Abstract
Evidence is growing that human exposures to pesticides are contributing in a myriad of complex ways to chronic disease. Regulatory and public health agencies have struggled for years with the definition of acceptable exposure thresholds. At the same time, scientists are trying to design studies so that a chemical is delivered at ‘environmentally relevant levels.’ The aim of this review is to: (1) explain the many factors that must be taken into account in determining environmentally relevant levels or doses; (2) improve the ability to properly translate results from laboratory studies into human-health risk assessment; (3) enhance opportunities to compare results across studies using different experimental designs, organisms and routes of exposure. We found that confusion over the relationship between concentrations, dosing levels, regulatory thresholds and ‘safe’ exposure levels is common. We provide recommendations to scientists and authors, peer reviewers and journal editors in the hope of advancing understanding of how to design, carry out, interpret and explain the real-world significance of both old and new lines of scientific inquiry.
Introduction
Evidence is growing that human exposures to chemicals are contributing in a myriad of complex ways to chronic disease etiology, as well as reproductive and developmental problems (Landrigan et al. Citation2018; Mesnage and Zaller Citation2021). The likelihood that chemical exposures will cause, accelerate or exacerbate disease in human populations is difficult to predict with the current battery of toxicity tests required by regulators. Pesticide risk-assessment tools and methods have largely failed to take advantage of rapid progress in mechanistic studies, epidemiology and genomic sequencing (Wang and Gray Citation2015; Benbrook et al. Citation2021).
The quest for consensus over whether, and under what circumstances a given chemical increases the risk of an adverse impact on public health is often delayed by contradictory evidence and/or alternative explanations of observed experimental results (Mebane et al. Citation2019). Historical examples include drugs such as thalidomide or diethylstilbestrol, and environmental contaminants including organochlorine pesticides, plasticizers, so-called ‘forever chemicals’ (poly, etc.) and heavy metals (EEA Citation2013). A recent case is the ongoing debate over the oncogenicity and genotoxicity of glyphosate-based herbicides (GBHs) and their active ingredient glyphosate. The US Environmental Protection Agency (EPA), the European Food Standards Agency (EFSA) and the International Agency for Research on Cancer (IARC) have reached divergent conclusions on whether exposures to GBHs increase cancer risks (Benbrook Citation2019).
Regulatory and public health agencies have struggled for years with how to incorporate new tools and information when carrying out or updating risk assessments, revisiting acceptable exposure thresholds and deciding whether to alter risk-mitigation requirements (Bopp et al. Citation2019; EFSA Citation2022). The gap is growing between the risk-assessment status quo in industry and regulatory agencies and the cutting-edge methods and analytical systems currently deepening understanding of how and why exposures to toxic substances trigger reproductive problems, developmental anomalies or chronic disease.
Scientists not affiliated with pesticide manufacturers often strive to design experiments in ways that enhance the relevance of reported results in protecting public health (Mesnage and Séralini Citation2018; Goumenou et al. Citation2021). Indeed, an increasing number of studies are designed to mimic real-life exposure scenarios, taking into account to one degree or another numerous confounding and complicating factors including multiple routes of exposure, bioaccumulation, simultaneous exposure to several chemicals and risk factors unique to certain population groups and individuals (e.g. microbiome health and genetic polymorphisms). Regardless of the approach or focus of pesticide risk-assessment science, progress in identifying policy changes and regulatory interventions to promote public health depends on:
Accurate identification of chemical hazards,
Understanding the magnitude of risks associated with various levels, timing, routes and durations of exposure in various population cohorts, including especially known, high-exposure cohorts,
Recognition of risks likely to arise from alternatives to a chemical or chemicals subject to stricter regulation and
Realistic assessment of the likely effectiveness of possible risk-mitigation measures.
A fundamental, but incomplete principle in toxicology is that the dose makes the poison (Hayes and Dixon Citation2017). In reality, the dose plus timing of exposures, tissues exposed, other exposures and stresses on the organism, life stage, general health and the genetics of the exposed organism ‘makes the poison’ (Festing and Vesell Citation1987; Myers et al. Citation2009; Dallmann et al. Citation2016). This reality is why pesticide risk-assessment science is growing so much more complex and contentious despite rapid advances in the underlying sciences. Over the last 50 years, the focus of pesticide risk assessment has been on just a few detectable, adverse health outcomes in mostly rat and mouse studies in which treatment groups were not even administered the formulated chemical that people, and especially mixer-loaders and applicators, are exposed to. In the decades ahead, pesticide risk-assessment science must evolve beyond dichotomous analyses hopefully relevant to, but too often not protective of all exposed populations to more nuanced appraisals of the degree of risk likely to stem from routine exposures taking into account a number of factors that are not typically assessed in pesticide toxicology testing and risk assessments.
In an effort to make laboratory studies more relevant to human-health risk assessments and regulatory decisions, scientists are trying to design studies so that a chemical is delivered in vivo to a test organism, or within an in vitro cell assay system at 'environmentally relevant levels’ or ‘realistic doses’ or ‘acceptable (to an regulatory agency) rates’ (Figure ). The ongoing and commendable effort to integrate new science from many different fields and approaches into practical recommendations to promote public health will benefit from a deepened understanding of what concepts like an ‘environmentally relevant dose,’ ‘realistic exposure level,’ and ‘relevant dose level’ really mean (Weltje and Sumpter Citation2017).
Figure 1. An increasing number of toxicity studies mention the use of environmentally relevant concentration(s)/dose(s). We analysed the number of PubMed abstracts and titles containing the words ‘environmentally relevant concentration’ or ‘environmentally relevant dose’ among the abstracts containing the word ‘toxicity’ (Performed on July 27st, 2022).
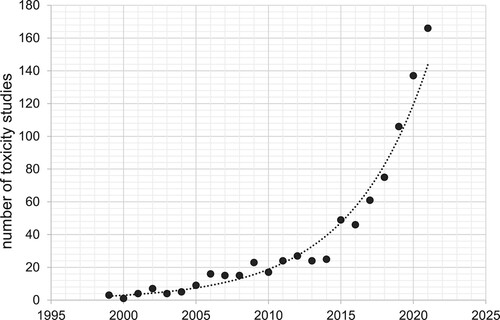
In this review, we explain how various standard concepts and measures of herbicide levels, doses, regulatory benchmarks and exposures should be deployed, explained and related one to the other. We do not aim to present a systematic review on the topic, but we critically commented a diverse set of laboratory toxicity studies designed to inform pesticide risk assessment. We focus on herbicides containing glyphosate, 2,4-dichlorophenoxyacetic acid (2,4-D) and dicamba as active ingredients. Use of these three herbicides has risen dramatically in recent years in areas where farmers have embraced herbicide-tolerant corn, soybean and cotton cultivars (Benbrook Citation2016). Rising herbicide use usually leads to new routes of exposure and higher body-burden levels as borne out in recent biomonitoring results (CDC Citation2022; Freisthler et al. Citation2022). Section 2 discusses what an ‘environmentally relevant’ dose or exposure level typically refers to. We describe important regulatory benchmarks and thresholds used around the world and the role of biomonitoring data in linking toxicology study results to public health outcomes. Section 3 describes several common mistakes in describing the relevance of experimental results to pesticide risk assessment and quantification of possible public health outcomes, with special focus on cutting-edge genotoxicity, epigenetic and microbiome studies. Last, section 4 provides recommendations to scientists and authors, peer reviewers and journal editors in the hope of advancing understanding of how to design, carry out, interpret and explain the real-world significance of both old and new lines of scientific inquiry.
Essential elements of an ‘environmentally relevant’ and/or ‘acceptable’ level of exposure
For the sake of clarity, our discussion herein focuses on herbicide applications, herbicide exposures to humans occurring as a result, and associated risks of adverse consequences in people. The basic concepts apply to all types of pesticides and chemicals, different organisms and a variety of adverse health or ecological impacts.
Many authors describe their herbicide dose levels, or dosage regime as ‘environmentally relevant.’ On what basis do they make this assertion? They typically do so on the basis of their belief that the low-end levels or doses in their experimental work are comparable to at least high-end levels of herbicide exposure experienced by humans in the real world.
There are important and often significant differences between the level of a herbicide detected in some compartment of the environment (water, food, the soil) in contrast to the delivered and absorbed dose of the herbicide in a test organism, or a person or an organism in the real world. Herbicide levels in biomonitoring studies are static concentrations expressed usually as mg or μg of the herbicide per kilogram of the food, water, biofluid, or tissue in which the herbicide is detected. Levels can charge quickly over time. Estimating exposure levels in an organism from biomonitoring results requires pharmacokinetic studies that accurately account for a chemical’s intake and rates of absorption, metabolism and excretion.
Typical, real-world herbicide exposures occur through four routes: food, drinking water and other beverages, dermal absorption and inhalation. A herbicide dose level via any of these routes, or cumulative exposures is a function of:
The person’s bodyweight,
The amount of food and beverages ingested, coupled with the concentration of the herbicide in all ingested foods and beverages,
The amount of formulated herbicide falling on a person’s skin and/or
The herbicide’s concentration (if any) in the air inhaled over the course of a day.
To normalize dose levels across people of different sizes, or from experimental animal studies to humans, the above four routes of herbicide exposure are usually reported as mg of herbicide per kilogram of bodyweight per day, or the familiar mg/kg/day. It is innacurate to refer to an experiment’s ‘dose rate’ and ‘levels’ interchangeably. Likewise, important differences occur between the total delivered dose in an experiment or a real-world setting, and the absorbed dose. For example, metabolism studies show that, in general, between 60% and 90% of ingested glyphosate passes through most mammals unabsorbed via faecal matter.
On the other hand, any herbicide measured in a person’s blood or urine had to enter the body and bloodstream somehow and most will ultimately leave the body primarily via urine. Exceptions can occur as a result of metabolism and/or binding in certain tissues (e.g. bone marrow in the case of glyphosate, or paraquat that reaches the brain and can stay in the brain for a long time). For these reasons, it is important to accurately report how a delivered dose enters the body, because the route of exposure drives the relationship between total delivered and absorbed dose, as well as the tissues or organs most likely to be impacted.
In the case of mammalian exposures to glyphosate and GBHs, it is generally assumed that about 20% of the glyphosate ingested via food or beverages is absorbed; about 3% of the glyphosate landing on skin is absorbed but significant uncertainty persists in the accuracy and applicability of these estimates despite the fact that for over 20 years glyphosate and GBHs have been by far the most heavily used pesticide globally (Benbrook Citation2016).
There are other complications in accurately quantifying and reporting total exposure levels, delivered does and absorbed doses, especially in the case of dietary exposures. For example, the portion of the glyphosate in the food and beverages a person ingests in a day that is absorbed as it passes through the GI tract is a function of the total amount of glyphosate ingested, the health of the person’s GI tract, and his or her microbiome (Mesnage, Teixeira et al. Citation2021; Mesnage, Calatayud et al. Citation2022). A total of 7 pharmacokinetic studies were performed and submitted to EFSA in order to obtain the extension of glyphosate registrations (EFSA Citation2015). There is a large variation between the different studies, but very few studies were done with low doses corresponding to what the human population is exposed to. In a recent study where twelve participants consumed a meal with a known amount of glyphosate, only 1% of the glyphosate dose was excreted in urine, suggesting that ‘typical’ absorption estimations applicable to dietary exposures (20% to 30%) may be markedly overestimated (Zoller et al. Citation2020). Further studies need to be performed to understand if glyphosate intestinal absorption becomes progressively saturated at incrementally higher doses. In addition, all these studies used gavage and thus have limited value in understanding absorption for dietary exposure scenarios. The difference between delivered and absorbed doses of glyphosate in animal feeding studies, coupled with technical glyphosate’s low acute toxicity, accounts for the very high ‘Maximum Tolerated Dose’ (MTD) in most of glyphosate’s two-year chronic feeding studies in rats and mice (i.e. high-dose levels in animal feed in the 10,000 ppm to 50,000 ppm range).
Likewise, the spatial and/or temporal distribution of measured levels of herbicides in the environment, or in food and beverages, often vary significantly. Hence, the delivered doses to organisms living within a particular ecosystem, including people, fluctuate and sometimes by wide margins. For example, herbicide levels in drinking water drawn from surface water sources tend to spike during peak herbicide-spray seasons and decline sharply over the winter (Kruć-Fijałkowska et al. Citation2022). Dietary exposures tend to be more stable over time and vary as a function of dietary choices and percent of organically grown food in a person’s diet (Curl et al. Citation2015).
The physical and chemical properties of formulated pesticides products can also impact the persistence of the herbicide in various environmental media (Kucharski and Sadowski Citation2011). These properties impact whether the herbicide bioaccumulates along certain food chains, the rate of dermal penetration, and whether the herbicide is slowly or rapidly metabolized (Brand and Mueller Citation2002). All of the above factors and properties must be taken into account in determining the differences between delivered and absorbed doses.
What makes a herbicide dose or level in the environment ‘acceptable’ or purportedly (and hopefully) ‘safe’?
A variety of health-based regulatory benchmark values have been derived based mostly on the results of registrant-submitted animal studies. The maximum level of total herbicide exposure that regulators regard as hopefully ‘safe’ is called an Acceptable Daily Intake (ADI) in Europe and a Reference Dose or Population Adjusted Dose in the U.S. This value is generally derived from laboratory animal toxicity studies conducted according to OECD or U.S. Environmental Protection Agency (EPA) guidelines.
The number of incrementally higher doses tested is an important parameter for toxicology studies used in human-health risk assessment. Experimental designs should ideally support assessment of whether there is a dose–response relationship in observed adverse health impacts. In the case of adverse effects thought to adhere to a linear dose–response relationship, a minimum of three doses is generally recommended in OECD and EPA guidelines. However, the dose response of chemicals causing toxic effects through altered hormone metabolism is not always linear, and hence requires a higher number of dose levels. This was the case of the CLARITY-BPA Core Study which administered 5 doses of bisphenol A to Sprague–Dawley rats in order to clarify the existence of health effects at low doses (Vandenberg et al. Citation2019).
Dose levels are not the only important parameter in an animal feeding study. The route of administration to test animals should be selected to reflect, as fully as possible, how humans will likely be exposed to the same chemical (Tudi et al. Citation2022). This is why studies deploying intraperitoneal or gavage dosing sometimes produce results that are questioned (Vandenberg et al. Citation2014). As a general rule, animal studies designed to mimic real-world exposures should be conducted using the same or a similar real-world routes of exposure, as well as formulated product instead of pure active ingredients, especially in the case of dermal and inhalation exposures. For example, the rat study of glyphosate and GBH dermal absorption and impacts on cancer by George et al. utilized an innovative model and dosing regime that maximized the study’s relevance in assessment of human applicator exposure and risk levels (George et al. Citation2010).
Regulators search for the lowest-observed-adverse-effect level (LOAEL) across all available toxicity studies. The next-lower dose that does not cause the adverse effect associated with the LOAEL is called the no-observed-adverse-effect level (NOAEL). By dividing the NOAEL by a standard safety factor of 100, regulators estimate a herbicide’s ADI/RfD. In some cases under U.S. law and EU policy, regulators add an additional safety factor to accommodate, for example, heightened risk of harm during pregnancy, or failure of a toxicology study to produce a NOAEL (i.e. the LOAEL is the lowest dose tested).
Most ADIs and RfDs governing general population dietary and water-based exposures are set based on chronic risks (cADI, cRfD) following exposure to pure active ingredient. To the extent surfactants and other coformulants are no longer present on or in food and beverages when eaten, this focus on just active ingredients is justified. Since cADI/cRfD exposure thresholds are derived from animal experiments typically conducted with no more than three dose levels, there is uncertainty in the determination of the dose–response relationship (Davis et al. Citation2011). More recently, Benchmark Doses (BMD) have become the preferred guidance values to derive human-health toxicity values. The BMD is a probabilistic approach that considers the shape of the dose–response curve to calculate a BMDL (‘benchmark dose lower confidence limit’). The BMD is essentially the hopefully ‘safe’ toxicity threshold. For example, a BMD5 would be the dose leading to an estimated 5% chance of an adverse impact, typically with 95% certainty.
BMDLs are often more reliable than cADIs/cRfDs that are set on the basis of NOAELs/LOAELs. This is because the calculation of BMDLs is less dependent on dose-regime selection and sample size (Davis et al. Citation2011). Although the BMDL is becoming the preferred approach within both U.S. and EU regulatory authorities, exposures to most herbicides are still managed to remain below applicable cADIs/cRfDs.
While cADIs/cRfDs set the maximum amount of a herbicide that a person can be exposed to in a day while meeting health-protection goals (i.e. in the US, ‘a reasonable certainty of no harm’), regulators also establish Maximum Contamination Levels (MCLs) for herbicides and other chemicals in drinking water. MCLs are set in a similar way as cADIs/cRfDs, and strive to assure that herbicide exposures via drinking water are not likely to push an individual over his or her personal cADI/cRfD.
The role of tolerance levels and maximum residue levels (MRLs)
Expected dietary exposures to herbicides and other pesticides are governed by the type and number of crops a herbicide is registered for use on, when the herbicide is applied in the crop growth cycle, rates and number of applications and allowable tolerance levels (also referred to as Maximum Residue Levels, or MRLs). Tolerances/MRLs govern the maximum, legal concentration of a pesticide that can be present in or on a given food item. Under US law and policy, tolerances are set to cover ∼125% of the maximum residue level expected in the harvested crop when it leaves the farm. Field studies are carried out in multiple locations deploying maximum label rates in order to quantify the likely maximum level of residue that is likely to be present in a crop sprayed with a legal, on-label application.
Accordingly in the US, tolerances are set based on unavoidable residue levels in food at harvest. Prior to approval of a tolerance, the US EPA must confirm that an analytical method is available to detect the pesticide at the proposed tolerance level, and that the tolerance is ‘supported’ by the EPA’s existing evaluation of total dietary exposure levels relative to the pesticide’s cRfD or BMDL. However, many tolerances have remained on the books for many years, if not decades, and were initially based on high-rate pesticide-use patterns sanctioned by then EPA-approved labels. But US label directions and typical use patterns change over time. There are typically large differences between maximum, allowed use of a pesticide on a given crop and how the pesticide is actually used. Moreover, the way a pesticide is used in the US or EU often differs markedly from the way it is used elsewhere. As a result, hundreds of EPA-established tolerances exceed actual residue levels in food at harvest by an order of magnitude or more. But in many cases, the EPA would not regard residues in food at or near the tolerance level as ‘safe,’ but since such elevated levels are rarely if ever detected, the agency places a low priority on revisiting the tolerances or lowering or revoking them. For this reason, it is not appropriate to assert that all published tolerances are set at ‘safe’ levels under US law (Benbrook et al. Citation2021). In addition, such tolerances allow high-risk residues to be present in food imported to the US and can place US growers at a competitive disadvantage when farmers abroad can continue to use high-risk pesticides in ways not allowed on US product labels.
It is also important to acknowledge uncertainties embedded in ADIs, RfDs, BMD, MCLs and tolerances governing residues in food. For food-use pesticides, approximately a dozen sub-chronic and chronic toxicity studies conducted mostly in rats and mice are required by regulators prior to setting a pesticide’s ADI/RfD/BMD/MCL. Such benchmark exposure levels are hopefully ‘safe’ for humans, but are ‘safe’ only to the extent that the existing studies on a pesticide’s active ingredient are capable of detecting all possible adverse impacts among people exposed to formulated pesticide products. Such an assumption, or assertion, requires a significant leap of faith.
Figure displays how many of the above factors impact the relationship between measured levels of glyphosate in the environment, in contrast to regulatory benchmarks.
Figure 2. Defining environmentally relevant doses or concentrations for glyphosate. High urinary concentrations correspond to the levels encountered in applicator studies, but also occasionally in some biomonitoring studies of the general population as in the recent National Health and Nutrition Examination Survey (CDC Citation2022) For the general public, estimated high-end environmental exposures originate mostly from dietary exposure. Exposures of bystanders or residents are poorly characterized. Environmentally relevant concentrations refers to the glyphosate concentrations which are typically found in the food chain or in environmental samples.
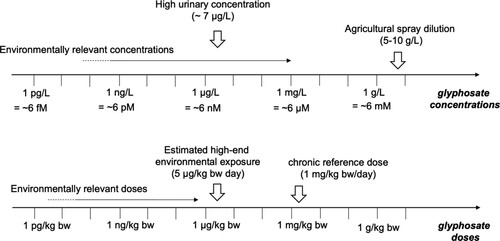
While some benchmarks are generally accepted in terms of glyphosate concentrations in different media, including people’s bodies and spray solution, the relationship between typical concentrations and delivered doses is complex and dependent on many factors unique to how a pesticide is applied or ingested and the health status of the exposed individual.
Biomonitoring and human exposure assessment
Measuring the presence of environmental pollutants in human biological fluids is a significant technical challenge. This only became possible in the last 20–30 years via major progress in the performance of analytical techniques such as mass spectrometry and nuclear magnetic resonance. Recent advances in Gas Chromatography coupled to Tandem Mass Spectrometry (GC/MS/MS) allow the measurement of blood concentrations for 60 persistent organic pollutants, including polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), polycyclic aromatic hydrocarbons (PAHs), dioxins/furans, as well as some pesticides (Macherone et al. Citation2015).
Although pesticide exposures have been linked to health effects after residential and occupational exposures (Cognez et al. Citation2019; Sagiv et al. Citation2019), few studies have focused on population-wide exposures and risk outcomes. A notable exception is the herbicide biomonitoring carried out as part of the National Health and Nutrition Examination Survey (NHANES) performed by the US Centers for Disease Control and Prevention (CDC). NHANES collects urine and blood samples from several thousand people on a recurring cycle, along with extensive demographic, dietary and health data (https://www.cdc.gov/exposurereport/).
Over the last two decades, NHANES has periodically tested urine for 17 sulfonylurea herbicides, atrazine, 2,4-D, as well as pyrethroid, organophosphorus insecticide and carbamate metabolites. For 2,4-D, urinary levels are available for samples collected in 1999–2000 and 2001–2002 by the NHANES. Approximately a quarter of the population studied had 2,4-D urinary levels below the limit of detection (0.2 µg/L), with the most exposed individuals having levels around 1 µg/L. The 95th percentile for 2,4-D urinary concentrations was 1.24–1.55 µg/L depending on the age group considered. There were significant associations between crop application of 2,4-D and the per cent of NHANES participants with detectable levels of 2,4-D in urine (Freisthler et al. Citation2022).
In Europe, the European Human Biomonitoring Initiative (project HBM4EU) is the largest study of human exposure to chemicals in Europe. It strives to incorporate biomonitoring data in the chemical risk-assessment process (Louro et al. Citation2019). More recently, pyrethroids, chlorpyrifos, dimethoate, glyphosate and fipronil were also included in the priority substance list. Interestingly, two pesticide coformulants (piperonyl butoxide and ethoxylated tallowamine) were also included in the HBM4EU priority list. Some independent studies were also performed to evaluate the exposure to these active ingredients (Mesnage, Bowyer et al. Citation2022), or develop biomonitoring methods for their coformulants (Mesnage, Mazzacuva et al. Citation2021).
By contrast, the monitoring of hair (Appenzeller et al. Citation2017; Grundler et al. Citation2021), adipose tissue (Jackson et al. Citation2017) and meconium (Ostrea et al. Citation2008) are increasingly used to provide insights on cumulative exposure. A notable example is a recent French study measuring pesticides in mothers’ hair samples and children's measurements at birth (Beranger et al. Citation2020). Among the 64 compounds measured, numerous associations between maternal hair concentrations and birth measurements were statistically significant. An extensive list of biological matrices usable at different life stages to track exposures is available (Barr et al. Citation2005).
Health risk evaluations from human biomonitoring
Human biomonitoring data can be used directly to estimate health outcomes in human populations. A classic example is the increase in lifetime mean blood lead levels from 1 to 10 μg/dL, an increase associated with a reduction of 7.4 IQ points (Lanphear et al. Citation2005). However, scenarios where both the health outcome and exposure levels can be studied in a human population are uncommon. Health-based guidance values allowing the estimation of health risk are generally based on toxicity tests performed in rodents before a product is released on the market.
When the cADI/cRfD is used to perform health risk evaluations, biomonitoring equivalent (BE) values are sometimes calculated in order to compare ‘environmental’ doses in humans to references doses in rodents. In this context, ‘environmental’ refers to ‘real-world’ exposure levels based on actual herbicide levels in food, water, the air and spray solution. A BE level is the concentration of a given chemical in urine or plasma which is expected when an average individual is exposed to the cADI/cRfD, see (Aylward and Hays Citation2008) for a 2,4-D example. Estimating the internal dose in humans requires pharmacokinetic knowledge to estimate the delivered dose required to produce a given level in urine or blood. When the internal dose reaching the bloodstream (or a target tissue) of a pesticide is estimated from urinary concentrations. It is necessary to incorporate average human physiological data in the model such as body weight, food or water consumption or urine excretion (EFSA Citation2012).
Health risk can be estimated from the results of animal studies even when information is not sufficient to adequately derive BE values. In a recent review of glyphosate biomonitoring studies, it was found that the highest glyphosate urinary levels detected were 7 μg/L (Gillezeau et al. Citation2019). If a concentration of 7 µg/L glyphosate is measured in urine and the person excretes 2 L of urine per day (EFSA Citation2012), daily excretion of glyphosate would be 14 µg per day via urine. Metabolism studies suggest that at least 80% of ingested glyphosate passes through the GI tract unabsorbed, exiting the body in faeces (Figure ). In addition, another 2% to 5% of ingested glyphosate is likely retained beyond 24 h in the body, mostly in bone marrow, liver and kidney tissues. Glyphosate does not bioaccumulate in mammals and metabolism is minimal given how quickly glyphosate exits the body, although some studies suggest that glyphosate can be metabolized by the gut microbiome (Mesnage and Antoniou Citation2020; Mesnage, Calatayud et al. Citation2022).
Figure 3. Overview of the chronic health risk assessment of dietary exposures to glyphosate – switching from biomonitoring to risk assessment. Considering that the high-end of glyphosate urinary levels resulting from environmental exposures is approximately 7 μg/L (Gillezeau et al. Citation2019), it can be estimated that environmental exposures to glyphosate result in an internal dose of 0.2 µg/kg/bw and an external dose of 1 µg/kg/bw. Glyphosate concentrations cannot be considered as environmentally relevant if they are far higher than these doses, except in the case of relatively high applicator or occupational exposures.
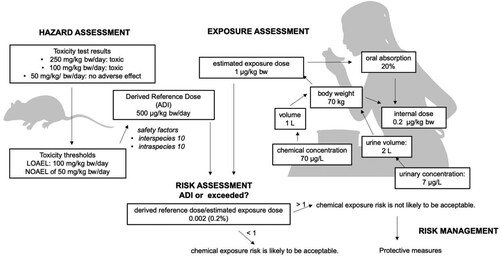
A 70 kg person would have an internal dose of 0.2 µg/kg body weight (internal dose = daily excretion [14 µg] / body weight [70 kg]) (Figure ). The corresponding external dose, based on the calculations described above, is 1 µg/kg body weight. This is because it is estimated that 80% of a glyphosate dose that enters the body via food/beverages passes through unabsorbed and exists the body in faeces. The 14 µg of glyphosate in urine is thus likely to originate from an exposure to 70 µg glyphosate [70 µg x 0.2]. However, recent studies suggest that for dietary exposures, as little as 1% of the delivered dose of glyphosate was excreted in urine (Zoller et al. Citation2020). In this case the estimated intake from human urine data is 1400 µg glyphosate [70 × 0.01]. The acceptable daily intake of glyphosate set by EFSA is 500 µg/kg body weight per day, suggesting a 500-fold margin of safety between current levels of exposure and the applicable EFSA cADI. However, this large margin-of-safety depends on glyphosate’s relatively high cADI/cRfD of 500 μg/kg/day in Europe (currently 1.0 mg/kg/day in the US).
If a new toxicology study emerges that leads EFSA to reduce glyphosate’s cADI to 5 μg/kg/day, the 500-fold safety margin would shrink to just 5-fold. Recent animal studies assessing the role of glyphosate-based herbicide exposures in triggering non-alcoholic fatty liver disease suggest that a cADI reduction well below 5μg/kg/day might actually be necessary to fully protect against damage to the liver (Mesnage et al. Citation2017). Other adverse health effects yet to be associated with GBH use and exposures may be recognized. For example, birth cohort studies focused on glyphosate and its primary breakdown product aminomethylphosphonic acid (AMPA) have reported associations with health outcomes such as breast cancer, aging, or preterm birth, at real-world levels of exposure occurring in several areas (Franke et al. Citation2021; Silver et al. Citation2021; Lucia Rachel et al. Citation2022). It is widely recognized that for many chemicals, adverse impacts on reproduction and children’s development occur at the lowest dose levels across all other endpoints (Council Citation1993).
From dietary exposures to internal doses
When biomonitoring data is not available, it is possible to estimate pesticide dietary exposures using standard daily intake levels for a variety of foodstuffs for which contaminating levels are known. This is performed in Europe using the EFSA pesticide Residues Intake Model PRIMO model (Brancato et al. Citation2018). PRIMO estimates pesticide external doses for a variety of dietary patterns.
Dietary risk assessment methods for pesticides are comparable in the US. Chronic exposures rely on food-consumption data from the NHANES ‘What We Eat in America’ survey (Steinfeldt et al. Citation2019). In PRIMO, food intake levels are derived from WHO cluster diets, as well as diets considered representative of different EU countries, for adults, toddlers or infants.
Pesticide exposure levels are modelled using the median residue value derived from residue trials, or maximum residues limits. These levels of residues reflect the concentration in pesticide residues which are expected in a crop cultivated according to good agricultural practices. They can thus be combined with national commodity intake levels to estimate the daily intake of a given active substance, as done with the pesticide residue data collected by the UK-FSA and USDA via the Dietary Risk Index (DRI) system (Benbrook and Davis Citation2020). These values can also be refined across food forms by considering data on the typical impact of food processing on residue levels in fresh food forms, coupled with available monitoring data on various food forms (e.g. fresh, frozen, canned, dried, juice, sauce). For glyphosate, International Estimates of Daily Intake (IEDI) (in % ADI) for the WHO GEMS/Food 17 Cluster Diets range between 1.7 and 4.9 µg/kg bw/day in a recent analysis (Stephenson and Harris Citation2016). In this case, the highest contributor to the total glyphosate intake was barley in the Irish diet. It is estimated that the Irish population consumes on average 65 grams of barley in breakfast cereals which may be contaminated by 5.85 mg/kg of glyphosate (Stephenson and Harris Citation2016). Barley consumption in Ireland results in a glyphosate daily intake of 0.38 µg/kg bw/day.
Challenges in the design and conduct of pesticide studies designed to inform risk assessment and promote public health
Guidelines exist to ensure that toxicity studies in animals and cell assay systems are technically sound and reproducible. But there are no formal or verified methods to determine whether such studies are likely to detect the adverse effect on humans likely to occur at the lowest intake level, especially among vulnerable and/or heavily exposed populations. Regulatory studies using doses several orders of magnitude higher than dietary doses, and unrealistic routes of exposures such as gavage continue to support nearly all pesticide dietary risk assessments.
Concentrations used in tissue culture experiments cannot be directly compared to doses arising from concentrations in the environment. A variety of factors drive the degree to which concentrations in formulated products, spray solutions, food, water, soil or air translate into delivered doses to an individual. Another set of complex factors then drive blood and tissue concentrations, longevity of exposures and health outcomes.
Evaluating the health effects of pesticides in different scenarios requires a good understanding of the concept of dose, as well as the differences between a dose level and a concentration. In brief, a concentration expresses the mass of molecules in a volume (e.g. grams per litre), or the number of molecules in a volume of liquid (e.g. moles per litre). A dose is typically normalized to take account of the weight of a given individual, the level in food or beverages consumed and the duration of the exposure (e.g. grams per kilogram body weight per day). As a consequence, a given dose can be achieved by eating or drinking different concentrations of chemicals depending on the body weight of the exposed individuals. In some cases, like for multi-component mixtures, it is also common to express a given level as a dilution (volume per volume, v/v). This v/v unit can also be expressed as a percentage (%) or as a part-per-million (1 ppm being a dilution of one in a million) for smaller quantities. Timing is also important. It is common to normalize exposure to daily intakes. Most references doses such as the acceptable daily intake are expressed as a daily dose (e.g. mg/kg bw/day).
Although this is common knowledge for toxicologists, concentrations and doses are sometimes not accurately communicated when authors with expertise in specific test methods, but not general toxicological principles and methods, test pesticides, describe their protocols and report experimental findings. For instance, In a glyphosate toxicity study (de Liz Oliveira Cavalli et al. Citation2013), authors indicated that glyphosate was tested at a low dose of 36 ppm in vitro, which could suggest that they tested a dose of 36 mg/kg body weight or a concentration of 36 ppm in some medium to which the test organism was exposed. A concentration of 0.036 g/L was actually tested in an in vitro system, and so does not reflect a daily dose of 36 mg/kg/day. Concentrations used in tissue cultures experiments cannot be directly compared to doses in animal studies and concentrations in the environment. The authors indicated that ‘It is important to emphasize that Roundup is used in agricultural work at dilutions ranging from 10,000 to 20,000 ppm (10 to 20 g/L), concentrations much higher than those described in our results.’ If the glyphosate was ingested, an agricultural worker with a body weight of 70 kg would have to drink approximately one litre of his 10 g/L spray to absorb 2 g of glyphosate (assuming 20% intestinal absorption), which would be diluted to a concentration of 0.036 g/L glyphosate at the tissue level assuming that glyphosate distributes evenly in different tissues.
Academic scientists are also free to create their own set of rules to define the relevance of their results. Authors sometimes claim that a study is relevant for public-health assessment in the hope such relevance will enhance the odds of favourable passage through peer review. For instance, a recent glyphosate toxicity study which tested 0.05% concentration of a Roundup formulation on breast cancer cell lines concluded that Roundup altered cell cycle and DNA repair ‘at much lower doses than the ones used in agriculture’ (Stur et al. Citation2019). This study cannot be used to inform regulators on the effects of glyphosate on the mammary gland because the authors do not discuss expected glyphosate concentrations in the mammary gland after expected, real-world exposures. A recent study investigated for the first time the transgenerational inheritance of glyphosate induced-obesity, prostate, kidney and ovarian disease in rats (Kubsad et al. Citation2019). The authors claimed that they used ‘an environmentally relevant exposure’ by administering daily intraperitoneal injections of glyphosate (25 mg/kg bw/day). This dose is at least 10,000 times too high to be qualified as ‘environmentally relevant’ following exposures via the human diet. In addition, intraperitoneal injections are not regarded as comparable to environmental exposure scenarios.
Other animal studies have used intraperitoneal administrations to assess the effects of transgenerational exposures which are more likely to occur through oral exposures (Kubsad et al. Citation2019). Although the use of this type of experimental design can be suitable to study biochemical mechanisms, they are less valuable when conducting health risk assessments. Overall, few studies are performed where the experimental design is tailored to specific exposure scenarios. In the next sections, we recommend a set of criteria which can be used in scientific studies to enhance their relevance.
The concentration of a pesticide ingredient in an experimental system that can be regarded as environmentally relevant will differ between application and exposure scenarios. Exposure levels are higher among human populations living in intensive cropping areas or urban habitats where pesticides are frequently used. It is also organism dependent. Environmentally relevant concentrations will be different for worms and soil fungi in agricultural fields regularly sprayed with pesticides compared to untreated cropland, different among organisms at various branches in the tree of life and different depending on the adverse impact of concern.
Caution must be exercised in attempts to draw hazard or risk conclusions from in vitro cell assays. In order to characterize their dosage levels as ‘environmentally relevant,’ some authors of in vitro studies erroneously compare their dose levels (e.g. 1 g/L) to the recommended 10–20 g/L spray solution concentrations on product labels. In a study aiming to characterize the effects of glyphosate on liver cells, a group from Italy exposed hepatoma tissue culture (HTC) cells to 1–10 mM Roundup. The authors qualified their dosage as ‘very low concentrations of Roundup.’ However, calculations using standard conversion factors reveal that a rat would need to be exposed to a diet contaminated by approximately 10 g/kg glyphosate to be exposed to an internal concentration of 1–10 mM Roundup (Malatesta et al. Citation2008).
Some authors also claim that the doses in their studies correspond to ‘environmental relevant’ levels because they tested permitted levels like the EFSA cADI or a tolerance level. The fact that a concentration of glyphosate is allowed in a certain exposure scenario (i.e. in soybeans) does not mean that the concentration is an accurate estimate of delivered or absorbed dose from any or all routes of exposure. In a study of glyphosate reprotoxicity (Milesi et al. Citation2018), the authors indicated that ‘The dose of 2 mg/kg bw/day is representative of the glyphosate residues found in soybean grains.’ Although a glyphosate concentration of 2 mg/kg may be found in soybeans, this does not mean that their consumption will automatically result in a dose of 2 mg/kg bw/day. A typical human being (70 kg) would need to eat 70 kg of soybeans contaminated by 2 mg/kg every day to achieve a dose level of 2 mg/kg bw/day. In other studies, maximum residue limits are confounded with actual environmental levels. This was the case in a long-term toxicity study investigating toxic effects of a GBH at the levels permitted by regulatory authorities in drinking water in the EU (0.1 µg/L) (Seralini et al. Citation2014). Levels of pesticides allowed in tap water in the EU are not established based on the results of toxicity studies and should not be used to claim that effects at 0.1 µg/L or above are a source of health risks.
Environmental pollutants such as pesticides are found at very low concentrations in human biological fluids. While food components, drugs, or endogenous compounds are found in blood at concentrations in the range of the µM level, pollutant concentrations are generally found at the nM level (Rappaport et al. Citation2014). We estimated in the previous sections that a realistic dose in studies aiming to mimic environmental exposure scenarios for glyphosate would be below 10 µg/kg bw/day. Such exposures would be predominantly via food and drinking water. Further research is needed to estimate comparable ‘environmentally relevant’ dose levels in the case of dermal and inhalation exposures among those handling and spraying GBHs, and especially among those spraying a GBH for several hours a day for many days per year over many years using small-scale application equipment.
A surprisingly common mistake is failure to fully and carefully describe the test substance. For example, some authors just report a test involving glyphosate without clarifying what chemical form was secured, or whether a pure active ingredient or formulated product was used (Sivikova and Dianovsky Citation2006; Chan et al. Citation2007; Hokanson et al. Citation2007). Ideally, authors should state not just the precise chemical formulation tested, but also provide full details on where the product was purchased, date of purchase and lot numbers when available.
Conclusion
We identify and describe gaps in the scientific literature regarding the concept of environmental relevance of exposures and doses in laboratory settings. Our aim is threefold: to explain the many factors that must be taken into account in determining environmentally relevant levels; second, to improve the ability to properly translate results from laboratory studies into human-health risk assessment; and third, to enhance opportunities to compare results across studies using different experimental designs, organisms and routes of exposure.
Clear definitions and full specification of details on test substances, formulations, organisms tested, how doses are delivered and pharmacokinetics are vital to fully appreciate the findings in a given study. We also provide an overview of the roll of concentrations and doses in chemical risk assessment, highlighting the emerging use of human biomonitoring data in the regulatory assessments. It is crucial to indicate in scientific studies what environments the concentrations are coming from, and which organisms and exposure routes are encompassed in a given study. We also recommend that Editors and reviewers should pay particular attention to misuse of exposure thresholds in defining ‘acceptable’ concentrations in various media in light of various routes of exposure.
This paper can serve as a point of departure for disciplinary experts compiling and vetting recommendations to help scientists design and carry out projects, report results and conduct peer-reviews. The goal is to better understand and more clearly define and approximate ‘environmentally relevant’ levels and doses of pesticides across the diversity of use patterns, exposure scenarios, test systems and human vulnerabilities.
Important next steps in regions with rising herbicide use should include focused, clinical studies of pregnant women and newborns designed to determine whether elevated levels of prenatal herbicide exposures are associated with heightened risk of adverse birth outcomes spanning low birth weight, pre-term delivery, birth defects and developmental abnormalities. The research objectives and protocols of the Heartland Study serve as an example of a clinical study designed to explore potential, herbicide-induced adverse birth outcomes (Benbrook et al. Citation2021).
Improving science reporting needs to be associated with an improvement of science translation for public audiences (Uhrig et al. Citation2020). Erroneous statements about environmental relevance have been publicized in a variety of media, sometimes inadvertently but sometimes with intent (Hansen Citation2016). This has been well documented for the case of tobacco (Baba et al. Citation2005). However, this is not unilateral. Pesticide manufacturers develop strategies to preserve their commercial interests while advocate groups deploy strategies to ban these same products. The way scientific findings are presented can also profoundly shape public reactions. Since most adults do not have experience in participating in scientific research activities, and have for most of all limited education on the specialized topics (Burke et al. Citation2022), it is important for scientists to adopt strategies which improve public acceptance of research information. For instance, mental models of how a person conceptualizes a phenomenon have been proposed to improve public understanding of the risks from infections by mosquito borne diseases (Southwell et al. Citation2018).
There is equally compelling need to more accurately compare regulatory-set, herbicide-exposure thresholds to levels of herbicides measured in the environment, food, sediment, the air and human biofluids and tissues. Such levels must be tracked to determine whether contemporary and legal herbicide uses are leading to exposures reliably below, roughly equal to, or sometimes above ‘acceptable’ regulatory benchmarks (e.g. cRfDs/ADIs). The path from measured levels of herbicides in various media, to estimates of exposure that can be compared to ‘acceptable’ doses derived from toxicology studies is fraught with technical complexity. The path is too often clouded by unstated assumptions and missing information. Imprecise characterization of test substances and dosing methods, routes and levels often complicate interpretation of results. Confusion over the relationship between concentrations, regulatory thresholds and ‘safe’ exposure levels is all too common and must be overcome to accelerate progress in identifying those pesticides and exposure scenarios worthy of regulatory interventions and those that do not based on current knowledge.
Author's contributions
RM and CMB designed and wrote this review.
Disclosure statement
CMB has and continues to serve as an expert witness in pesticide litigation, including cases involving Roundup and non-Hodgkin lymphoma and paraquat and Parkinsons disease. RM has served as a consultant on glyphosate risk assessment issues as part of litigation in the US over glyphosate health effects. RM is a section editor of the section ‘Environmental Toxicology and Health’ in ‘All Life’.
Data availability statement
This manuscript is a review and do not contain experimental data. All the results reviewed are from published studies.
Ethical statement
The research presented in this manuscript did not involve any animal or human participants.
References
- Appenzeller BMR, Hardy EM, Grova N, Chata C, Faÿs F, Briand O, Schroeder H, Duca RC. 2017. Hair analysis for the biomonitoring of pesticide exposure: comparison with blood and urine in a rat model. Arch Toxicol. 91(8):2813–2825. DOI:10.1007/s00204-016-1910-9
- Aylward LL, Hays SM. 2008. Biomonitoring equivalents (BE) dossier for 2,4-dichlorophenoxyacetic acid (2,4-D) (CAS No. 94-75-7). Regul Toxicol Pharmacol. 51(3 Suppl):S37–S48. DOI:10.1016/j.yrtph.2008.05.006
- Baba A, Cook DM, McGarity TO, Bero LA. 2005. Legislating “sound science": the role of the tobacco industry. Am J Public Health. 95(Suppl 1):S20–S27. DOI:10.2105/ajph.2004.050963
- Barr DB, Wang RY, Needham LL. 2005. Biologic monitoring of exposure to environmental chemicals throughout the life stages: requirements and issues for consideration for the national children's study. Environ Health Perspect. 113(8):1083–1091. DOI:10.1289/ehp.7617
- Benbrook C, Perry MJ, Belpoggi F, Landrigan PJ, Perro M, Mandrioli D, Antoniou MN, Winchester P, Mesnage R. 2021. Commentary: novel strategies and new tools to curtail the health effects of pesticides. Environ Health. 20(1):87. DOI:10.1186/s12940-021-00773-4
- Benbrook CM. 2016. Trends in glyphosate herbicide use in the United States and globally. Environ Sci Eur 28(1):3. DOI:10.1186/s12302-016-0070-0
- Benbrook CM. 2019. How did the US EPA and IARC reach diametrically opposed conclusions on the genotoxicity of glyphosate-based herbicides? Environ Sci Eur. 31(1):2. DOI:10.1186/s12302-018-0184-7
- Benbrook CM, Davis DR. 2020. The dietary risk index system: a tool to track pesticide dietary risks. Environ Health. 19(1):1–18.
- Béranger R, Hardy EM, Binter AC, Charles MA, Zaros C, Appenzeller BMR, Chevrier C. 2020. Multiple pesticides in mothers’ hair samples and children's measurements at birth: results from the French national birth cohort (ELFE). Int J Hyg Environ Health. 223(1):22–33. DOI:10.1016/j.ijheh.2019.10.010
- Bopp SK, Kienzler A, Richarz AN, van der Linden SC, Paini A, Parissis N, Worth AP. 2019. Regulatory assessment and risk management of chemical mixtures: challenges and ways forward. Crit Rev Toxicol. 49(2):174–189. DOI:10.1080/10408444.2019.1579169
- European Food Safety Authority (EFSA); Brancato A, Brocca D, Ferreira L, Greco L, Jarrah S, Leuschner R, Medina P, Miron I, Nougadere A, et al. 2018. Use of EFSA pesticide residue intake model (EFSA PRIMo revision 3). EFSA J. 16(1):e05147. DOI:10.2903/j.efsa.2018.5147
- Brand RM, Mueller C. 2002. Transdermal penetration of atrazine, alachlor, and trifluralin: effect of formulation. Toxicol Sci. 68(1):18–23.
- Burke A, Okrent A, Hale K, Gough N. 2022. The State of US Science & Engineering 2022. National Science Board Science & Engineering Indicators. NSB-2022-1. National Science Foundation.
- CDC. 2022. National Health and Nutrition Examination Survey - Glyphosate (GLYP) - Urine (SSGLYP_H). https://www.ncdcgov/Nchs/Nhanes/2013-2014/SSGLYP_Hhtm.
- Chan YC, Chang SC, Hsuan SL, Chien MS, Lee WC, Kang JJ, Wang SC, Liao JW. 2007. Cardiovascular effects of herbicides and formulated adjuvants on isolated rat aorta and heart. Toxicol In Vitro. 21(4):595–603. DOI:10.1016/j.tiv.2006.12.007
- Cognez N, Warembourg C, Zaros C, Metten MA, Bouvier G, Garlantézec R, Charles MA, Béranger R, Chevrier C. 2019. Residential sources of pesticide exposure during pregnancy and the risks of hypospadias and cryptorchidism: the French ELFE birth cohort. Occup Environ Med. 76(9):672–679. DOI:10.1136/oemed-2019-105801
- Council NR. 1993. Pesticides in the diets of infants and children.
- Curl CL, Beresford SA, Fenske RA, Fitzpatrick AL, Lu C, Nettleton JA, Kaufman JD. 2015. Estimating pesticide exposure from dietary intake and organic food choices: the Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health Perspect. 123(5):475–483. DOI:10.1289/ehp.1408197
- Dallmann R, Okyar A, Lévi F. 2016. Dosing-time makes the poison: circadian regulation and pharmacotherapy. Trends Mol Med. 22(5):430–445.
- Davis JA, Gift JS, Zhao QJ. 2011. Introduction to benchmark dose methods and U.S. EPA's Benchmark Dose Software (BMDS) version 2.1.1. Toxicol Appl Pharmacol. 254(2):181–191. DOI:10.1016/j.taap.2010.10.016
- de Liz Oliveira Cavalli VL, Cattani D, Heinz Rieg CE, Pierozan P, Zanatta L, Benedetti Parisotto E, Wilhelm Filho D, Mena Barreto Silva FR, Pessoa-Pureur R, Zamoner A. 2013. Roundup disrupts male reproductive functions by triggering calcium-mediated cell death in rat testis and Sertoli cells. Free Radical Biol Med. 65:335–346. DOI:10.1016/j.freeradbiomed.2013.06.043
- EEA. 2013. Late lessons from early warnings: science, precaution, innovation. ISBN 978-92-9213-349-8. DOI:10.2800/70069
- EFSA. 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA J. 10(3):2579. DOI:10.2903/j.efsa.2012.2579
- EFSA. 2015. Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J. 13(11):4302. DOI:10.2903/j.efsa.2015.4302
- EFSA. 2022. Guidance on the assessment of exposure of operators, workers, residents and bystanders in risk assessment of plant protection products. EFSA J. 20(1):e07032.
- Festing MF, Vesell ES. 1987. Genetic factors in toxicology: implications for toxicological screening. CRC Crit Rev Toxicol. 18(1):1–26.
- Franke AA, Li X, Shvetsov YB, Lai JF. 2021. Pilot study on the urinary excretion of the glyphosate metabolite aminomethylphosphonic acid and breast cancer risk: The multiethnic cohort study. Environ Pollut. 277:116848. DOI:10.1016/j.envpol.2021.116848
- Freisthler MS, Robbins CR, Benbrook CM, Young HA, Haas DM, Winchester PD, Perry MJ. 2022. Association between increasing agricultural use of 2,4-D and population biomarkers of exposure: findings from the National Health and Nutrition Examination Survey, 2001–2014. Environ Health. 21(1):23. DOI:10.1186/s12940-021-00815-x
- George J, Prasad S, Mahmood Z, Shukla Y. 2010. Studies on glyphosate-induced carcinogenicity in mouse skin: a proteomic approach. J Proteomics. 73(5):951–964. DOI:10.1016/j.jprot.2009.12.008
- Gillezeau C, van Gerwen M, Shaffer RM, Rana I, Zhang L, Sheppard L, Taioli E. 2019. The evidence of human exposure to glyphosate: a review. Environ Health. 18(1):2. DOI:10.1186/s12940-018-0435-5
- Goumenou M, Djordjevic AB, Vassilopoulou L, Tsatsakis AM. 2021. Endocrine disruption and human health risk assessment in the light of real-life risk simulation. In: Tsatsakis AM, editor. Toxicological risk assessment and multi-system health impacts from exposure. Elsevier; p. 147–162.
- Grundler F, Séralini G-E, Mesnage R, Peynet V, Wilhelmi de Toledo F. 2021. Excretion of heavy metals and glyphosate in urine and hair before and after long-term fasting in humans. Front Nutr. 8. DOI:10.3389/fnut.2021.708069
- Hansen A. 2016. The changing uses of accuracy in science communication. Public Underst Sci. 25(7):760–774. DOI:10.1177/0963662516636303
- Hayes AW, Dixon D. 2017. Cornerstones of toxicology. Toxicol Pathol. 45(1):57–63. DOI:10.1177/0192623316675768
- Hokanson R, Fudge R, Chowdhary R, Busbee D. 2007. Alteration of estrogen-regulated gene expression in human cells induced by the agricultural and horticultural herbicide glyphosate. Hum Exp Toxicol. 26(9):747–752. DOI:10.1177/0960327107083453
- Jackson E, Shoemaker R, Larian N, Cassis L. 2017. Adipose tissue as a site of toxin accumulation. Compr Physiol. 7(4):1085–1135. DOI:10.1002/cphy.c160038
- Kruć-Fijałkowska R, Dragon K, Drożdżyński D, Górski J. 2022. Seasonal variation of pesticides in surface water and drinking water wells in the annual cycle in western Poland, and potential health risk assessment. Sci Rep. 12(1):3317. DOI:10.1038/s41598-022-07385-z
- Kubsad D, Nilsson EE, King SE, Sadler-Riggleman I, Beck D, Skinner MK. 2019. Assessment of glyphosate induced epigenetic transgenerational inheritance of pathologies and sperm epimutations: generational toxicology. Sci Rep. 9(1):6372. DOI:10.1038/s41598-019-42860-0
- Kucharski M, Sadowski J. 2011. Behaviour of metazachlor applied with additives in soil: laboratory and field studies. J Food Agric Environ. 9(3/4 part 2):723–726.
- Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu NN, Baldé AB, Bertollini R, Bose-O'Reilly S, Boufford JI, et al. 2018. The Lancet Commission on pollution and health. Lancet. 391(10119):462–512. DOI:10.1016/S0140-6736(17)32345-0
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, et al. 2005. Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. Environ Health Perspect. 113(7):894–899. DOI:10.1289/ehp.7688
- Louro H, Heinälä M, Bessems J, Buekers J, Vermeire T, Woutersen M, van Engelen J, Borges T, Rousselle C, Ougier E, et al. 2019. Human biomonitoring in health risk assessment in Europe: current practices and recommendations for the future. Int J Hyg Environ Health. 222(5):727–737. DOI:10.1016/j.ijheh.2019.05.009
- Lucia RM, Huang WL, Pathak KV, McGilvrey M, David-Dirgo V, Alvarez A, Goodman D, Masunaka I, Odegaard AO, Ziogas A, et al. 2022. Association of glyphosate exposure with blood DNA methylation in a cross-sectional study of postmenopausal women. Environ Health Perspect. 130(4):047001. DOI:10.1289/EHP10174
- Macherone A, Daniels S, Maggitti A, Churley M, McMullin M, Smith M. 2015. Measuring a slice of the exposome: targeted GC-MS/MS analysis of persistent organic pollutants POPs) in small volumes of human plasma. In: 63rd AMS conference on mass spectrometry and allied topics, St Louis, MO, Abstract TP, Vol. 309.
- Malatesta M, Perdoni F, Santin G, Battistelli S, Muller S, Biggiogera M. 2008. Hepatoma tissue culture (HTC) cells as a model for investigating the effects of low concentrations of herbicide on cell structure and function. Toxicol In Vitro. 22(8):1853–1860. DOI:10.1016/j.tiv.2008.09.006
- Mebane CA, Sumpter JP, Fairbrother A, Augspurger TP, Canfield TJ, Goodfellow WL, Guiney PD, LeHuray A, Maltby L, Mayfield DB, et al. 2019. Scientific integrity issues in environmental toxicology and chemistry: improving research reproducibility, credibility, and transparency. Integr Environ Assess Manag. 15(3):320–344. DOI:10.1002/ieam.4119
- Mesnage R, Antoniou MN. 2020. Computational modelling provides insight into the effects of glyphosate on the shikimate pathway in the human gut microbiome. Curr Res Toxicol. 1:25–33. DOI:10.1016/j.crtox.2020.04.001
- Mesnage R, Bowyer RCE, El Balkhi S, Saint-Marcoux F, Gardere A, Ducarmon QR, Geelen AR, Zwittink RD, Tsoukalas D, Sarandi E, et al. 2022. Impacts of dietary exposure to pesticides on faecal microbiome metabolism in adult twins. Environ Health. 21(1):46. DOI:10.1186/s12940-022-00860-0
- Mesnage R, Calatayud M, Duysburgh C, Marzorati M, Antoniou MN. 2022. Alterations in infant gut microbiome composition and metabolism after exposure to glyphosate and roundup and/or a spore-based formulation using the SHIME® technology. Gut Microbiome. 1–30. DOI:10.1017/gmb.2022.5
- Mesnage R, Mazzacuva F, Caldwell A, Halket J, Antoniou MN. 2021. Urinary excretion of herbicide co-formulants after oral exposure to roundup MON 52276 in rats. Environ Res. 197:111103. DOI:10.1016/j.envres.2021.111103
- Mesnage R, Renney G, Seralini GE, Ward M, Antoniou MN. 2017. Multiomics reveal non-alcoholic fatty liver disease in rats following chronic exposure to an ultra-low dose of roundup herbicide. Sci Rep. 7:39328. DOI:10.1038/srep39328
- Mesnage R, Séralini G-E. 2018. Editorial: toxicity of pesticides on health and environment. Front Public Health. 6. DOI:10.3389/fpubh.2018.00268
- Mesnage R, Teixeira M, Mandrioli D, Falcioni L, Ducarmon QR, Zwittink RD, Mazzacuva F, Caldwell A, Halket J, Amiel C, et al. 2021. Use of shotgun metagenomics and metabolomics to evaluate the impact of glyphosate or roundup MON 52276 on the gut microbiota and serum metabolome of sprague-dawley rats. Environ Health Perspect. 129(1):17005. DOI:10.1289/EHP6990
- Mesnage R, Zaller JG. 2021. Herbicides: chemistry, efficacy. In: Mesnage R, Zaller JG, editors. Toxicology, and environmental impacts. Elsevier.
- Milesi MM, Lorenz V, Pacini G, Repetti MR, Demonte LD, Varayoud J, Luque EH. 2018. Perinatal exposure to a glyphosate-based herbicide impairs female reproductive outcomes and induces second-generation adverse effects in Wistar rats. Arch Toxicol. 92(8):2629–2643. DOI:10.1007/s00204-018-2236-6
- Myers JP, Zoeller RT, vom Saal FS. 2009. A clash of old and new scientific concepts in toxicity, with important implications for public health. Environ Health Perspect. 117(11):1652–1655.
- Ostrea EM Jr, Bielawski DM, Posecion NC Jr, Corrion M, Villanueva-Uy E, Jin Y, Janisse JJ, Ager JW. 2008. A comparison of infant hair, cord blood and meconium analysis to detect fetal exposure to environmental pesticides. Environ Res. 106(2):277–283. DOI:10.1016/j.envres.2007.08.014
- Rappaport SM, Barupal DK, Wishart D, Vineis P, Scalbert A. 2014. The blood exposome and its role in discovering causes of disease. Environ Health Perspect. 122(8):769–774. DOI:10.1289/ehp.1308015
- Sagiv SK, Bruno JL, Baker JM, Palzes V, Kogut K, Rauch S, Gunier R, Mora AM, Reiss AL, Eskenazi B. 2019. Prenatal exposure to organophosphate pesticides and functional neuroimaging in adolescents living in proximity to pesticide application. Proc Natl Acad Sci U S A. 116(37):18347–18356. DOI:10.1073/pnas.1903940116
- Séralini GE, Clair E, Mesnage R, Gress S, Defarge N, Malatesta M, Hennequin D, de Vendômois JS. 2014. Republished study: long-term toxicity of a roundup herbicide and a roundup-tolerant genetically modified maize. Environ Sci Eur. 26(1):14. DOI:10.1186/s12302-014-0014-5
- Silver MK, Fernandez J, Tang J, McDade A, Sabino J, Rosario Z, Vélez Vega C, Alshawabkeh A, Cordero JF. 2021. Prenatal exposure to glyphosate and its environmental degradate, aminomethylphosphonic acid (AMPA), and preterm birth: a nested case-control study in the PROTECT Cohort (Puerto Rico). Environ Health Perspect. 129(5):57011. DOI:10.1289/ehp7295
- Sivikova K, Dianovsky J. 2006. Cytogenetic effect of technical glyphosate on cultivated bovine peripheral lymphocytes. Int J Hyg Environ Health. 209(1):15–20. DOI:10.1016/j.ijheh.2005.07.005
- Southwell BG, Ray SE, Vazquez NN, Ligorria T, Kelly BJ. 2018. A mental models approach to assessing public understanding of Zika virus, Guatemala. Emerg Infect Dis. 24(5):938–939. DOI:10.3201/eid2405.171570
- Steinfeldt L, Goldman J, Moshfegh A. 2019. Usual intake of food patterns components by older adults from WWEIA. NHANES. 2013–2016. (P18-119-19). Curr Develop Nutr. 3(Suppl 1). DOI: 10.1093/cdn/nzz039.P18-119-19.
- Stephenson CL, Harris CA. 2016. An assessment of dietary exposure to glyphosate using refined deterministic and probabilistic methods. Food Chem Toxicol. 95:28–41. DOI: 10.1016/j.fct.2016.06.026.
- Stur E, Aristizabal-Pachon AF, Peronni KC, Agostini LP, Waigel S, Chariker J, Miller DM, Thomas SD, Rezzoug F, Detogni RS, et al. 2019. Glyphosate-based herbicides at low doses affect canonical pathways in estrogen positive and negative breast cancer cell lines. PLoS One. 14(7):e0219610. DOI:10.1371/journal.pone.0219610
- Tudi M, Li H, Li H, Wang L, Lyu J, Yang L, Tong S, Yu QJ, Ruan HD, Atabila A, et al., 2022. Exposure routes and health risks associated with pesticide application. Toxics. 10:6. DOI:10.3390/toxics10060335
- Uhrig JD, Lewis MA, Poehlman JA, Southwell BG. 2020. Beyond evidence reporting: evidence translation in an era of uncertainty. The Medical Care Blog [online publication of Medical Care]. https://www.themedicalcareblog.com/evidence-translation/.
- Vandenberg LN, Hunt PA, Gore AC. 2019. Endocrine disruptors and the future of toxicology testing—lessons from CLARITY–BPA. Nat Rev Endocrinol. 15(6):366–374.
- Vandenberg LN, Welshons WV, Vom Saal FS, Toutain PL, Myers JP. 2014. Should oral gavage be abandoned in toxicity testing of endocrine disruptors? Environ Health. 13(1):46. DOI:10.1186/1476-069x-13-46
- Wang B, Gray G. 2015. Concordance of noncarcinogenic endpoints in rodent chemical bioassays. Risk Anal. 35(6):1154–1166. DOI:10.1111/risa.12314
- Weltje L, Sumpter JP. 2017. What makes a concentration environmentally relevant? critique and a proposal. Environ Sci Technol. 51(20):11520–11521. DOI:10.1021/acs.est.7b04673
- Zoller O, Rhyn P, Zarn JA, Dudler V. 2020. Urine glyphosate level as a quantitative biomarker of oral exposure. Int J Hyg Environ Health. 228:113526. DOI:10.1016/j.ijheh.2020.113526
