Abstract
Owing to tumor heterogeneity and individual physiological variations, there remains a necessity to enhance the current prognosis prediction system for metastatic gastric cancer (MGC). This study aimed to establish a prognostic model for patients diagnosed with MGC. Pertinent information pertaining to MGC patients was extracted and assessed from the Surveillance, Epidemiology, and End Results (SEER) database between 2010 and 2016. The Kaplan-Meier method and Cox regression analysis were employed to identify potential prognostic factors. Furthermore, a nomogram model containing possible prognostic factors associated with overall survival (OS) was constructed using R software. A total of 2975 patients from the SEER database were included in this investigation. N-stage, grade, surgery, radiotherapy, chemotherapy, brain metastasis, bone metastasis, liver metastasis, and lung metastasis were identified as independent prognostic factors for OS. These variables were subsequently incorporated into the nomogram model. Calibration curves demonstrated a good correlation between nomogram predictions and actual observations. The nomogram model exhibits the potential to estimate the individualized survival of MGC patients.
Introduction
Gastric cancer (GC) remains a severe global disease, accounting for more than 1,089,103 new cases and 768,793 deaths in 2020 (Sung et al. Citation2021). Clinical investigations have revealed that the incidence of GC is comparatively higher in Asia, Latin America, and Eastern Europe, in contrast to North America and Western Europe (Bray et al. Citation2015). This geographical variance may be attributed to population-specific genetic risk factors, dietary influences, and Helicobacter pylori (H. pylori) infection. Despite advancements in therapeutic strategies that have substantially improved the prognosis of early-stage GC, the overall survival (OS) rates for patients with metastatic gastric cancer (MGC) remain unsatisfactory (Yagi et al. Citation2000; Thun et al. Citation2010). Currently, clinicians rely on the tumor, lymph node, metastasis (TNM) staging system by American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) to assess the prognosis of GC patients. However, it is important to consider other factors, such as age, sex, race, and differentiation, as they can significantly impact patient survival outcomes. Consequently, researchers have aimed to incorporate novel prognostic markers with anatomical staging in order to accurately predict individualized survival outcomes. Nomograms, statistical prediction models, have recently demonstrated superior prognostic capabilities compared to traditional staging systems in certain cancer types. Compared to the TNM staging system, nomograms weight each relevant variable and present them in a more intuitive and precise manner. In this study, we sought to predict the prognostic outcomes of MGC patients by analyzing clinicopathological characteristics extracted from the Surveillance, Epidemiology, and End Results (SEER) database, based on this statistical predictive model.
Materials and methods
Data source
The medical records of MGC patients between 2010 and 2016 were obtained from the SEER database. The inclusion criteria contain: (1) patients with pathologically confirmed MGC; (2) patients without a history of histologically diagnosed second primary tumor; (3) patients with available clinicopathological and follow-up data, including age, sex, race, marital status, tumor location, TNM stage, tumor grade, histologic type, surgery, radiotherapy, metastasis site, and survival status. In our study, a signed SEER research data agreement form was provided to the SEER program and we were given approval to access and analyze SEER database, which is a public database that does not require ethical approval.
Statistical analyses
SPSS 13.0 and R software version 3.6.1 (http://www.rproject.org) were used to conduct statistical analyses. Specifically, the Kaplan-Meier method was employed for survival analysis, while the log-rank test was utilized to assess differences among groups. Univariate and multivariate Cox proportional hazard models were used to identify variables associated with OS and cancer-specific survival (CSS). Subsequently, a nomogram model was constructed based on selected variables to predict patient OS using R software. The discriminative abilities of the model were assessed using the concordance index (C-index). Furthermore, calibration curves were plotted using the Hosmer-Lemeshow test for validating accuracy and reliability (Kramer and Zimmerman Citation2007). Two-sided p values < 0.05 were considered statistically significant.
Results
Baseline patient characteristics
2975 MGC patients from the SEER database between 2010 and 2016, who met the screening criteria, were involved in this analysis. These patients were randomly divided into a training cohort (70%) and a validation cohort (30%). Among the patients, 1263 cases (42.5%) were female, and 1893 cases (63.6%) were of Caucasian ethnicity. The most frequent primary tumor site observed in this analysis was the pylorus, accounting for 35.5% of cases. A majority of patients, 79.2%, exhibited middle and low differentiation, while 23.3% were classified as T3 stage. Multiple metastatic sites were present in 46% of patients, with the liver being the most common site of metastasis, observed in 1071 cases (36%). Lung metastases were diagnosed in 293 patients (9.8%), while 244 patients (8.2%) had bone metastases. Brain metastases were detected in only 19 patients (0.6%).
Survival analysis
To screen variables associated with survival, we employed Kaplan-Meier analysis. As shown in Figure. S1, there were statistically significant differences in OS based on age, T-stage, N-stage, tumor grade, active treatment (including surgery, chemotherapy, and radiotherapy), and metastatic site (including liver, lung, bone, and brain metastasis) (all P < 0.01). Additionally, CSS was significantly correlated with patients’ age, marital status, N-stage, histologic type, treatment strategies (including surgery, chemotherapy, and radiotherapy), and metastatic status (lung and bone metastasis) (all P < 0.01) (Figure S2).
Univariate and multivariate cox hazard regression analysis, and nomogram construction
The hazard ratios for CSS and OS, based on variables in the univariate and multivariate Cox proportional hazard models, are presented in Table . In the multivariate Cox proportional hazard model, N-stage, histologic type, surgery, radiotherapy, chemotherapy, bone metastasis, and lung metastasis were identified as independent prognostic factors for CSS. Subsequently, we analyzed potential predictors of OS in MGC patients. N-stage, grade, surgery, radiotherapy, chemotherapy, brain metastasis, bone metastasis, liver metastasis, and lung metastasis were identified as independent prognostic factors for OS. Using these variables, we constructed a nomogram model to predict 1-year and 3-year OS. As shown in Figure , each variable was assigned a score on a point scale. By summing the scores of all selected variables, we calculated the individual patient's probability of 1-year and 3-year OS. Notably, chemotherapy demonstrated the most significant contribution, followed by surgery and tumor grade.
Figure 1. Nomogram for predicting overall survival in patients with metastasis gastric cancer. Notes: All the points assigned on the top point scale for each factor are summed together to generate a total point score. The total point score is projected on the bottom scales to determine the overall survival rate in an individual.
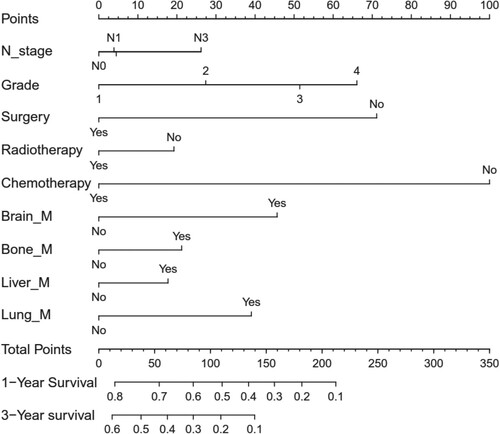
Table 1. Prognostic variables for overall survival and cancer-specific survival in MGC patients.
Nomogram validation
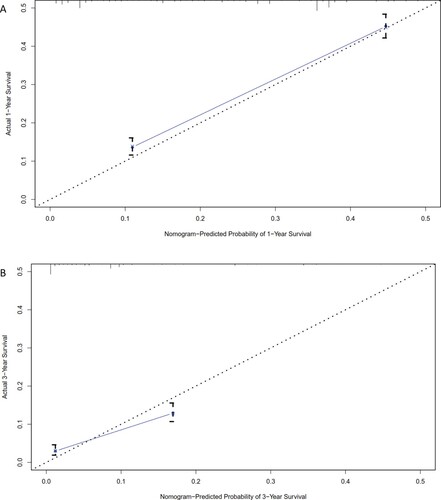
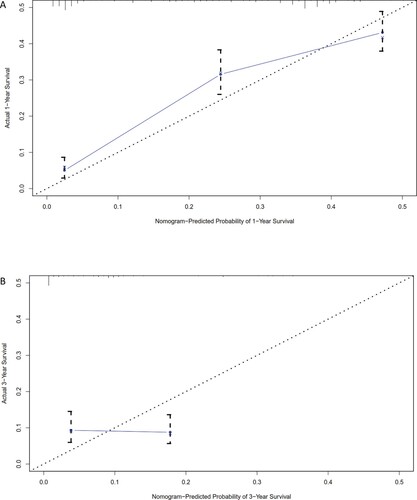
To assess the accuracy of our predictions, we performed a calibration plot. The calibration curves revealed a strong correlation between the predicted values and the actual observations in both the training (Figure ) and the validation (Figure ) cohorts, with deviations consistently within a 10% margin of error. Furthermore, the Harrell's C-index of our system was calculated to be 0.73, indicating an acceptable accuracy for prediction.
Discussion
GC is a prevalent disease with unsatisfactory prognosis across the world. Despite therapeutic advancements in recent years, such as curative surgery combined with chemoradiotherapy, improving prognosis for GC patients (Liu and Chen Citation2011; Bilici Citation2014; Gadde et al. Citation2015; Izuishi and Mori Citation2020), the management of metastatic disease remains an ongoing challenge for clinicians. A previous study by Zhu et al. (Zhu et al. Citation2020) developed a nomogram model to predict the likelihood of early death (survival time ≤3 months) in patients with MGC. Additionally, Ebinger et al. (Citation2016) found that palliative gastrectomy could modestly enhance OS in MGC patients. To the best of our knowledge, our study was the first to focus on identifying prognostic variables associated with 1-year and 3-year OS in MGC patients based on the SEER database. Through assigning appropriate weights to the relevant variables, we aimed to establish a statistical prediction model that could facilitate clinicians in prognostic evaluation and personalized treatment decisions. While competing risk regression is commonly employed in SEER data analysis, we opted for the Kaplan-Meier method and Cox regression model for survival analysis, considering the high mortality rate associated with metastatic disease and the limited number of competing events. We identified nine independent prognostic factors associated with OS and developed a nomogram prognostic model accordingly. Furthermore, the accuracy of our prediction system was validated using two statistical methods in both the training and validation cohorts. Our findings demonstrated that our prognostic model exhibits acceptable prediction accuracy, as evidenced by a good C-index and calibration curve. This quantitative approach offers a more precise and personalized estimation of survival outcomes compared to the TNM staging system for MGC patients.
Previous studies have consistently reported age as an independent prognostic factor in both early and advanced GC (Saito et al. Citation2006; Liang et al. Citation2013; Song et al. Citation2016). Nevertheless, our multivariate analysis did not find age to be statistically significant and therefore it was excluded from our nomogram model. These discrepancies could be attributed to the relatively short survival time of patients with MGC, which may have obscured the impact of age-related factors such as comorbidity and treatment tolerability.
Most studies have suggested that advanced stages of the primary lesion in GC are often associated with a higher likelihood of distant metastasis (Han et al. Citation2012; Bando et al. Citation2020). Interestingly, our study did not find a significant correlation between T-stage and OS and CSS in MGC patients. It may be related with the exclusion of tumor size from our analysis due to the missing data and the reduced statistical power. Further research is warranted to explore the relationship between the primary lesion and tumor progression.
According to our nomogram, adjuvant chemotherapy had the greatest impact on the prognosis of MGC patients. The hazard ratio for patients who did not receive chemotherapy was 3.21 (95% CI: 2.88-3.57). Several clinical trials have also demonstrated that palliative chemotherapy improves prognosis by extending survival for several months compared to supportive care alone (Wagner et al. Citation2006; Takashima et al. Citation2009; Chen et al. Citation2013; Iacovelli et al. Citation2014). Moreover, recent phase III trials and meta-analyses have recommended second-line chemotherapy for patients who did not respond well to first-line treatment (Pyrhönen et al. Citation1995; Glimelius et al. Citation1997; Wagner et al. Citation2006; Takashima et al. Citation2009; Chen et al. Citation2013; Iacovelli et al. Citation2014). However, it is important to note that the chemotherapy effect extracted from the SEER database may be subject to bias, as indicated in the SEER data use agreement (https://seer.cancer.gov/data-software/documentation/seerstat/nov2021/treatment-limitations-nov2021.html). Additionally, the lack of information on adjuvant treatment in the model may overlook a critical aspect of cancer management that influences survival outcomes. Therefore, further analysis is necessary to elucidate the true impact of chemotherapy in MGC patients.
The role of palliative gastrectomy in MGC patients is still a topic of debate, as its purpose may vary between symptom relief and survival benefit. Several studies have examined the association between palliative gastrectomy and prognosis in MGC patients, but the exact role remains to be addressed (Chen et al. Citation2013; Mohri et al. Citation2014; Gadde et al. Citation2015; Tiberio et al. Citation2015). Hartgrink et al. (Citation2002) analyzed 285 MGC cases that underwent palliative gastrectomy and suggested potential benefits for MGC patients. Sun et al. (Citation2013) in a meta-analysis of 3003 patients from 14 related articles, found that palliative gastrectomy significantly improved OS in MGC patients (HR 0.62; 95% CI 0.49-0.78, P < 0.01). However, some studies have reported inconclusive results regarding the advantages of palliative resection (Martin et al. Citation2002; Gold et al. Citation2007). In our study, we identified surgery as an independent prognostic factor for MGC patients, with patients who underwent gastrectomy showing a survival advantage over those receiving supportive care. It is important to acknowledge that selection biases, such as comorbidities and metastatic tumor burden, were inevitable in our analysis. Properly designed randomized trials are still necessary to elucidate the prognostic role of palliative gastrectomy in MGC patients.
In our analysis, radiotherapy also showed a correlation with a survival advantage in MGC patients. Similar to chemotherapy, the overall specificity of radiotherapy in the SEER database was high, with a sensitivity of 80%. Considering that radiotherapy was only administered to a small proportion of MGC patients, we deemed the bias to be acceptable. The primary goal of radiotherapy for MGC patients was local palliation, such as controlling symptoms of bleeding and obstruction. In particular, radiotherapy has often been employed to manage gastric bleeding in the context of poor general conditions. Hiramoto et al. (Citation2018) conducted a retrospective analysis in 23 gastric cancer patients who underwent palliative radiation. The response rates for bleeding and obstruction were 88.8% and 80%, respectively. The median event-free survival times from the initiation of radiation were 103 days for the bleeding group and 104 days for the obstruction group, respectively. Asakura et al. (Citation2011) reported a response rate of 73% (22/30) in patients treated with radiotherapy, among which 50% (11/22) had rebleeding occur. Generally speaking, patients who received radiotherapy often have a poor prognosis. However, with the development of chemotherapeutic agents, the role of palliative radiation therapy in the management of gastric bleeding will become more significant. Of note, chemoradiotherapy may emerge as an effective strategy for the treatment of T4 patients with M1 in the future.
Several limitations should be addressed when interpreting the results. Firstly, the SEER database, from which we obtained our data, may contain inherent coding errors and biases, as it relies on information reported by various institutions. Secondly, although we conducted internal validation, external validation in independent cohorts is still necessary to further evaluate the practical applicability and generalizability of our nomogram model. Thirdly, genetic information, which could potentially influence the prediction accuracy, was not available in the SEER database. Future studies incorporating genetic data may provide additional insights into prognostic evaluation.
Conclusion
In conclusion, our study successfully developed and validated a prognostic prediction model for MGC patients. This nomogram has the potential to provide valuable reference information for clinicians in evaluating the prognosis of MGC patients. However, further research and validation are still needed to enhance the accuracy and applicability of the model.
Author contributions
(I) Conception and design: Jianhui Li, Wenhan Li, and Yao Tang; (II) Administrative support: Jianhui Li; (III) Provision of study materials: Danfang Wang, Wenhan Li; (IV) Collection and assembly of data: Yao Tang, Danfang Wang; (V) Data analysis and interpretation: Danfang Wang; (VI) Manuscript writing: Jianhui Li, Wenhan Li; (VII) Final approval of manuscript: all authors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in Science Data Bank, at https://doi.org/10.57760/sciencedb.07169.
Additional information
Funding
References
- Asakura H, Hashimoto T, Harada H, Mizumoto M, Furutani K, Hasuike N, Matsuoka M, Ono H, Boku N, Nishimura T. 2011. Palliative radiotherapy for bleeding from advanced gastric cancer: is a schedule of 30 Gy in 10 fractions adequate? J Cancer Res Clin Oncol. 137(1):125–130. doi:10.1007/s00432-010-0866-z.
- Bando E, Ji X, Kattan MW, Seo HS, Song KY, Park CH, Bencivenga M, de Manzoni G, Terashima M. 2020. Development and validation of a pretreatment nomogram to predict overall survival in gastric cancer. Cancer medicine.
- Bilici A. 2014. Treatment options in patients with metastatic gastric cancer: current status and future perspectives. World J Gastroenterol. 20(14):3905–3915. doi:10.3748/wjg.v20.i14.3905.
- Bray F, Ferlay J, Laversanne M, Brewster DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E, Swaminathan R, Antoni S, et al. 2015. CancerIncidence inFiveContinents: Inclusion criteria, highlights from Volume X and the global status of cancer registration. Int J Cancer. 137(9):2060–2071. doi:10.1002/ijc.29670.
- Chen L, Song MQ, Lin HZ, Hao LH, Jiang XJ, Li ZY, Chen YX. 2013. Chemotherapy and resection for gastric cancer with synchronous liver metastases. World J Gastroenterol. 19(13):2097–2103. doi:10.3748/wjg.v19.i13.2097.
- Chen WW, Wang F, Xu RH. 2013. Platinum-based versus non-platinum-based chemotherapy as first line treatment of inoperable, advanced gastric adenocarcinoma: a meta-analysis. PLoS One. 8(7):e68974.
- Ebinger SM, Warschkow R, Tarantino I, Schmied BM, Güller U, Schiesser M. 2016. Modest overall survival improvements from 1998 to 2009 in metastatic gastric cancer patients: a population-based SEER analysis. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 19(3):723-734.
- Gadde R, Tamariz L, Hanna M, Avisar E, Livingstone A, Franceschi D, Yakoub D. 2015. Metastatic gastric cancer (MGC) patients: Can we improve survival by metastasectomy? A systematic review and meta-analysis. J Surg Oncol. 112(1):38–45. doi:10.1002/jso.23945.
- Glimelius B, Ekström K, Hoffman K, Graf W, Sjödén PO, Haglund U, Svensson C, Enander LK, Linné T, Sellström H, et al. 1997. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 8(2):163–168. doi:10.1023/A:1008243606668.
- Gold JS, Jaques DP, Bentrem DJ, Shah MA, Tang LH, Brennan MF, Coit DG. 2007. Outcome of patients with known metastatic gastric cancer undergoing resection with therapeutic intent. Ann Surg Oncol. 14(2):365–372. doi:10.1245/s10434-006-9059-z.
- Han DS, Suh YS, Kong SH, Lee HJ, Choi Y, Aikou S, Sano T, Park BJ, Kim WH, Yang HK. 2012. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol. 30(31):3834–3840. doi:10.1200/JCO.2012.41.8343.
- Hartgrink HH, Putter H, Klein Kranenbarg E, Bonenkamp JJ, van de Velde CJ. 2002. Value of palliative resection in gastric cancer. Br J Surg. 89(11):1438–1443. doi:10.1046/j.1365-2168.2002.02220.x.
- Hiramoto S, Kikuchi A, Tetsuso H, Yoshioka A, Kohigashi Y, Maeda I. 2018. Efficacy of palliative radiotherapy and chemo-radiotherapy for unresectable gastric cancer demonstrating bleeding and obstruction. Int J Clin Oncol. 23(6):1090–1094. doi:10.1007/s10147-018-1317-0.
- Iacovelli R, Pietrantonio F, Farcomeni A, Maggi C, Palazzo A, Ricchini F, de Braud F, Di Bartolomeo M. 2014. Chemotherapy or targeted therapy as second-line treatment of advanced gastric cancer. A systematic review and meta-analysis of published studies. PLoS One. 9(9):e108940. doi:10.1371/journal.pone.0108940.
- Izuishi K, Mori H. 2020. Recent strategies for treating stage IV gastric cancer: roles of palliative gastrectomy, chemotherapy, and radiotherapy. J Gastrointestin Liver Dis. 25(1):87–94. doi:10.15403/jgld.2014.1121.251.rv2.
- Kramer AA, Zimmerman JE. 2007. Assessing the calibration of mortality benchmarks in critical care: The Hosmer-Lemeshow test revisited*. Crit Care Med. 35(9):2052–2056. doi:10.1097/01.CCM.0000275267.64078.B0.
- Liang YX, Deng JY, Guo HH, Ding XW, Wang XN, Wang BG, Zhang L, Liang H. 2013. Characteristics and prognosis of gastric cancer in patients aged ≥ 70 years. World J Gastroenterol. 19(39):6568–6578. doi:10.3748/wjg.v19.i39.6568.
- Liu J, Chen L. 2011. Current status and progress in gastric cancer with liver metastasis. Chin Med J. 124(3):445–456.
- Martin RC, Jaques DP, Brennan MF, Karpeh M. 2002. Achieving R0 resection for locally advanced gastric cancer: is It worth the risk of multiorgan resection? J Am Coll Surg. 194(5):568–577. doi:10.1016/S1072-7515(02)01116-X.
- Mohri Y, Tanaka K, Ohi M, Saigusa S, Yasuda H, Toiyama Y, Araki T, Inoue Y, Kusunoki M. 2014. Identification of prognostic factors and surgical indications for metastatic gastric cancer. BMC Cancer. 14:409. doi:10.1186/1471-2407-14-409.
- Pyrhönen S, Kuitunen T, Nyandoto P, Kouri M. 1995. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 71(3):587–591. doi:10.1038/bjc.1995.114.
- Saito H, Osaki T, Murakami D, Sakamoto T, Kanaji S, Tatebe S, Tsujitani S, Ikeguchi M. 2006. Effect of age on prognosis in patients with gastric cancer. ANZ J Surg. 76(6):458–461. doi:10.1111/j.1445-2197.2006.03756.x.
- Song P, Wu L, Jiang B, Liu Z, Cao K, Guan W. 2016. Age-specific effects on the prognosis after surgery for gastric cancer: A SEER population-based analysis. Oncotarget. 7(30):48614–48624. doi:10.18632/oncotarget.9548.
- Sun J, Song Y, Wang Z, Chen X, Gao P, Xu Y, Zhou B, Xu H. 2013. Clinical significance of palliative gastrectomy on the survival of patients with incurable advanced gastric cancer: a systematic review and meta-analysis. BMC Cancer. 13:577. doi:10.1186/1471-2407-13-577.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. 2021. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71(3):209–249. doi:10.3322/caac.21660.
- Takashima A, Yamada Y, Nakajima TE, Kato K, Hamaguchi T, Shimada Y. 2009. Standard first-line chemotherapy for metastatic gastric cancer in Japan Has Met the global standard: evidence from recent phase III trials. Gastrointestinal Cancer Research: Gcr. 3(6):239–244.
- Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM. 2010. The global burden of cancer: priorities for prevention. Carcinogenesis. 31(1):100–110. doi:10.1093/carcin/bgp263.
- Tiberio GA, Baiocchi GL, Morgagni P, Marrelli D, Marchet A, Cipollari C, Graziosi L, Ministrini S, Vittimberga G, Donini A, et al. 2015. Gastric cancer and synchronous hepatic metastases: is it possible to recognize candidates to R0 resection? Ann Surg Oncol. 22(2):589–596. doi:10.1245/s10434-014-4018-6.
- Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. 2006. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 24(18):2903–2909. doi:10.1200/JCO.2005.05.0245.
- Yagi Y, Seshimo A, Kameoka S. 2000. Prognostic factors in stage IV gastric cancer: univariate and multivariate analyses. Gastric Cancer: Official Journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 3(2):71–80.
- Zhu Y, Fang X, Wang L, Zhang T, Yu D. 2020. A predictive nomogram for early death of metastatic gastric cancer: A retrospective study in the SEER database and China. J Cancer. 11(18):5527–5535. doi:10.7150/jca.46563.