Abstract
PNO1 (Dim2 or Rrp2 or YOR145), a highly conserved regulator of ribosome assembly from yeast to mammals, is involved in forming the 90S pre-ribosome and plays an essential role in the late stage of 40S small subunit maturation. Recent studies have found that PNO1 is involved in the progression of a variety of tumors and is highly expressed in colorectal, lung, esophageal, glioma, and breast cancer and is associated with poor prognosis. In tumors, PNO1 mainly promotes cell proliferation, invasion, and autophagy and inhibits apoptosis by regulating the P53 pathway, FAK/AKT pathway, Notch signaling pathway, and MAPK signaling pathway. The high expression of PNO1 in various tumors may be reduced by the regulation of early B cell factor 1 (EBF1), transcription factor MYC, miR-340-5p, and the drug celecoxib inhibiting tumor proliferation, invasion and migration, and autophagy, promoting apoptosis. This article reviews the structure and function of PNO1, related molecular pathways, and its regulatory role in tumor formation and discusses its possibility as a molecular target for tumor therapy.
Introduction
Cancer cells are characterized by the uncontrolled and massive proliferation of cells (Evan and Vousden Citation2001), which requires extensive protein synthesis, thus increasing ribosome biogenesis (Ruggero Citation2012). Ribosome biogenesis, involving the de novo synthesis of ribosomes, is a complex and essential process within cells, including transcription and processing of ribosomal RNA, production of ribosomal proteins, and assembly of nuclear export of ribosomal subunits. Biogenesis in eukaryotes is complex and depends on more than 200 assembly factors. During the process of ribosomal maturation, assembly factors such as helicase, ATPase, GTPase, and kinase are inserted at different times and released from the ribosomal particles. Studies have shown that ribosomal biogenesis affects cell growth and proliferation by affecting the ability of cells to synthesize proteins. Many diseases, such as cancer, are relevant to the disorder of this vital process (Ruggero and Pandolfi Citation2003; Algra and Rothwell Citation2012; Cao et al. Citation2016). Over-expressed ribosomal assembly factors and specific ribosomal proteins promote ribosomal biogenesis, regulate the progression of some human malignant tumors, and are linked to poor prognosis (Pogue-Geile et al. Citation1991; Kondoh et al. Citation2001; Kasai et al. Citation2003; Bee et al. Citation2006; Lai and Xu Citation2007; Wang et al. Citation2009; Bee et al. Citation2011; Song et al. Citation2011). If it is supposed that up-regulated ribosomal biogenesis can promote the proliferation of cancer cells, then down-regulated ribosomal biogenesis will lead to cell cycle arrest, inhibit cell proliferation, induce apoptosis, and achieve the objective of inhibiting tumor growth (Boon et al. Citation2001; Deisenroth and Zhang Citation2010; Stumpf and Ruggero Citation2011; Armistead and Triggs-Raine Citation2014).
PNO1 (Dim2/Rrp2/YOR145), a highly conserved regulator of ribosome assembly from yeast to mammals, is involved in the transition from pre-90S to 40S and the final stage of maturation of the 40S small subunit in the cytoplasm (Turowski et al. Citation2014; Sturm et al. Citation2017; Ameismeier et al. Citation2020). In both eukaryotes and prokaryotes, the PNO1 family exhibits significant conservation. Nucleus-localized PNO1 regulates the biosynthesis of ribosomes and proteasomes, mainly concentrated in the nucleolus. Studies showed that PNO1 was mainly expressed in the liver and lung, and a small amount was expressed in the thymus and testis, while no expression was found in the heart and brain, skeletal muscle, small intestine, and colon (Tone and Toh Citation2002; Miura et al. Citation2004; Vanrobays et al. Citation2004; Zhou et al. Citation2004; Lin et al. Citation2020). In 2002, research showed that PNO1 acts as a molecular chaperone to form a complex with NOB1 and localize in the nucleus (Tone and Toh Citation2002). At the last stage of the maturation of the 40S small subunit in the cytoplasm, RKIO1 (a member of the atypical eukaryotic protein kinase family, which is highly conserved and participates in the development of all systems) shifts and digests PNO1 to dissociate it from the endonuclease NOB1, which stimulates the cleavage of NOB1 at the 3′ end of pre-18SrRNA, and plays an essential role in ribosomal biogenesis (Senapin et al. Citation2003; Vanrobays et al. Citation2008; Shen et al. Citation2019). Recent studies have found that PNO1 is involved in the progression of a variety of tumors and is highly expressed in colorectal cancer, lung cancer, esophageal cancer, glioma, and breast cancer, and is associated with poor prognosis (Shen et al. Citation2019; Liu et al. Citation2020; Chen et al. Citation2021; Wang et al. Citation2021 may). Therefore, a promising anticancer strategy might inhibit ribosome biosynthesis by targeting the ribosome assembly factor PNO1.
Main text
The structure and function of PNO1
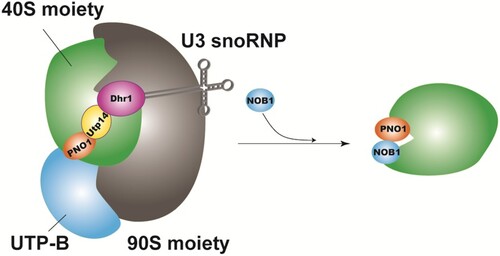
PNO1, the human homologous protein of the NOB1 gene, also known as Dim2, or Rrp2, or YOR145 (Zemp and Kutay Citation2007), is a highly conserved regulator of ribosome assembly from yeast to mammals. The human PNO1 gene is located on chromosome 2q14 and consists of five introns and seven exons (Miura et al. Citation2004). The full-length cDNA sequence of PNO1 is 1637 bp, containing a 759 bp open reading frame, encoding a protein of 252 or 248 amino acids (Miura et al. Citation2004; Woolls et al. Citation2011). KH domains exist in many different proteins involved in countless different biological processes, including splicing, transcriptional regulation, and translational control (Valverde et al. Citation2008), whereas PNO1 contains two conserved KH domains (KH1, KH2) in sequence, in which KH2 contains the GXXG motifs necessary for RNA/DNA binding, but KH1 lacks the typical GXXG RNA-binding motifs and instead participates in protein–protein interactions (Woolls et al. Citation2011; Zheng et al. Citation2014). The KH1 domain of PNO1 provides a binding site for the endonuclease NOB1 (Sturm et al. Citation2017). Ribosomes are where cells synthesize proteins, and are known as ‘the protein factory of cells,’ responsible for translating the genetic code in mRNA and catalyzing protein synthesis in organisms. Ribosomes are composed of two ribonucleoprotein subunits, large and small. In eukaryotes, ribosome biosynthesis begins with forming a large precursor particle, the 90S pre-ribosome, from which pre-40S and pre-60S particles are generated (Dragon et al. Citation2002; Grandi et al. Citation2002). In 90S biogenesis, PNO1 localizes to the pre-90S ribosome by interacting with the UTP-B module to receive and transmit information about the correct assembly state of the nascent ribosome. UTP-B is a module with ‘antenna’-like properties which can glean information on the assembly state from unusual regions of 90S granules. Following proper 90S assembly and coordinated pre-rRNA processing, the information can be transported from UTP-B by PNO1 to nearby Utp14 (cofactor and activator of Dhr1 helicase activity which catalyzes the removal of U3 snoRNP from 90S) (Sturm et al. Citation2017). In this way, the Dhr1 helicase can induce dissociation of U3 snoRNP from their pre-rRNA binding sites by unwinding the RNA heteroduplex, resulting in a pre-90S to 40S transition (Martin et al. Citation2013) (Figure ).
Figure 1. Model of PNO1 (Dim2) involvement during the transition from the 90S to the 40S pre-subunit (Turowski et al. Citation2014; Sturm et al. Citation2017).
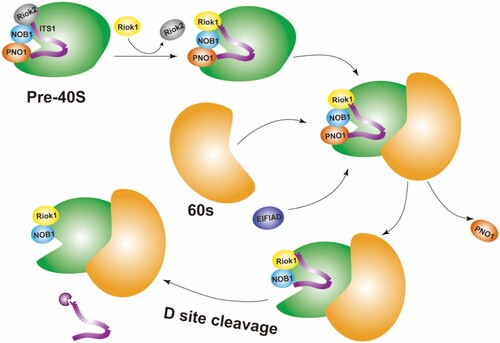
PNO1 is not only involved in early ribosome maturation but also plays an important role in the late stage of small subunit maturation. On the one hand, it is involved in the final assembly of the 40S small subunit (Ameismeier et al. Citation2020). The formation of the small subunit ends with the final 18S rRNA precursor 18S-E being cleaved by the endonuclease NOB1 at position 3 (D site 18), which flanks residues of the internal transcribed spacer 1 (ITS1) (a combination of transcription factors, which has the characteristics of conservative phylogeny and may play a role in ribosomal maturation) and all remaining biogenesis factors are rapidly released (Woolford and Baserga Citation2013; Cerezo et al. Citation2019). Previous structures of the human 40S precursor indicate that PNO1 binds to the 3′ end of the 18S rRNA with its cognate protein NOB1 (Ameismeier et al. Citation2018 jun), and NOB1 exists in an inactive conformation (Sloan et al. Citation2019). During the final stage of small subunit maturation, with the participation of the eukaryotic translation initiation factor 1A domain protein (EIF1AD) and the mature 60S subunit, the atypical kinase RIOK1 (a member of the atypical eukaryotic protein kinase family, which is highly conserved and participates in the development of all systems) coordinates the conformational maturation of 18S rRNA and the release of PNO1, thereby activation of the endonuclease NOB1 cleaves 20S pre-rRNA to generate 18S rRNA (Ameismeier et al. Citation2020) (Figure ).
Figure 2. Model for the involvement of PNO1 in the final stage of 40S small subunit maturation (Turowski et al. Citation2014; Sturm et al. Citation2017).
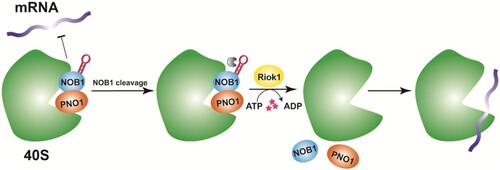
On the other hand, PNO1, together with NOB1 and the kinase RIOK1, establish a quality checkpoint that prevents premature translation of immature ribosomes. PNO1 stabilizes NOB1 structure and prevents mRNA recruitment to the 40S ribosome of immature 20S pre-rRNA, thus preventing premature release of the immature 40S subunit into the translation pool. Kinase RIOK1 utilizes the energy generated by ATP hydrolysis to release PNO1 and NOB1 from nascent ribosomes, thereby regulating their entry into the translation pool in an ATPase-dependent manner. RIOK1-NOB1-PNO1 plays a vital role by ensuring that only fully mature ribosomes enter the translation pool (Parker et al. Citation2019) (Figure ).
Figure 3. RIOK1-NOB1-PNO1 checkpoint mechanism model (Parker et al. Citation2019).
The role and mechanism of PNO1 in tumor
Recent studies have shown that PNO1 is not only involved in the normal physiological function of ribosomes but also is closely related to the occurrence and development of tumors. High expression of PNO1 is considered a marker of poor prognosis in various tumors and promotes cell proliferation, invasion, and autophagy mainly by regulating the p53 pathway, FAK/AKT pathway, Notch signal pathway, and MAPK signal pathway (Table and Figure ).
Figure 4. Regulation of PNO1-related pathways. (EBF1: early B cell factor 1; MYC: transcription factor) (Dai et al. Citation2019; Shen et al. Citation2019; Liu et al. Citation2020; Chen et al. Citation2021; Han et al. Citation2021; Wang et al. Citation2021 may).
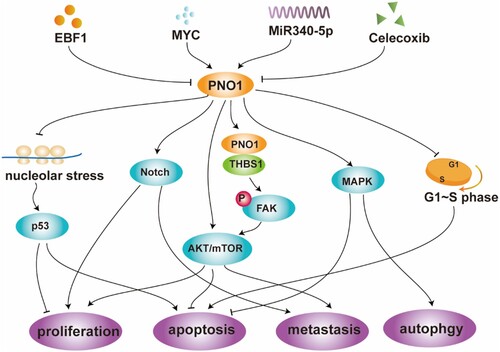
Table 1. The role and related pathways of PNO1 in different tumors.
PNO1 regulates the p53 pathway through the nuclear stress pathway to promote tumor cell proliferation and inhibit apoptosis
PNO1 regulates cell proliferation mainly by regulating P53-related protein in colorectal cancer. Activation of the well-known tumor suppressor p53 induces the transcription of various genes leading to cell cycle arrest, inhibition of cell proliferation, and induction of apoptosis (Morgado-Palacin et al. Citation2012; Liu et al. Citation2016; Shen et al. Citation2019). Various cellular injuries can activate p53, including the blockade of ribosome biogenesis (Zhang and Lu Citation2009; Deisenroth and Zhang Citation2010). Experiments have demonstrated that PNO1 knockdown reduces the number of 18S rRNA, 40S subunit, 60S subunit, and 80S ribosomes, resulting in defects in ribosome biosynthesis (Shen et al. Citation2019), which triggers a p53-dependent cellular stress response called ‘nucleolar stress’ or ‘ribosomal stress’ (Deisenroth and Zhang Citation2010). Nuclear stress promotes the binding of the ribosomal proteins RPL1/(rp)L11 and RPL5/(rp)L5 as well as 5S rRNA to MDM2. It inhibits its ubiquitin ligase activity on p53, resulting in reduced degradation and ubiquitination of p53, promoting p53 accumulation (Deisenroth and Zhang Citation2010; Golomb et al. Citation2014; Goudarzi et al. Citation2014), thereby inhibiting cell proliferation. In addition, inhibition of endogenous PNO1 increases the percentage of colorectal cancer cells apoptosis and increases the activities of caspase-3 and caspase-9. PNO1 knockdown also increases the percentage of cells in the G0-G1 phase and decreases the percentage of cells in the S phase. Inhibition of endogenous PNO1 can induce apoptosis during the G1-S transition phase of the cell cycle and inhibit tumor growth in vivo and in vitro (Shen et al. Citation2019). Early B cell factor 1 (EBF1) in B cell development helps drive DNA demethylation and chromatin remodeling, thereby controlling the transcription of various genes (Liao Citation2009; Bohle et al. Citation2013). In colorectal cancer, EBF1 overexpression significantly decreases the levels of PNO1 mRNA and protein and increases the levels of p53 and p21 protein (Shen et al. Citation2019), inhibiting tumor proliferation by inhibiting PNO1-mediated p53/p21 signaling pathway activation. Furthermore, EBF1 overexpression induces cell cycle arrest in the G0/G1 phase and increases apoptosis (Shen et al. Citation2020).
PNO1 promotes glioma cell proliferation and metastasis by regulating the FAK pathway
In many tumor cells, tyrosine kinase FAK promotes the development of the malignant phenotype of the tumor through over-expression (Zhou et al. Citation2019). Thrombospondin 1 (THBS1) is one of the critical components of the extracellular matrix and is involved in regulating the development of tumors, including gliomas (Gahtan et al. Citation1999; Adams and Lawler Citation2004 jun; Firlej et al. Citation2011). In glioma cells U251, co-immunoprecipitation experiments showed that PNO1 interacts with THBS1 and regulates the expression of THBS1, leading to the phosphorylation of FAK and Akt, participating in the activation of the FAK/Akt pathway and significantly promoting the proliferation and metastasis of glioma cells (Chen et al. Citation2021). The proliferation and metastasis of PNO1 overexpression in glioma can be attenuated or even reversed by simultaneous silencing of THBS1. Transcription factor MYC is a key integrator of growth-regulatory and oncogenic signaling pathways (Kress et al. Citation2015). In glioma cells, overexpression of MYC increases the activity of the PNO1 promoter. In contrast, MYC knockdown significantly reduces the expression levels of PNO1 and THBS1 mRNA and protein, resulting in the phosphorylation of FAK and Akt, significantly reduced cell viability, anti-apoptosis capacity, and invasion capacity through the PNO1/THBS1/FAK/Akt signaling pathway (Chen et al. Citation2021).
PNO1 promotes tumor cell proliferation, migration, and invasion by regulating the Notch signaling pathway
Cell differentiation, proliferation, apoptosis, and metastasis in colorectal cancer, lung cancer, breast cancer, and other tumors are regulated by the Notch signaling pathway. Cancer cells can also enhance the ability of invasion and migration, stem cell-like characteristics, and therapeutic resistance through epithelial–mesenchymal transformation (EMT) (Yuan et al. Citation2014; Jin et al. Citation2017; Wang et al. Citation2018). Results on the GSEA dataset (Gene Set Enrichment Analysis) suggest that PNO1 may act as an oncogene to promote lung adenocarcinoma (LUAD) progression through the Notch signaling pathway and that PNO1 expression is positively correlated with EMT (Liu et al. Citation2020). In LUAD, PNO1 knockdown decreases the protein levels of Notch2 (Notch pathway ligand), Notch4 (Notch intracellular domain), and Hey1 (Notch target gene). In addition, PNO1 can induce the up-regulation of EMT-related marker E-cadherin, and the down-regulation of EMT-related marker N-cadherin and vimentin are also regulated by PNO1. Downregulation of PNO1 can inhibit the proliferation, migration, and invasion of LUAD cells by inhibiting the Notch signaling pathway that regulates EMT. The xenotransplantation model and lung metastasis experiment showed that the proliferation and metastasis of LUAD in vivo might be encouraged by the expression of PNO1 (Liu et al. Citation2020). There is a precise molecular upstream mechanism in LUAD, negative regulation of PNO1 by miR-340-5p. By directly binding to PNO1 3′ untranslatable region (UTR), miR-340-5p inhibits the expression of PNO1 in LUAD and plays an essential role in the progression of LUAD through the Notch signaling pathway (Liu et al. Citation2020).
PNO1 regulates tumor cell proliferation, invasion, and apoptosis by inducing AKT/mTOR, NF-κB and other signaling
AKT signaling pathway regulates many processes under physiological and pathological conditions, including metabolism, proliferation, cell survival, growth, and angiogenesis (Lien et al. Citation2016; Manning and Toker Citation2017). In in vitro and in vivo experiments, PNO1 knockdown has been shown to significantly reduce protein kinase B (AKT)/rapamycin (mTOR) signaling, reduced tumor volume, tumor weight, and lung metastasis, and significantly inhibit the growth and metastasis of hepatoma cells (Dai et al. Citation2019). In esophageal cancer tissues, knockdown of PNO1 inhibits cell proliferation, migration, and invasion. It promotes cell apoptosis, which may function by regulating the expression of AKT1, Twist, MYC, mTOR, matrix metalloproteinase 2 (MMP2), NF-κB p65 and CTNNB1 (β-catenin 1). Furthermore, in nude mice, smaller tumor volumes are observed after PNO1 knockdown (Wang et al. Citation2021 may). Celecoxib may also exert its anti-tumor activity by inhibiting PNO1. Celecoxib is a member of the cyclooxygenase-2 (COX-2) selective non-steroidal anti-inflammatory drug (NSAID) family. In tumors, celecoxib can inhibit proliferation (Gao et al. Citation2016), apoptosis (Toriyama et al. Citation2016), angiogenesis (Gao et al. Citation2016 Oct), and invasion (Behr et al. Citation2015) and helps slow the progression of liver, lung, breast, and prostate tumors (Stasinopoulos et al. Citation2013; Vosooghi and Amini Citation2014; Suri et al. Citation2016). In hepatocellular carcinoma, celecoxib significantly reduces the level of PNO1 in tumor tissue, inhibits the growth of hepatoma cells in vitro and in vivo (Dai et al. Citation2019), and in a mouse xenograft tumor model, celecoxib has shown efficacy against hepatocellular carcinoma (Chu et al. Citation2018). AKT/mTOR signaling helps mediate the oncogenic effects of PNO1 (Dai et al. Citation2019).
PNO1 promotes autophagy and inhibits apoptosis of tumor cells by activating the MAPK signal pathway
Autophagy can double regulate the progress of cancer (Poillet-Perez and White Citation2019). On the one hand, autophagy can promote tumorigenesis and angiogenesis by providing nutrition and energy. On the other hand, autophagy can inhibit inflammation and promote genomic stability, thus serving as an inhibitor of early cancer development (Wu et al. Citation2012; White Citation2015; Amaravadi et al. Citation2016; Singh et al. Citation2018). Autophagy has served as the entry point to treat cancer in clinical trials (Barnard et al. Citation2014; Chude and Amaravadi Citation2017 jun 16). The principal pathway of cancer autophagy may include Erk/MAPK signaling pathway (Gao et al. Citation2019; Yue et al. Citation2019; Xiong et al. Citation2020). Activation of the Erk/MAPK signal pathway can promote the proliferation, invasion, and metastasis of hepatoma cells (Liu et al. Citation2016; Chen et al. Citation2019; Li et al. Citation2019). In hepatocellular carcinoma, it has been found that the over-expression of PNO1 inhibits the apoptosis of hepatocellular carcinoma cells by activating the MAPK signal pathway to promote autophagy, and the down-regulation of PNO1 interferes with the downstream of autophagy-related markers p62, Beclin-1, LC3B, Atg5 and Atg7 (Han et al. Citation2021).
Perspectives
The above results not only highlight the potential carcinogenic role of PNO1 and the possibility of being used as a biomarker for early diagnosis and prognosis of tumors, but also prove the rationality of finding small molecular inhibitors targeting PNO1 as a new cancer treatment strategy, and are expected to provide new targets for individualized cancer therapy and prognostic value for the identification of patients.
Statements & declarations
Competing interests
The authors report there are no competing interests to declare.
Acknowledgment
Thanks to all the working partners and funding agencies who participated and contributed to this project.
Author contributions
Wanyi Liu, Yongqiu Zeng, and Guicheng Kuang were involved in drafting the paper and the conception, design, analysis, and interpretation of data. Binbin Yang, Hanlin Liu, Yangpin Lv, and Yang Xiong revised the paper critically for intellectual content. All authors agree on the final approval of the version to be published and that all authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing does not apply to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Adams JC, Lawler J. 2004 Jun. The thrombospondins. Int J Biochem Cell Biol. 36(6):961–968.
- Algra AM, Rothwell PM. 2012 May. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomized trials. Lancet Oncol. 13(5):518–527.
- Amaravadi R, Kimmelman AC, White E. 2016 Sep 1. Recent insights into the function of autophagy in cancer. Genes Dev. 30(17):1913–1930.
- Ameismeier M, Cheng J, Berninghausen O, Berninghausen O, et al. 2018 Jun. Visualizing late states of human 40S ribosomal subunit maturation. Nature. 558(7709):249–253.
- Ameismeier M, Zemp I, van den Heuvel J, et al. 2020 Nov. Structural basis for the final steps of human 40S ribosome maturation. Nature. 587(7835):683–687.
- Armistead J, Triggs-Raine B. 2014 May 2. Diverse diseases from a ubiquitous process: the ribosomopathy paradox. FEBS Lett. 588(9):1491–1500.
- Barnard RA, Wittenburg LA, Amaravadi RK, et al. 2014 Aug. Phase I clinical trial and pharmacodynamic evaluation of combination hydroxychloroquine and doxorubicin treatment in pet dogs treated for spontaneously occurring lymphoma. Autophagy. 10(8):1415–1425.
- Bee A, Brewer D, Beesley C, et al. 2011. siRNA knockdown of ribosomal protein gene RPL19 abrogates the aggressive phenotype of human prostate cancer. PLoS One. 6(7):e22672.
- Bee A, Ke Y, Forootan S, et al. 2006 Apr 1. Ribosomal protein l19 is a prognostic marker for human prostate cancer. Clin Cancer Res. 12(7 Pt 1):2061–2065.
- Behr CA, Hesketh AJ, Barlow M, et al. 2015 Oct. Celecoxib inhibits Ewing sarcoma cell migration via actin modulation. J Surg Res. 198(2):424–433.
- Bohle V, Doring C, Hansmann ML, et al. 2013 Mar. Role of early B-cell factor 1 (EBF1) in Hodgkin lymphoma. Leukemia. 27(3):671–679.
- Boon K, Caron HN, van Asperen R, et al. 2001 Mar 15. N-myc enhances the expression of a large set of genes functioning in ribosome biogenesis and protein synthesis. EMBO J. 20(6):1383–1393.
- Cao Y, Nishihara R, Wu K, et al. 2016 Jun 1. Population-wide impact of long-term Use of aspirin and the risk for cancer. JAMA Oncol. 2(6):762–769.
- Cerezo E, Plisson-Chastang C, Henras AK, et al. 2019 Jan. Maturation of pre-40S particles in yeast and humans. Wiley Interdiscip Rev RNA. 10(1):e1516.
- Chen J, Ji T, Wu D, et al. 2019 May 29. Human mesenchymal stem cells promote tumor growth via MAPK pathway and metastasis by epithelial mesenchymal transition and integrin alpha5 in hepatocellular carcinoma. Cell Death Dis. 10(6):425.
- Chen X, Guo ZQ, Cao D, et al. 2021 Mar 4. MYC-mediated upregulation of PNO1 promotes glioma tumorigenesis by activating THBS1/FAK/Akt signaling. Cell Death Dis. 12(3):244.
- Chu TH, Chan HH, Hu TH, et al. 2018 Jun. Celecoxib enhances the therapeutic efficacy of epirubicin for novikoff hepatoma in rats. Cancer Med. 7(6):2567–2580.
- Chude CI, Amaravadi RK. 2017 Jun 16. Targeting autophagy in cancer: update on clinical trials and novel inhibitors. Int J Mol Sci. 18(6):1279.
- Dai H, Zhang S, Ma R, et al. 2019 Sep 30. Celecoxib inhibits hepatocellular carcinoma cell growth and migration by targeting PNO1. Med Sci Monit. 25:7351–7360.
- Deisenroth C, Zhang Y. 2010 Jul 29. Ribosome biogenesis surveillance: probing the ribosomal protein-Mdm2-p53 pathway. Oncogene. 29(30):4253–4260.
- Dragon F, Gallagher JE, Compagnone-Post PA, et al. 2002 Jun 27. A large nucleolar U3 ribonucleoprotein required for 18S ribosomal RNA biogenesis. Nature. 417(6892):967–970.
- Evan GI, Vousden KH. 2001 May 17. Proliferation, cell cycle and apoptosis in cancer. Nature. 411(6835):342–348.
- Firlej V, Mathieu JR, Gilbert C, et al. 2011 Dec 15. Thrombospondin-1 triggers cell migration and development of advanced prostate tumors. Cancer Res. 71(24):7649–7658.
- Gahtan V, Wang XJ, Ikeda M, et al. 1999 Jun. Thrombospondin-1 induces activation of focal adhesion kinase in vascular smooth muscle cells. J Vasc Surg. 29(6):1031–1036.
- Gao JH, Wen SL, Feng S, et al. 2016 Oct. Celecoxib and octreotide synergistically ameliorate portal hypertension via inhibition of angiogenesis in cirrhotic rats. Angiogenesis. 19(4):501–511.
- Gao L, Dou ZC, Ren WH, et al. 2019 Oct 3. CircCDR1as up-regulates autophagy under hypoxia to promote tumor cell survival via AKT/ERK(1/2)/mTOR signaling pathways in oral squamous cell carcinomas. Cell Death Dis. 10(10):745.
- Golomb L, Volarevic S, Oren M. 2014 Aug 19. P53 and ribosome biogenesis stress: the essentials. FEBS Lett. 588(16):2571–2579.
- Goudarzi KM, Nister M, Lindstrom MS. 2014. mTOR inhibitors blunt the p53 response to nucleolar stress by regulating RPL11 and MDM2 levels. Cancer Biol Ther. 15(11):1499–1514.
- Grandi P, Rybin V, Bassler J, et al. 2002 Jul. 90S pre-ribosomes include the 35S pre-rRNA, the U3 snoRNP, and 40S subunit processing factors but predominantly lack 60S synthesis factors. Mol Cell. 10(1):105–115.
- Han Z, Liu D, Chen L, et al. 2021 May 28. PNO1 regulates autophagy and apoptosis of hepatocellular carcinoma via the MAPK signaling pathway. Cell Death Dis. 12(6):552.
- Jin L, Vu T, Yuan G, et al. 2017 Oct 15. STRAP promotes Stemness of human colorectal cancer via epigenetic regulation of the NOTCH pathway. Cancer Res. 77(20):5464–5478.
- Kasai H, Nadano D, Hidaka E, et al. 2003 May. Differential expression of ribosomal proteins in human normal and neoplastic colorectum. J Histochem Cytochem. 51(5):567–574.
- Kondoh N, Shuda M, Tanaka K, et al. 2001 Jul-Aug. Enhanced expression of S8, L12, L23a, L27 and L30 ribosomal protein mRNAs in human hepatocellular carcinoma. Anticancer Res. 21(4A):2429–2433.
- Kress TR, Sabo A, Amati B. 2015 Oct. MYC: connecting selective transcriptional control to global RNA production. Nat Rev Cancer. 15(10):593–607.
- Lai MD, Xu J. 2007 Mar. Ribosomal proteins and colorectal cancer. Curr Genomics. 8(1):43–49.
- Li Z, Lu J, Zeng G, et al. 2019 Oct 17. MiR-129-5p inhibits liver cancer growth by targeting calcium calmodulin-dependent protein kinase IV (CAMK4). Cell Death Dis. 10(11):789.
- Liao D. 2009 Dec. Emerging roles of the EBF family of transcription factors in tumor suppression. Mol Cancer Res. 7(12):1893–1901.
- Lien EC, Lyssiotis CA, Juvekar A, et al. 2016 May. Glutathione biosynthesis is a metabolic vulnerability in PI(3)K/Akt-driven breast cancer. Nat Cell Biol. 18(5):572–578.
- Lin C, Yuan H, Wang W, et al. 2020 Jan. Importance of PNO1 for growth and survival of urinary bladder carcinoma: role in core-regulatory circuitry. J Cell Mol Med. 24(2):1504–1515.
- Liu D, Lin L, Wang Y, et al. 2020 Jun 1. PNO1, which is negatively regulated by miR-340-5p, promotes lung adenocarcinoma progression through notch signaling pathway. Oncogenesis. 9(5):58.
- Liu J, Wei X, Wu Y, et al. 2016 Aug. Giganteaside D induces ROS-mediated apoptosis in human hepatocellular carcinoma cells through the MAPK pathway. Cell Oncol (Dordr). 39(4):333–342.
- Liu K, Chen HL, Wang S, et al. 2016 Jun 27. High expression of RIOK2 and NOB1 predict human Non-small cell lung cancer outcomes. Sci Rep. 6:28666.
- Manning BD, Toker A. 2017 Apr 20. AKT/PKB signaling: navigating the network. Cell. 169(3):381–405.
- Martin R, Straub AU, Doebele C, et al. 2013 Jan. DExd/H-box RNA helicases in ribosome biogenesis. RNA Biol. 10(1):4–18.
- Miura M, Hirose M, Miwa T, et al. 2004 Jan 7. Cloning and characterization in Pichia pastoris of PNO1 gene required for phosphomannosylation of N-linked oligosaccharides. Gene. 324:129–137.
- Morgado-Palacin L, Llanos S, Serrano M. 2012 Feb 1. Ribosomal stress induces L11- and p53-dependent apoptosis in mouse pluripotent stem cells. Cell Cycle. 11(3):503–510.
- Parker MD, Collins JC, Korona B, et al. 2019 Dec. A kinase-dependent checkpoint prevents escape of immature ribosomes into the translating pool. PLoS Biol. 17(12):e3000329.
- Pogue-Geile K, Geiser JR, Shu M, et al. 1991 Aug. Ribosomal protein genes are overexpressed in colorectal cancer: isolation of a cDNA clone encoding the human S3 ribosomal protein. Mol Cell Biol. 11(8):3842–3849.
- Poillet-Perez L, White E. 2019 Jun 1. Role of tumor and host autophagy in cancer metabolism. Genes Dev. 33(11–12):610–619.
- Ruggero D. 2012 Sep 11. Revisiting the nucleolus: from marker to dynamic integrator of cancer signaling. Sci Signal. 5(241):e38.
- Ruggero D, Pandolfi PP. 2003 Mar. Does the ribosome translate as cancer? Nat Rev Cancer. 3(3):179–192.
- Senapin S, Clark-Walker GD, Chen XJ, et al. 2003 May 15. RRP20, a component of the 90S preribosome, is required for pre-18S rRNA processing in Saccharomyces cerevisiae. Nucleic Acids Res. 31(10):2524–2533.
- Shen A, Chen Y, Liu L, et al. 2019 May 1. EBF1-Mediated upregulation of ribosome assembly factor PNO1 contributes to cancer progression by negatively regulating the p53 signaling pathway. Cancer Res. 79(9):2257–2270.
- Shen Z, Chen Y, Li L, et al. 2020. Transcription factor EBF1 over-expression suppresses tumor growth in vivo and in vitro via modulation of the PNO1/p53 pathway in colorectal cancer. Front Oncol. 10:1035.
- Singh SS, Vats S, Chia AY, et al. 2018 Mar. Dual role of autophagy in hallmarks of cancer. Oncogene. 37(9):1142–1158.
- Sloan KE, Knox AA, Wells GR, et al. 2019 Feb. Interactions and activities of factors involved in the late stages of human 18S rRNA maturation. RNA Biol. 16(2):196–210.
- Song MJ, Jung CK, Park CH, et al. 2011 Nov. RPL36 as a prognostic marker in hepatocellular carcinoma. Pathol Int. 61(11):638–644.
- Stasinopoulos I, Shah T, Penet MF, et al. 2013. COX-2 in cancer: Gordian knot or Achilles heel? Front Pharmacol. 4:34.
- Stumpf CR, Ruggero D. 2011 Aug. The cancerous translation apparatus. Curr Opin Genet Dev. 21(4):474–483.
- Sturm M, Cheng J, Bassler J, et al. 2017 Dec 20. Interdependent action of KH domain proteins Krr1 and Dim2 drive the 40S platform assembly. Nat Commun. 8(1):2213.
- Suri A, Sheng X, Schuler KM, et al. 2016 Jun 28. The effect of Celecoxib on tumor growth in ovarian cancer cells and a genetically engineered mouse model of serous ovarian cancer. Oncotarget. 7(26):39582–39594.
- Tone Y, Toh EA. 2002 Dec 15. Nob1p is required for biogenesis of the 26S proteasome and degraded upon its maturation in Saccharomyces cerevisiae. Genes Dev. 16(24):3142–3157.
- Toriyama S, Horinaka M, Yasuda S, et al. 2016 Sep. A Histone Deacetylase inhibitor, OBP-801, and Celecoxib Synergistically inhibit the cell growth with apoptosis via a DR5-Dependent pathway in bladder cancer cells. Mol Cancer Ther. 15(9):2066–2075.
- Turowski TW, Lebaron S, Zhang E, et al. 2014 Oct 29. Rio1 mediates ATP-dependent final maturation of 40S ribosomal subunits. Nucleic Acids Res. 42(19):12189–12199.
- Valverde R, Edwards L, Regan L. 2008 Jun. Structure and function of KH domains. FEBS J. 275(11):2712–2726.
- Vanrobays E, Gelugne JP, Caizergues-Ferrer M, et al. 2004 Apr. Dim2p, a KH-domain protein required for small ribosomal subunit synthesis. RNA. 10(4):645–656.
- Vanrobays E, Leplus A, Osheim YN, et al. 2008 Oct. TOR regulates the subcellular distribution of DIM2, a KH domain protein required for cotranscriptional ribosome assembly and pre-40S ribosome export. RNA. 14(10):2061–2073.
- Vosooghi M, Amini M. 2014 Mar. The discovery and development of cyclooxygenase-2 inhibitors as potential anticancer therapies. Expert Opin Drug Discov. 9(3):255–267.
- Wang D, Xu J, Liu B, et al. 2018 Feb. IL6 blockade potentiates the anti-tumor effects of gamma-secretase inhibitors in Notch3-expressing breast cancer. Cell Death Differ. 25(2):330–339.
- Wang G, Li Q, Li C, et al. 2021 May. Knockdown of PNO1 inhibits esophageal cancer progression. Oncol Rep. 45(5):85.
- Wang M, Hu Y, Stearns ME. 2009 Jan 12. RPS2: a novel therapeutic target in prostate cancer. J Exp Clin Cancer Res. 28:6.
- White E. 2015 Jan. The role for autophagy in cancer. J Clin Invest. 125(1):42–46.
- Woolford JL, Jr., Baserga SJ. 2013 Nov. Ribosome biogenesis in the yeast Saccharomyces cerevisiae. Genetics. 195(3):643–681.
- Woolls HA, Lamanna AC, Karbstein K. 2011 Jan 28. Roles of Dim2 in ribosome assembly. J Biol Chem. 286(4):2578–2586.
- Wu WK, Coffelt SB, Cho CH, et al. 2012 Feb 23. The autophagic paradox in cancer therapy. Oncogene. 31(8):939–953.
- Xiong Q, Liu A, Ren Q, et al. 2020 May 14. Cuprous oxide nanoparticles trigger reactive oxygen species-induced apoptosis through activation of erk-dependent autophagy in bladder cancer. Cell Death Dis. 11(5):366.
- Yuan X, Wu H, Han N, et al. 2014 Dec 5. Notch signaling and EMT in non-small cell lung cancer: biological significance and therapeutic application. J Hematol Oncol. 7:87.
- Yue J, Jin S, Gu S, et al. 2019 Dec. High concentration magnesium inhibits extracellular matrix calcification and protects articular cartilage via Erk/autophagy pathway. J Cell Physiol. 234(12):23190–23201.
- Zemp I, Kutay U. 2007 Jun 19. Nuclear export and cytoplasmic maturation of ribosomal subunits. FEBS Lett. 581(15):2783–2793.
- Zhang Y, Lu H. 2009 Nov 6. Signaling to p53: ribosomal proteins find their way. Cancer Cell. 16(5):369–377.
- Zheng S, Lan P, Liu X, et al. 2014 Aug 15. Interaction between ribosome assembly factors Krr1 and Faf1 is essential for formation of small ribosomal subunit in yeast. J Biol Chem. 289(33):22692–22703.
- Zhou GJ, Zhang Y, Wang J, et al. 2004 Jun. Cloning and characterization of a novel human RNA binding protein gene PNO1. DNA Seq. 15(3):219–224.
- Zhou J, Yi Q, Tang L. 2019 Jun 11. The roles of nuclear focal adhesion kinase (FAK) on cancer: a focused review. J Exp Clin Cancer Res. 38(1):250.
