Inclusion and exclusion criteria
The study participants from Makoni were all permanent residents of the Makoni district and attended Bandanyenje primary school. The study participants from Mount Darwin were all permanent residents of the Mount Darwin district and attended Bemberi primary school. Primary school-aged children were chosen for the study as they constitute the high-risk age group for schistosomiasis in endemic areas (WHO Citation2017). The participants who could not provide samples of urine, for various reasons were excluded from the study. All the children attending each of the primary schools were eligible for the study. Only permanent inhabitants of the villages, who reported to be never treated for any infection with helminths, were included in the study. Severely sick children were not included. Participants, who appeared malnourished and unhealthy or presented symptoms of active schistosomiasis, were excluded from the study.
Data collection
Questionnaire
To assess the prevalence and burden of schistosomiasis among the SAC, a questionnaire, developed in English and translated into the local language, was used to collect the data from the participants. The questionnaire sought answers about demographic data (age, sex), social habits (frequency of encounters with surrounding waters) and general self-reported or legal guardian-indicated health conditions (rectal pain, frequent tiredness, diarrhoea, abdominal pain and vomiting) among the questions asked. The questionnaire included anthropometric measurements: weight, height and mid-upper arm circumference (MUAC).
Sample collection
A single urine sample jar with the sample identity was provided to each child. The children were instructed to collect a midstream urine sample to reduce the risk of sample contamination with bacteria from the skin around the urethra. The urine samples were collected between 1000 and 1400 h to ensure the maximum collection of the eggs. The samples were processed within two hours of collection to avoid eggs’ disintegration if the time mentioned was exceeded.
Parasitological diagnosis
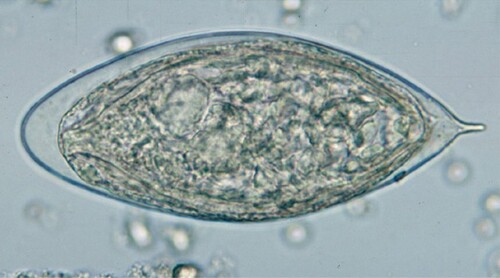
Urine samples collected were sent to the laboratory for the examination of S. haematobium eggs using the urine filtration technique. Urine samples (10 ml) were thoroughly mixed and the urine specimen was filtered through a 12-µm pore-sized nitrile filter membrane (Sigma-Aldrich, USA) and put onto a microscope slide indicated with the participant’s research identity. Iodine (10%) was used to stain the slides and examined for S. haematobium eggs under the laboratory compound microscope using a 10X objective lens. S. haematobium eggs were structurally identified by their oval shape and characteristic terminal spine. The number of eggs was counted per slide.
Anthropometric measurement
The body mass was measured in kilograms (kg) to the nearest 0.1 kg using a weighing scale with children without shoes and wearing light clothing. A stadiometer was used to measure the height in metres (m). The MUAC was measured in centimetres (cm) on the left arm midpoint between the shoulder and the tip of the elbow, with the arm relaxed and hanging down the body, using MUAC tape. The World Health Organization (WHO) AnthroPlus software v.3.0.1 (http://www.whoint/childgrowth/en/) was used to assess growth and nutritional status. The software generated scores for specific nutritional and growth measures, such as stunting by height for age (HAZ) and underweight by weight for age (WAZ) and body mass index age (BAZ). When Z scores were less than or equal to −2, measurements were considered abnormal. The children were considered malnourished when the MUAC was less than 12.5 cm.
Data analysis
The data gathered were entered into a spreadsheet database using Microsoft Excel (Redmond, WA, USA). The data were analysed using IBM Statistical Package for Social Sciences (IBM SPSS) for Windows, version 23.0 (IBM SPSS Corp., Armonk, N.Y., USA) and Microsoft Excel. Data analysis was done for 1000 students: 500 SAC from Bemberi Primary School and 500 SAC from Bandanyenje Primary School. Age for SAC was categorized into the following age groups 6-7, 8–9 and 10-13.
Descriptive statistics was used to describe the general characteristics of this study: age, gender, height, weight and MUAC of the SAC employed in this study in terms of their means, modes, median, minimum, maximum and standard deviations. Infection status was reported as negative when no eggs were observed in the urine samples and positive if one or more eggs were observed in the urine sample. A scoring system used on SPSS for schistosomiasis infection and gender was 0 denoted not infected and females and 1 denoted infected and males, respectively.
Frequency distribution analysis expressed as graphs was used to describe the distribution of age, gender, weight and MUAC of the 1000 SAC that is 500 SAC from Bemberi and 500 SAC from Bandanyenje Primary School employed in this study.
Pearson’s Chi-Square test statistics and Spearman’s Correlation test were used to define the prevalence, statistical significance, association, and strength of the relationship between the schistosomiasis infection against gender, age, height, weight and MUAC. Spearman correlation coefficient measures the strength and direction of the relationships where if rs value was interpreted based on the rule of thumb.
Mann Whitney (U) Test was used to compare the differences between S. haematobium infection in relation to gender, age, height, weight and MUAC of children from Bemberi and Bandanyenje Primary School.
The widespread of schistosomiasis among SAC was calculated as Prevalence = (number of SAC infected by schistosomiasis/total number of SAC) *100. The burden was estimated as the ratio of the proportion of infected children with morbidity (stunting or underweight) to the proportion of uninfected individuals with morbidity.
Ethical statement
The Provincial Medical Director, District Medical Director and village headman granted permission to conduct the study. Ethical approval was received from the University of Zimbabwe’s Institute Review Board (UZIRB) and the Medical Research Council of Zimbabwe (MCRZ) (MRCZ/A/1710). The permission to conduct the school-based cross-sectional study was granted by Provincial Education Director, District Education Officer, headmasters and teachers. Parents and legal guardians were asked for permission by filling and signing written consent forms. The recruitment was voluntary; hence, urine samples were obtained from willing children. Before enrolment, the study aims, objectives and procedures were explained to the parents and guardians.
Results
Prevalence of S. haematobium infection
Of 500 SAC from Bemberi, who participated in the study, 12.60% were infected with S. haematobium. From 500 SAC from Bandanyenje who participated in the study, 12% of the students were infected with S. haematobium as shown in Table .
Table 1. Interpretation of the size of Spearman correlation coefficient by the rule of thumb.
Tables show the total prevalence of SAC infected with schistosomiasis, prevalence based on gender, age, height, weight, MUAC of the 6-13-year-old children from Bemberi Primary School in Mount Darwin District and Bandanyenje Primary School in Makoni District in Zimbabwe. Higher prevalence was observed in males 14.88% and 13% from Bemberi and Bandanyenje primary schools, respectively. The highest prevalence based on age was observed among 10-13-year-olds with 17.72% and 12% for Bandanyenje and Bemberi primary schools, respectively. Bemberi’s primary school showed the highest prevalence of schistosomiasis based on height for height group 1.51-1.71 m (33.33%) and Bandanyenje highest prevalence of 100% in the height category of > 1.71 m. The highest prevalence based on weight was observed in the weight category 35.5-45.5 kg with a prevalence of 27.66% and 17.24% for Bemberi and Bandanyenje primary schools, respectively. The MUAC category of <15.5 cm showed the highest prevalence of schistosomiasis at 22.94% and 24.39% for Bemberi and Bandanyenje primary schools, respectively.
Table 2. Total prevalences of S. haematobium infection for the 6–13-year-old SAC from Bemberi Primary and Bandanyenje Primary Schools.
Table 3. S. haematobium prevalence of infection based on gender among the 6–13-year-old children from Bemberi Primary School and Bandanyenje Primary School.
Table 4. Prevalence of S. haematobium infection based on different age groups of SAC at Bemberi Primary School and Bandanyenje Primary School.
Table 5. Prevalence of S. haematobium infection based on height of SAC at Bemberi Primary School in Mount Darwin District and Bandanyenje Primary School in Makoni District.
Table 6. Prevalence of S. haematobium infection based on the weight from Bemberi Primary School in Mount Darwin District and Bandanyenje Primary School in Makoni District.
Table 7. Prevalence of S. haematobium infection based on MUAC from Bemberi Primary School and Bandanyenje Primary School in Mount Darwin and Makoni Districts.
General characteristics of the study population
Overall, 1000 SAC aged 6–13 years were enrolled in this study, 500 SAC from Bemberi Primary School and 500 SAC from Bandanyenje Primary school. From Bemberi Primary School, the ratio of infected to non-infected in percentage was 12.60: 87.40. The population comprised 51.60% females and 49.40% males with a mean age of 9 years (±1.7 SD), a mean height of 1.30 m (±0.10 SD), a mean weight of 27.25 kg (±5.56 SD) and a mean MUAC of 16.90 cm (±1.81 SD). From Bandanyenje Primary School, 12% of the SAC were infected with schistosomiasis and 88% were not infected. The study population comprised 55.40% males and 44.60% females with a mean age of 10 years (±1.97 SD), a mean height of 1.35 m (±0.13 SD), a mean weight of 29.70 kg (±7.45 SD) and a mean MUAC of 17.18 cm (±1.62 SD).
Table shows the basic features included in this study which were used to simplify the data used in the prevalence and burden of schistosomiasis in the two districts. These variables include minimum, which is the smallest value of a certain variable, maximum the highest value of a certain variable in the population, meaning which is the average of all the values used, median which is the value found in the middle of all the set values in sequential order, mode the most occurring value in the set of values, a standard deviation which gives an accurate and detailed estimate of dispersion of the values. Standard error of the mean measures the dispersion of the sample mean from the true population mean.
Table 8. Different variable distribution among 6–13 years old school-aged children from Bemberi Primary and Bandanyenje Primary School analysed using IBM SPSS for windows version 23.0.
Significance and correlation
Cross-tabulation reports were used to show the relationship between gender, age, height, weight and MUAC in relation to S. haematobium infection. It is mostly used for categorical data and hence was used for gender, age, height, weight and MUAC which was divided into groups. Relationship significance was then determined using Pearson’s Chi-Square.
Pearson’s Chi-Square
Pearson Chi-Square Test was used to test the association between the independent variables used in the study. The study was done at a 95% confidence interval and the results were significant at p < 0.05. The degrees of freedom were obtained by calculating DF = n-1 where n is the number of categories in the group of the specified general characteristics of this study. Null hypothesis (H0) denoted that there is no relationship between the variables (gender, age, height, weight and MUAC) with S. haematobium infection.
Table shows that the relationship between S. haematobium infection and age, height, weight and MUAC of SAC from Bemberi Primary school was statistically significant. However, for gender, the association was not significant since p > 0.05 and H0 were accepted. The Chi-Square showed a statistically significant relationship between urinary infection and height and MUAC for SAC from Bandanyenje primary school. However, the test showed that the relationship between gender, age and weight was statistically insignificant as shown with a p > 0.05, and, therefore, H0 was accepted.
Table 9. The Chi-Ssquare relationship between gender, age, height, weight and MUAC in relation to S. haematobium infection from Bemberi and Bandanyenje Primary School. Data were analysed for 500 SAC aged 6–13 years old using IBM SPSS for windows version 23.0. The significant association is p < 0.05.
The Mann Whitney (U) Test
Mann Whitney (U) Test was used to compare the relationship between the S. haematobium infection and gender, age, height, weight and MUAC. The null hypothesis denoted that there was no relationship between S. haematobium infection and the general characteristics of the study and the alternative hypothesis denoted that there is a relationship between the variables. A sSignificant association was done at a 95% confidence interval.
The Mann Whitney (U) Test in Table shows that the association between ages, height, weight and MUAC with S. haematobium infection was statistically significant and, therefore, H0 was rejected for Bemberi Primary school. However, the association between gender and urinary infection was insignificant since p > 0.05 hence H0 was accepted.
Table 10. The Mann Whitney (U) Test showing the association between gender, age, height, weight and MUAC in relation to S. haematobium infection. The test was performed at a 95% Confidence interval with a significant association of p < 0.05.
The test showed that the association between gender, age, height and weight from Bandanyenje primary school in relation to schistosomiasis was statistically insignificant. Hence, H0 was accepted. However, the test showed that the association between S. haematobium infection and MUAC was statistically significant therefore, H0 was rejected.
Spearman’s Correlation
Spearman’s Correlation was used to measure the strength of the relationship between the variables in relation to S. haematobium infection. Table shows that for Bemberi Primary school, with relation to urinary schistosomiasis, there was no relationship between S. haematobium infection and gender. However, a very weak positive relationship was demonstrated between age, height and weight, and a very weak negative relationship was observed between S. haematobium and MUAC. The relationship was significant for age, height, weight and MUAC, however, insignificant for gender.
Table 11. The correlation between gender, age, height, weight and MUAC of SAC aged 6–13 years old against S. haematobium infection at Bemberi and Bandanyenje Primary Schools. Significant association p < 0.05.
From Bandanyenje Primary School, there was no relationship between the variables gender, age, height, weight and a very weak negative relationship of MUAC with S. haematobium infection. The relationship was significant for MUAC only and insignificant for gender, age, height and weight.
Burden of schistosomiasis
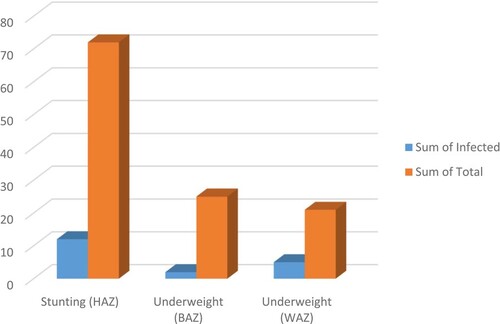
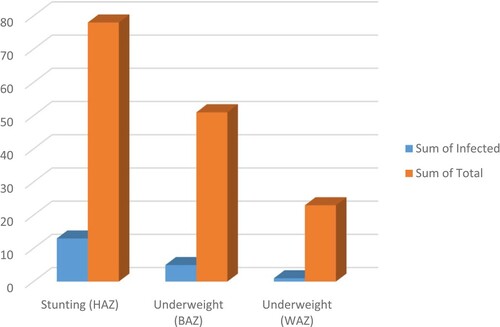
The burden for schistosomiasis among the SAC from Bemberi and Bandanyenje Primary School was determined as a measure of morbidity that is stunting, underweight and malnutrition. Table shows that the mean Z scores, for the variables HAZ indicating stunting and WAZ and BAZ as an indicator for underweight, were all greater than −2 (>−2) for both schools.
Table 12. The mean Z scores for HAZ, WAZ and BAZ to showing the distribution of stunting and underweight as a result of schistosomiasis among the children.
Table shows the burden of schistosomiasis as a measure of morbidity among the SAC from the two schools Figure .
Table 13. The burden of schistosomiasis among the SAC from Bemberi and Bandanyenje Primary Schools.
Morbidity due to schistosomiasis
Stunting and being underweight were observed among other children included in this study. Figure and Figure show the total number of children with morbidity and those with morbidity as an influence of S. haematobium infection at Bemberi and Bandanyenje Primary school, respectively. Stunting was the dominant morbidity feature in both school children.
Discussion
General characteristics and methodology
The study showed a high prevalence of urinary schistosomiasis among the children from the two schools. The variation of infection may be due to different environmental conditions, life, behaviour and nutrition of the children in these two districts.
The two districts were chosen for the study because these areas are surrounded by small perennial rivers, which are a conducive environment for the aquatic cycle of the S. haematobium (Banhela et al. Citation2017). The SAC, aged 6–13 years, were used in this particular study as it is the age group that is mainly affected with schistosomiasis due to their playful activities involving such as swimming, fishing and bathing (Hajissa et al. Citation2018).
A sample size of 500 SAC from each school was used as compared to the calculated sample size of 363. This was an added advantage because the larger the sample size the more accurate data will be since there are fewer chances of random error (Kaplan et al. Citation2014). However, a one-day sample for urine was used; therefore, there was a probability that the real prevalence results were underestimated (Midzi et al. Citation2014).
Descriptive statistics was used to simplify the data used in this study such as age, weight and MUAC in terms of mean, standard deviation, and median, mode, minimum and maximum. as shown in Table .
Prevalence of schistosomiasis among the SAC
The total prevalence of S. haematobium was in the range with previously reported studies and the prevalence of schistosomiasis in Mount Darwin and Makoni Districts was ≥10 but ≤ 50% (Midzi et al. Citation2014).
The findings of this study showed that S. haematobium prevalence was more in males than females, as shown in Table . This can be explained by the behaviour of boys, which put them at risk of being infected with schistosomiasis such as swimming, fishing or bathing in water bodies, which may be infested with cercariae (Phillips et al. Citation2018). These findings were similar yo previous results reported earlier in South-Eastern Nigeria (Amaechi Citation2014) and South West Cameroon (Hajissa et al. Citation2018). However, some studies have reported that the prevalence of infection in females was higher than males because of socio-cultural factors that may expose them to water contact such as laundry and fetching of water (Sady et al. Citation2013; Eyong et al. Citation2020).
The highest prevalence of schistosomiasis based on age from Bemberi primary school and Bandanyenje primary school was observed among 10–13-year-old age group with Bemberi having a high infection of 17. 72% and Bandanyenje has a prevalence of 12%. The explanation can be due to the increased mobility of children at this age leading to high chances of frequent water contact and activities among children in that age group (Amaechi Citation2014). This finding was like some of the studies previously reported; (Sady et al. Citation2013; Kabuyaya et al. Citation2017; Hajissa et al. Citation2018).
Approximately normal weight per age according to WHO for 6–13 years old ranges from 20 to 50 kg and the normal height ranges from 1.15 to 1.56 m of which most children in this prevalence study fell in this range. However, the prevalence of schistosomiasis based on height, weight and MUAC was of no significance due to the undistributed sample size among different categories of the variables understudy hence, for some categories, there was a small sample size giving higher prevalence values. All schoolchildren from Bemberi and Bandanyenje primary schools had MUAC greater than 12.50 cm indicating that there was no malnutrition as indicated by the WHO/UNICEF standard detection of malnutrition. The results were similar to the ones reported by Bishop and Akoh Citation2017.
Significance and correlation of general characteristics in relation to urinary infection
Pearson Chi-Square and Mann Whitney (U) Test were used to test the independence of general characteristics (gender, age, height, weight and MUAC) in relationship with S. haematobium infection. These tests were used because they are best suited for nonparametric data and the data used in this study were randomly selected. Age, height, weight and MUAC of the SAC from Bemberi Primary school in relation to urinary infection were statistically significant as shown by Chi-Square and U-test since p < 0.05. Therefore, H0 was rejected indicating that there is a relationship between age, height, weight and MUAC with S. haematobium infection. However, both tests showed statistical insignificance for gender, hence H0 was accepted indicating that gender is independent of S. haematobium infection.
Pearson Chi-Square and Mann Whitney Test were performed and showed that MUAC has p < 0.05 showing statistical insignificance, hence H0 was rejected explaining that there was a relationship between MUAC and urinary schistosomiasis. However, gender, height, weight and MUAC had p > 0.05 indicating statistical insignificance and H0 was accepted showing that the variables were independent of S. haematobium infection.
Spearman’s correlation showed that children from Bemberi had no relationship between gender and urinary schistosomiasis; however, a very weak positive relationship between age, height and weight with S. haematobium was observed. A very weak negative relationship was observed for urinary schistosomiasis and MUAC. The correlation results from Bandanyenje showed that there was no relationship between gender, age, height and weight with urinary schistosomiasis. However, the correlation results of S. haematobium infection showed a very weak negative relationship with MUAC.
The burden of schistosomiasis among the SAC
The burden of schistosomiasis among SAC from Bemberi and Bandanyenje Primary Schools was explained as a morbidity measure. The values were calculated using WHO standards and the WHO reference 2007. The HAZ indicated stunting, while WAZ and BAZ indicated underweight. However, the WAZ reference data were not significant for ages older than 10 years because this indicator does not distinguish between height and body mass at a time when many children are going through puberty and may appear to have an abnormal weight for their age when they are simply tall.
The overall data obtained showed that the Z scores for HAZ (stunting), BAZ and WAZ (underweight) were greater than −2. The results showed that there was no morbidity among the SAC from both schools and the percentage of children with morbidity was too small to make an effect on the data used in the study.
Stunting was the dominant feature caused by S. haematobium infection among SAC. Previous studies indicated that one of the effects of schistosomiasis among the SAC was stunted growth (Lothe et al. Citation2018; Osakunor et al. Citation2018). Some impacts of stunting, including poor health and poor school performance, will lead to impaired physical and mental development and a continuous life cycle of poverty which results in a deficit of productivity in adulthood (Dekker et al. Citation2010).
Conclusion and recommendations
The prevalence of S. haematobium from both schools was generally high. There is an urgent need to carry out a nationwide survey to help in planning, coordinating, and evaluating schistosomiasis control activities. School-aged children from Mount Darwin and Makoni Districts are to be treated with praziquantel biannually. The government of Zimbabwe is recommended to implement school-based health education programmes.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
Raw data are available in the repository: https://zenodo.org/record/7496465#.Y7AQOXZBzrc.DOI: https://doi.org/10.5281/zenodo.7496465
Additional information
Funding
References
- Adenowo A, Oyinloye BE, Ogunyinka BI, Kappo AP. 2015. Impact of human schistosomiasis in sub-Saharan Africa. Braz J Infect Dis. 19(2):196–205.
- Afrifa J, Gyedu D, Ofori GE, Essien-Baido S, Mensah-Essilfie I. 2017. Haematological profile and intensity of urogenital schistosomiasis in Ghanaian children. J Environ Public Health. 2017. 1–5.
- Akindele AA, Adedeji OA, Amoo B, Adekunle AA, Adedokun SA, Bolaji OS, Ojurongbe O. 2020. Epidemiology and burden of Schistosoma haematobium infection among school children in Osun State, Nigeria. Am J Biomed Sci. 12(3):173–182.
- Amaechi EC. 2014. Urinary schistosomiasis among school age children in some rural communities of Abia State. South Eastern Nigeria. Anim Res Int. 11(2):1953–1957.
- Banhela N, Taylor M, Zulu SG, Strabo LS, Kjetland EF, Gunderson SG. 2017. Environmental factors influencing the distribution and prevalence of Schistosoma haematobium in school attenders of Ilembe and uThungulu Health districts, KwaZulu-Natal Province. South Africa. Southern Afr J Infect Dis. 32(4):132–137.
- Bishop HG, Akoh RI. 2018. Risk factors, symptoms and effects of urinary schistosomiasis on anthropometric indices of school children in Zaria, Kaduna State. Nigeria. Open Access J Sci. 2(1):61–65.
- Dekker LH, Mora-Plazas M, Marin C, Baylin A, Villamor E. 2010. Stunting associated with poor socioeconomic and maternal nutrition status and respiratory morbidity in Colombian schoolchildren. Food Nutr Bull. 31(2):242–250.
- Doowuese Y, Chigor VN, Eze EA, Akosu DD, Onwuka AU, Okorie CN. 2019. Schistosomiasis in sub- Sahara Africa: causes, consequences and control measures for a leading neglected tropical disease. Int J Sci. 8(07):20–27.
- Eyong ME, Ewane EJ, Dilonga HM, Patrict IV, Nicholas T. 2020. Prevalence, infection intensity and risk factors of schistosomiasis and soil transmitted Helminthiasis among school ages children in Tiko Health District. SouthWest Cameroon: A Community-Based Cross Sectional Study. Int J Tropical Dis Health. 41(7):12–29.
- Hajissa K, Abd Elhafiz MA, Eshag HA, Alfadel A, Nahied E, Dahab R, Mohamed Z. 2018. Prevalence of schistosomiasis and associated risk factors among school children in Um-Asher Area, Khartoum, Sudan. BMC Res Notes. 11(1):1–5.
- Ismail SA, Kamal W, Salem HK. 2016. Schistosoma Prevalence World-Wide.
- Kabuyaya M, Chimbari MJ, Manyangadze T, Mukaratirwa S. 2017. Efficacy of praziquantel on Schistosoma haematobium and re-infection rates among school going children in the Ndumo area of uMkhanyakude district, KwaZulu-Natal, South Africa. Infect Dis Poverty. 6(1):1–9.
- Kaplan RM, Chambers DA, Glasgow RE. 2014. Big data and large sample size: a cautionary note on the potential for bias. Clin Transl Sci. 7(4):342–346.
- Lothe A, Zulu N, Oyhus AO, Kjetland EF, Taylor M. 2018. Treating schistosomiasis among South African high school pupils in endemic area, a qualitative study. BMC Infect Dis. 18(1):1–10.
- McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN. 2018. Schistosomiasis. Nat Rev Dis Prim. 4:13.
- Midzi N, Mduluza T, Chimbari MJ, Tshuma C, Charimari L, Mhlanga G, Manangazira P, Munyati SM, Phiri I, Mutambu SL, et al. 2014. Distribution of schistosomiasis and soil transmitted helminthiasis in Zimbabwe: towards a national plan of action for control and elimination. PLoS Negl Trop Dis. 8(8):1–13.
- Ministry of Health and Child Welfare. 2012. National schistosomiasis and soil transmitted helminth report; Ministry of Health and Child Welfare report services. Zimbabwe, pp16.
- Mnkugwe RH, Minzi OS, Kinungh’i SM, Kamuhabwa AA, Aklillu E. 2020. Prevalence and correlates of intestinal schistosomiasis infection among school aged children in North-Western Tanzania. PloS one. 15(2):1–17.
- Osakunor DNM, Mduluza T, Midzi N, Chase-Topping M, Mutsaka-Makuvaza MJ, Chimponda T, Mutapi F. 2018. Reinfection of urogenital schistosomiasis in pre-school children in a highly endemic district in Northern Zimbabwe: a 12 months’ compliance study. Infect Dis Poverty. 7(1):1–16.
- Phillips AE, Gazzinelli-Guimaraes PH, Aurelio HO, Dhanani N, Ferro J, Nala R, Fenwick A. 2018. Urogenital schistosomiasis in Cabo Delgado, northern Mozambique: baseline findings from the SCORE study. Parasit Vectors. 11(1):1–10.
- Sady H, Al-Mekhlafi HM, Mahdy MA, Lim A, Mahmud R, Surin J. 2013. Prevalence and associated factors of schistosomiasis among children in Yemen: implications for an effective control programme. PLoS Negl Trop Dis. 7(8):1–10.
- Taylor P, Makura O. 1985. Prevalence and distribution of schistosomiasis in Zimbabwe. Ann Trop Med Parasitol. 79(3):287–299.
- Tchueme LAT, Rollinson D, Stothard JR, Molyneux D. 2017. Moving from control to elimination of schistosomiasis in sub-saharan Africa: time to change and adapt strategies. Infect Dis Poverty. 6(1):1–14.
- World Health Organisation. 2016. https://www.who.int/.healthinfo/globalburdendisease/estimates/en/index1.html.