 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In densely populated, highly urbanized regions such as Zhejiang Province (eastern China), species are under enormous environmental pressures resulting from human activities, land use changes, and habitat fragmentation, which affect species composition and species diversity. Although eastern China is one of the most concentrated distribution areas of endemic bryophytes in East Asia, these species are also faced with the threats of habitat inadequacy. In order to get more information on bryophyte habitat and diversity, we investigated species composition, habitat preference, as well as species diversity differences of bryophytes under different habitat conditions in Jiulongshan National Forest Park (JNFP). In particular, the results showed that 108 specimens of bryophyte were collected in total, including 46 species in 32 genera of 21 families. Furthermore, we had found 12 species of bryophytes that had never been recorded before in Zhejiang Province. Remarkably, 40.74% of bryophytes were found to grow on natural rocks, followed by understories and roadside habitats. In addition, the Patrick richness index (R) varied most significantly among different habitats (p < 0.001). In a conservation perspective, natural rocks, roadways and understories should be preserved to protect bryophyte diversity.
Introduction
Bryophytes are the second largest group of land plants, with a unique place in plant evolutionary history and ecosystems (Shaw et al. Citation2011; Patiño and Vanderpoorten Citation2018; Wang et al. Citation2019). Numerous bryophyte species show strong adaptability (Harmens et al. Citation2007), growing in environmental conditions that are unbearable for most of the other land plants. Mosses also present drought tolerance and the ability to preserve soil and reduce water loss during ecological restoration, indicating that they can be used as pioneer plants for ecological restoration (Wen et al. Citation2015; Jing et al. Citation2018; Yuan et al. Citation2021; Ren et al. Citation2021b). In addition, bryophytes are a significant part of ecosystem biodiversity and make up an important component of species richness and plant biomass in forests (Sun et al. Citation2013). Although bryophytes are small, they have a huge surface area, strong adsorption capacity, and cation exchange capacity, and they are often large mat-like clusters (Yang et al. Citation2020). Furthermore, they also play a prominent role in nutrient biogeochemical cycling, seed germination, seedling growth, and plant colonization (Zamfir Citation2000; Turetsky Citation2003; Lin et al. Citation2006). Bryophytes can also be used as indicators of overall plant diversity and restoration status in some cases (Ren et al. Citation2021a). Because of their special physiological characteristics, bryophytes are extremely sensitive to environmental changes and are often used as indicator plants to monitor environmental pollution and climate changes, and to reflect the micro-habitat and microclimate of the ecosystem (Frego Citation2007; Palo et al. Citation2013; He et al. Citation2016).
Bryophyte species richness even far exceeds that of vascular plants in humid artificial forests in temperate regions (Gignac Citation1992). Hence, the regional investigation is very important for further study on bryophyte taxonomy and diversity (He and Jia Citation2017). However, bryophytes are still rarely taken into account in biodiversity surveys relative to their vascular counterparts (Sun et al. Citation2013). Bryophytes are widely distributed and play a vital role in forest ecosystems, such as in the forest ecosystems of eastern China. Eastern China is one of the most concentrated distribution areas of endemic bryophytes in East Asia (Wu and Jia Citation2006; Ye Citation2007). However, although bryophytes in eastern China are very abundant, they are also faced with the threats of a warming climate, rapid habitat loss, habitat disturbance, and particularly land-use change (He et al. Citation2016; Callaghan Citation2020; Riffo-Donoso et al. Citation2021). Furthermore, tourism development also has a great negative impact on the habitat environment of bryophytes (Hernández et al. Citation2019). More specifically, the development of tourism resources (such as felling trees and building roads) directly interferes with the balance of the ecosystem and the survival of bryophytes in scenic areas. Therefore, as a response to changes in habitat conditions, sensitive bryophytes inevitably suffer the threat (Zhao et al. Citation2020).
Consequently, understanding habitat preferences of bryophytes in high-intensity human activity areas in eastern China is useful to get more information on bryophyte niche which can be afterwards used at a small scale to help subtropical forest restoration. To reach this goal we aimed here to (1) explore the composition and compile a checklist of bryophytes, (2) find out the habitat preferences and distribution characteristics of bryophytes, and (3) reveal differences of bryophyte diversity index in various habitats in Jiulongshan National Forest Park (JNFP). By analyzing the habitat preference of bryophytes in JNFP and the diversity of bryophytes in different habitats, this study is expected to play an important role in guiding the research and formulation of ecological restoration strategies, and at the same time, lay a foundation for the formulation of bryophyte species conservation strategies and the conservation of forest ecosystems and other species in the region.
Methods
Study area
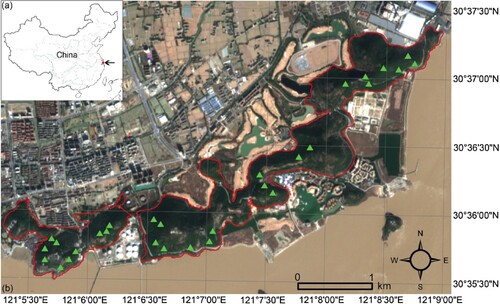
JNFP is the remnant of Tianmu Mountain in western Zhejiang, located in the coastal area of eastern China (Figure (a)), in the south of Pinghu city, Zhejiang Province. The region has a subtropical monsoon climate, with obvious differences between the four seasons. The annual average temperature in this area is 15.7 °C, and the annual average precipitation is 1197.6 mm. The forest park has rich ecological resources and a special geographical location and is also considered as an important ecological barrier connecting land and sea. The forest park was approved as a national forest park by the former Ministry of Forestry in 1997, with a total area of 424.1 hm2. The highest elevation of JNFP is 161 m. The area is rich in plant resources, with 462 terrestrial vascular plant species belonging to 316 genera of 107 families. The vegetation type of the forest park is mainly subtropical evergreen broad-leaved forest, which provides complex and diverse habitats for bryophytes.
Figure 1. (a) Location of JNFP in China; (b) The boundary of the JNFP (solid red line) and the location of the sampling site (green triangle). The original layer comes from Google Earth (https://www.google.com/earth/index.html).
Data compilation
In April 2021 and September 2021, a preliminary extensive survey collecting data on bryophyte composition and bryophyte diversity was carried out in 29 sites (Figure (b)). Based on the niche of bryophyte species, we investigated bryophytes in JNFP according to the gradients of altitude, forest type, and soil type. In this study, we divided habitats characteristics into five types, including the concrete stone walls, natural rocks, roadsides, tree bases and understories (Zechmeister et al. Citation2003; Jing et al. Citation2018). We used the method of stratified random sampling to conduct spot sampling in the forest park. Fifty-seven plots (50 × 25 cm) were recorded to describe community composition and habitat conditions of areas occupied by bryophytes, generally following the method of Callaghan (Citation2018). Sample locations were selected to represent the full range of habitat conditions occupied by the bryophyte. The presence and abundance of bryophytes were recorded according to the data collection method of Brunialti et al. (Citation2010), and the distribution information and habitat types of bryophytes were also recorded.
Data analyses
All statistical analyses in this study were performed by using the software R4.1.0 (R Core Team Citation2022). In addition, in order to determine if there were significant differences between the diversity indices, we performed a one-way ANOVA analysis at p = 0.001. Then, we performed a post hoc test (Tukey’s HSD test) to indicate which indices were different from the others. Furthermore, we also used ‘ggplot2’, ‘ggpubr’ and ‘ggsignif’ software packages to draw visual graphics. The habitat dependence of bryophytes was analyzed by cluster analysis which was carried out by complete method with the help of the ‘pheatmap’ package (Kolde Citation2019). The Patrick richness index (R), Shannon-Wiener diversity index (H), Simpson dominance index (D), and Pielou evenness index (J) of bryophytes were calculated by the ‘vegan’ package (Oksanen et al. Citation2022) of R software. The equations used were as follows (Whittaker Citation1972):
Results
Species checklist
In the field survey, we collected 108 bryophyte specimens in the JNFP containing 46 species of bryophytes (from 21 families) in total, mainly represented by moss species. Specifically, these bryophytes included 39 species of mosses (27 genera of 16 families) and 7 species of liverworts (5 genera of 5 families) (Table ). Among the bryophytes, Hypnaceae (4 genera and 6 species), Pottiaceae (4 genera and 5 species), Brachytheciaceae (3 genera and 5 species), and Fissidentaceae (1 genus and 4 species) were the most popular families (Figure (a)). In addition, 11 families contained only one species, accounting for the largest proportion (52.38%) (Figure (b)). Furthermore, the collected data showed that, Hypnaceae was the only family containing more than five species (Figure (b)).
Figure 2. Composition of Bryophytes in JNFP. (a) The number of bryophyte species contained in dominant families. (b) The number of families (contains different species numbers) and their ratio to the total number of families.
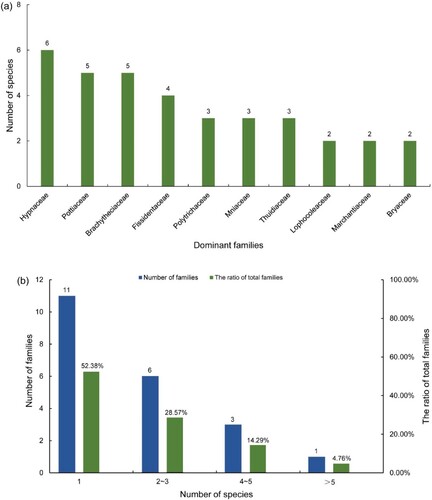
Table 1. Species checklist of bryophytes in the JNFP.
New geographical distribution
According to this survey, twelve species of bryophytes were newly recorded in Zhejiang Province. Among them, two species were liverworts, namely Cephalozia connivens (Figure (e)) and Hetreoscyphus tridentatus (Figure (h)). In addition, the other ten new records were mosses, including Atrichum angustatum (Figure (a)), Atrichum rhystophyllum (Figure (b)), Bryhnia brachycladula (Figure (c)), Bryum radiculosum (Figure (d)), Fissidens anomalus (Figure (f)), Grimmia longirostris (Figure (g)), Hygroamblystegium tenax (Figure (i)), Hypnum calcicola (Figure (j)), Hypnum callichroum (Figure (k)), and Plagiomnium confertidens (Figure (l)).
Figure 3. Bryophyte species newly recorded in Zhejiang Province. (a) Atrichum angustatum; (b) Atrichum rhystophyllum; (c) Bryhnia brachycladula; (d) Bryum radiculosum; (e) Cephalozia connivens; (f) Fissidens anomalus; (g) Grimmia longirostris; (h) Hetreoscyphus tridentatus; (i) Hygroamblystegium tenax; (j) Hypnum calcicola; (k) Hypnum callichroum; (l) Plagiomnium confertidens. The source of the species photograph was taken in the field in JNFP.

Habitat characteristics
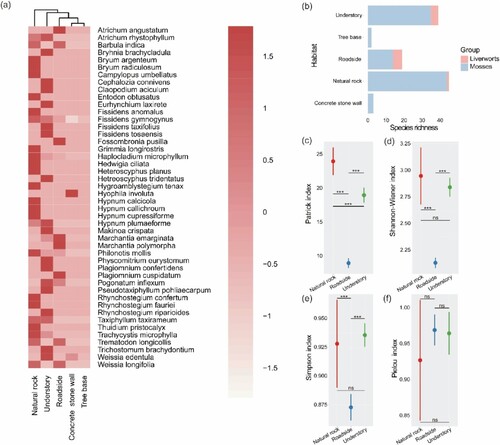
Out of 46 species of bryophytes, 3 species were found in at least three habitat-types, 10 in two habitat-types, and the remaining 33 in only one habitat. Among all the collected bryophytes, the habitat of natural rocks had the most bryophytes, followed by the habitats of understories and roadsides. Moreover, few bryophytes were distributed in the habitats of concrete stone walls and tree bases (Figure (a and b)).
Figure 4. (a) Clustering distribution map of different bryophytes in different habitats. (b) Bryophyte richness in different habitats. (c) Comparison of bryophyte species richness index in different habitats. (d) Comparison of bryophyte Shannon-Wiener diversity index in different habitats. (e) Comparison of bryophyte Simpson dominance index in different habitats. (f) Comparison of bryophyte Pielou evenness index in different habitats.
Note: due to the other two habitat types (concrete stone wall, tree base) having very few species, we only analyzed and compared the diversity of habitats (natural rock, roadside and understory) with relatively high species. In addition, ns represents not significant; *** represents a 0.001 level of significance.
The bryophyte diversity among different habitats
The results of the one-way analysis of variance showed that there was a significant difference (p < 0.001) between the Patrick richness index (R) of bryophyte species in the primary habitats (Figure (c)). Furthermore, there was also some significant differences (p < 0.001) between the Shannon-Wiener diversity index (H) and Simpson dominance index (D) of bryophyte species in the primary habitats (Figure (d and e)). However, concerning the Pielou evenness index (J), there was no significant difference between each habitat (Figure (f)). Specifically, in terms of the Patrick richness index (R), the habitat of natural rocks was significantly higher (p < 0.001) than roadsides and understories (Figure (c)). In addition, in terms of the Shannon-Wiener diversity index (H), the habitats of natural rocks and understories were significantly higher (p < 0.001) than roadsides (Figure (d)). Analogously, in terms of Simpson dominance index (D), the habitats of natural rocks and understories were significantly higher (p < 0.001) than roadsides (Figure (e)).
Discussion
Bryophyte distribution and habitat preference
Bryophyte distribution is influenced by many factors, both locally and regionally, such as climatic factors (e.g. precipitation, temperature, humidity), geographical structure (e.g. exposure, size, and microtopography), substrate chemistry (e.g. pH and nutrient status) and composition of the forest (Weibull and Rydin Citation2005; Sun et al. Citation2013; Coelho et al. Citation2021). The difference in habitat conditions was one of the reasons for the heterogeneity of bryophyte composition and distribution (Koncz et al. Citation2018), because the change of habitat conditions would cause the change of microenvironment (such as temperature, light and humidity), thus affecting the composition and diversity of bryophytes (Yang et al. Citation2020). In this study, in which the overall bryophyte richness was used as a significant indicator of their habitat preferences, we found that bryophytes were mainly distributed in habitats of natural rocks, understories, and roadsides. Among them, the largest number of bryophytes lived on natural rocks, which was consistent with the results of Cong et al. (Citation2020). Habitat preferences vary among moss species. The spatial patterns of bryophyte species diversity and community structure in response to different habitats may be determined by the bryophytes’ biological properties (Jiang et al. Citation2018). The gloomy, dank habitats gave some hygrophilic species favorable conditions. For example, most of the species of Thuidiaceae and Fissidentaceae were often found on exposed dripping natural rock surfaces, which was also consistent with the research results of He et al. (Citation2012).
Conservation implications
As an important part of the terrestrial ecosystem, bryophytes play an important ecological function in soil and water conservation, the global carbon cycle, and forest community succession and regeneration (Turetsky Citation2003; Soudzilovskaia et al. Citation2011; Tuba et al. Citation2011; Xing et al. Citation2022). It is well known that habitat quality plays a vital role in biodiversity conservation. Hence, the habitats preferred by bryophytes should be highly valued and protected. In the natural habitat, although the nutrient content of the substrate is low, the growth of bryophytes is guaranteed by the appropriate slope, light, canopy density, less human disturbance and the strong adaptability of bryophytes (Wu et al. Citation2019). This study shows that bryophytes have a significant preference for natural rock habitats, which may be due to the better moist conditions and aeration structure of natural rocks, as well as less human disturbance, which is beneficial to the growth of bryophytes (Wu et al. Citation2019). In addition, the forest margins or roadsides and understories also play an important role in maintaining the diversity and stability of bryophytes. Regarding our results, we should pay attention to preserve natural rock, understory and roadside habitats in order to protect bryophyte diversity. In addition, it is also suggested that the Jiulongshan National Forest Park Management Administration should minimize pollution and damage to the bryophyte habitat environment while developing tourism. It is also of great significance for the study and conservation of bryophyte diversity in this area. It is expected that the results of this study can not only provide basic reference materials for the future biodiversity conservation of JNFP, but also provide some theoretical guidance for the rational utilization and protection of vegetation in this area.
Conclusions
In this study, we have complemented the distribution data of bryophytes in JNFP. The results show that 108 bryophyte specimens have been collected in total, including 46 species in 32 genera of 21 families, of which, mosses contains 39 species in 27 genera of 16 families, liverworts includes 7 species in 5 genera of 5 families. In addition, we have also found 12 species of bryophytes that had never been recorded before in Zhejiang Province. In addition, natural rock habitats usually have a high biodiversity index. Remarkably, 40.74% of bryophytes were found to grow on natural rocks, followed by understories and roadside habitats. In a conservation perspective, natural rocks, roadways and understories should be preserved to protect bryophyte diversity.
Author contributions
Conceptualization, P.Y., J.W.; methodology, P.Y., X.Q., J.W.; formal analysis, P.Y.; investigation, P.Y., J.W., X.Z.; data curation, P.Y.; writing – original draft preparation, P.Y.; writing – review and editing, J.W.; supervision, J.W., X.Q.; project administration, J.W., X.Q., X.Z.; funding acquisition, J.W. All authors have read and agreed to the published version of the manuscript.
Permission for sample collection
Permissions were obtained from the Jiulongshan National Forest Park Management Administration of Pinghu City, Zhejiang Province for the sample collection carried out in this study.
Acknowledgements
We would like to thank Dr. Qiang He from the Institute of Botany, Chinese Academy of Sciences, who gave us great help in field sampling and specimen identification. We are grateful to Ms. Rui Meng for her assistance in revising the English expression of the paper. The research team would also like to thank the invited reviewers for their targeted and constructive suggestions.
Data availability statement
The species classification data that support the findings of this study are available in Flora of China at http://www.iplant.cn/. All the specimens are preserved in the Herbarium, Institute of Botany, Chinese Academy of Sciences. The raw data that support the findings of this study are openly available in figshare at https://doi.org/10.6084/m9.figshare.23282828.v2.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Brunialti G, Frati L, Aleffi M, Marignani M, Rosati L, Burrascano S, Ravera S. 2010. Lichens and bryophytes as indicators of old-growth features in Mediterranean forests. Plant Biosyst. 144(1):221–233. doi:10.1080/11263500903560959.
- Callaghan DA. 2018. Status, conservation and ecology of the exceptionally rare metallophyte cornish path-moss (Ditrichum cornubicum Paton). J Bryol. 40(4):358–370. doi:10.1080/03736687.2018.1480695.
- Callaghan DA. 2020. Survival of the critically endangered Ditrichum cornubicum and dependence on conservation management intervention. J Bryol. 43(2):175–180. doi:10.1080/03736687.2020.1834958.
- Coelho MCM, Gabriel R, Hespanhol H, Borges PAV, Ah-Peng C. 2021. Bryophyte diversity along an elevational gradient on Pico Island (Azores, Portugal). Diversity (Basel). 13(4):162. doi:10.3390/d13040162.
- Cong MY, Xu YY, Tang LY. 2020. Analysis on diversity of bryophytes in volcanic lava platform of Jingpo Lake World Geopark. J Plant Resour Environ. 29(6):57–65.
- Frego KA. 2007. Bryophytes as potential indicators of forest integrity. For Ecol Manag. 242(1):65–75. doi:10.1016/j.foreco.2007.01.030.
- Gignac LD. 1992. Niche structure, resource partitioning, and species interactions of mire bryophytes relative to climatic and ecological gradients in western Canada. Bryologist. 105(3):334–338.
- Harmens H., Norris D. A., Koerber G. R., Buse A., Steinnes E., Ruhlíng A. 2007. Temporal trends in the concentration of arsenic, chromium, copper, iron, nickel, vanadium and zinc in mosses across Europe. Atmos. Environ. 41(31):6673–6687. doi:10.1016/j.atmosenv.2007.03.062.
- He Q, Jia Y. 2017. Assessing the threat status of China’s bryophytes. Biodivers Sci. 25(7):774–780. doi:10.17520/biods.2016205.
- He X, He KS, Hyvönen J. 2016. Will bryophytes survive in a warming world? Perspect Plant Ecol Evol Syst. 19:49–60. doi:10.1016/j.ppees.2016.02.005.
- He ZX, Yan YH, Ma QX, Lu QY. 2012. The bryophyte diversity of the Danxia landform in Hunan, China. Biodivers Sci. 20(4):522–526.
- Hernández RH, Kluge J, Ah-Peng C, Gonzalez-Mancebo JM. 2019. Natural and human-impacted diversity of bryophytes along an elevational gradient on an oceanic island (La Palma, Canarias). PloS One. 14(4):e0213823. doi:10.1371/journal.pone.0213823.
- Jiang TT, Yang XC, Zhong YL, Tang QM, Liu Y, Su ZY. 2018. Species composition and diversity of ground bryophytes across a forest edge-to-interior gradient. Sci Rep. 8:11868. doi:10.1038/s41598-018-30400-1.
- Jing L, Lu JG, Xia W. 2018. Diversity of bryophytes in urban area of Nanjing, China. Chin J Appl Ecol. 29(6):1797–1804.
- Kolde R. 2019. pheatmap: pretty heatmaps. Version 1.0.12. https://cran.r-project.org/web/packages/pheatmap/.
- Koncz P, Hermanutz L, Marino P, Wheeler J, Cranston B. 2018. Bryophyte community diversities and expected change under a warming climate in contrasting habitats of the Torngat Mountains, Labrador. Bryologist. 121(2):174–182. doi:10.1639/0007-2745-121.2.174.
- Lin F, Hao ZQ, Ye J, Jiang P. 2006. Effects of bryophytes in dark coniferous forest of Changbai Mountains on three conifers seed germination and seedling growth. Chin J Appl Ecol. 17(8):1398–1402.
- Oksanen J, Simpson GL, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, Solymos P, Stevens MHH, Szoecs E, et al. 2022. vegan: community ecology package. Version 2.6-4. https://cran.r-project.org/web/packages/vegan/index.html.
- Palo A, Ivask M, Liira J. 2013. Biodiversity composition reflects the history of ancient semi-natural woodland and forest habitats—compilation of an indicator complex for restoration practice. Ecol Indic. 34(6):336–344. doi:10.1016/j.ecolind.2013.05.020.
- Patiño J, Vanderpoorten A. 2018. Bryophyte biogeography. Crit Rev Plant Sci. 37(2):175–209. doi:10.1080/07352689.2018.1482444.
- R Core Team. 2022. R: a language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org.
- Ren H, Wang F, Ye W, Zhang Q, Han T, Huang Y, Chu G, Hui D, Guo Q. 2021a. Bryophyte diversity is related to vascular plant diversity and microhabitat under disturbance in karst caves. Ecol Indic. 120:106947. doi:10.1016/j.ecolind.2020.106947.
- Ren J, Liu F, Luo Y, Zhu J, Luo X, Liu R. 2021b. The pioneering role of bryophytes in ecological restoration of manganese waste residue areas, southwestern China. J Chem. 2021:9969253.
- Riffo-Donoso V, Osorio F, Fonturbel FE. 2021. Habitat disturbance alters species richness, composition, and turnover of the bryophyte community in a temperate rainforest. For Ecol Manag. 496:119467. doi:10.1016/j.foreco.2021.119467.
- Shaw AJ, Szovenyi P, Shaw B. 2011. Bryophyte diversity and evolution: windows into the early evolution of land plants. Am J Bot. 98(3):352–369. doi:10.3732/ajb.1000316.
- Soudzilovskaia N. A., Graae B. J., Douma J. C., Grau O., Milbau A., Shevtsova A., Wolters L., Cornelissen Jhc. 2011. How do bryophytes govern generative recruitment of vascular plants?. New Phytol. 190(4):1019–1031. doi:10.1111/j.1469-8137.2011.03644.x.
- Sun SQ, Wu YH, Wang GX, Zhou J, Yu D, Bing HJ, Luo J. 2013. Bryophyte species richness and composition along an altitudinal gradient in Gongga Mountain, China. PLoS One. 8(3):e58131. doi:10.1371/journal.pone.0058131.
- Tuba Z., Slack N. G., Stark L. R. 2011. Bryophyte Ecology and Climate Change. Cambridge: Cambridge University Press.
- Turetsky MR. 2003. The role of bryophytes in carbon and nitrogen cycling. Bryologist. 106(3):395–409. doi:10.1639/05.
- Wang ZM, Ye W, Xing FW. 2019. Bryophyte diversity on a tropical continental island (Hainan, China): potential vulnerable species and environmental indicators. J Bryol. 41(4):350–360. doi:10.1080/03736687.2019.1653557.
- Weibull H, Rydin H. 2005. Bryophyte species richness on boulders: relationship to area, habitat diversity and canopy tree species. Biol Conserv. 122(1):71–79. doi:10.1016/j.biocon.2004.07.001.
- Wen L, Song TQ, Du H, Wang KL, Peng WX, Zeng FP, Zeng ZX, He TG. 2015. The succession characteristics and its driving mechanism of plant community in karst region, Southwest China. Acta Ecologica Sinica. 35(17):5822–5833.
- Whittaker RH. 1972. Evolution and measurement of species diversity. Taxon. 21:213–251. doi:10.2307/1218190.
- Wu PC, Jia Y. 2006. The regionalization and distribution types of the bryophytes in China. J Plant Resour Environ. 15(1):1–8.
- Wu Q, Lu ZH, Li CN, Jiang W, Zhou YH, Gao SP, Zhou YH, Yu XF. 2019. Research on the bryophytes’ diversity and microhabitat in Wenjiang Park of Sichuan Province. J Yunnan Agric Univ (Nat Sci). 34(3):458–465.
- Xing SC, Tang LY, Dai Z, Tu SW, Chen X, Zhang JH, Li HQ, Peng T, Wang J. 2022. Bryophyte diversity in Shitai County and Qingyang County, Anhui Province. Biodivers Sci. 30(1):116–123.
- Yang XC, Huang RX, Zhou Q, Xu MF, Tang QM, Su ZY. 2020. Association of bryophytes with site factors and indicator species in agroforestry ecotone of east Guangdong. J Agric Sci Technol. 22(4):177–186.
- Ye W. 2007. Study on the mosses flora and biodiversity of Fujian, China [master’s thesis]. Xiamen: Xiamen University.
- Yuan CJ, Xiong KN, Rong L, Weng YF. 2021. Research progress on the biodiversity during the ecological restoration of karst rocky desertification. Earth Environ. 49(3):336–345.
- Zamfir M. 2000. Effects of bryophytes and lichens on seedling emergence of alvar plants: evidence from greenhouse experiments. Oikos. 88(3):603–611. doi:10.1034/j.1600-0706.2000.880317.x.
- Zechmeister HG, Tribsch A, Moser D, Peterseil J, Wrbka I. 2003. Biodiversity ‘hot spots’ for bryophytes in landscapes dominated by agriculture in Austria. Agric Ecosyst Environ. 94(2):159–167. doi:10.1016/S0167-8809(02)00028-2.
- Zhao DX, Wang C, Sun ZK, Hao ZZ. 2020. Epiphytic bryophyte diversity and its influencing factors. Acta Ecologica Sinica. 40(8):2523–2532.
