ABSTRACT
Macroalgal biomass (seaweed) is one of the most abundant and valuable renewable resources globally. Lately, the conversion of this into biofuels has gained considerable attention as an alternative to fossil fuels. In addition, the success in exploiting this feedstock hinges on its distinctive characteristics and the method employed to isolate its specific active components. Within the framework of a circular economy, invasive Sargassum species present themselves as promising candidates for bioethanol production. Ghana’s bioethanol production feedstocks are mostly from edible crops in competition for human consumption or lignocellulosic biomass with high lignin content. This suggests utilisation of this invasive and valuable bioresource is still not recognised in the country. The present communication reviews Sargassum invasiveness (availability), chemical composition, processes and opportunities in bioethanol production in a potential country, Ghana. This aligns with the clean and sustainable energy fulfillment as outlined in Ghana’s energy transition framework policy and SDG 7.
KEYWORDS:
1. Introduction
The continuous growth of the world’s population leading to an increase in energy demand translates to extensive and rapid dependence on fossil resources. However, these resources keep on depleting daily, with their utilisation causing global warming issues that need to be addressed. To meet these energy demands and protect the environment burst, we search for renewable resources, biofuels that are sustainable, environmentally friendly, cost-effective and efficient with less greenhouse emissions (Bibi et al., 2016; Jambo et al., Citation2016; Miyuranga et al., Citation2022). Biofuels are renewable energy sources produced from crops, algal and animal feedstock. They are mainly classified into solid, liquid and gaseous biofuels. Some examples of solid biofuels include charcoal and wood. Bioethanol, biodiesel and bio-oil are liquid biofuels. Other potential liquid biofuels reported in the literature as an alternative to bioethanol are 2-methylfuran (MF) and 2,5-dimethylfuran (DMF). These furan-derived compounds have a high octane number, energy and low oxygen content, with the ability to overcome some drawbacks of bioethanol (Hoang & Pham, Citation2021; Hoppe et al., Citation2016; Jężak et al., Citation2016; Qian et al., Citation2015). Biogas, biomethane and syngas fall under gaseous biofuels (Balwan & Kour, Citation2021; Mishra et al., Citation2023). These renewable feedstocks mostly synthesise biomolecules using carbon dioxide and sunlight from the atmosphere, which are subsequently converted to these biofuels and other chemicals (Gengiah et al., Citation2023; Wei et al., Citation2013). Liquid biofuels, that is, bioethanol and biodiesel, have been reported to form about 40% of the global total energy consumption (Oo & Kywe, Citation2019). Bioethanol is a sustainable fuel produced from sugar, starch, lignocellulosic and algae biomass. Biomass is a renewable organic material obtained from crops, algae and wastes. With the use of different pathways, this material is processed to produce bioethanol. Distinctly, bioethanol has received extensive attention owing to its biodegradable nature and less greenhouse gas emissions. More importantly, it is a viable renewable fuel with a high heat of vaporisation, high octane number and cleaner combustion with less carbon dioxide emissions resulting in a cleaner environment and one that can be commercially produced (Hoang & Pham, Citation2021). Compared to gasoline, bioethanol contains a negligible amount of sulfur, and mixing of these two fuels helps to decrease sulfur content as well as the emission of sulfur oxide, which is a carcinogen and can contribute to acid rain. Also, the anti-knocking characteristic of this fuel as a blending component is of high importance in the transportation industry, as it is known to help prevent early ignition and knocking, ensuring an effective engine performance (Agboola et al., Citation2023; Niphadkar et al., Citation2017; Owusu et al., Citation2023). Furthermore, it is estimated to lead the biofuel ladder by 2050, presenting opportunities for socioeconomic development (Guo et al., Citation2015; Jambo et al., Citation2016; Mohapatra et al., Citation2017; Silalertruksa et al., Citation2012; H. Zabed et al., Citation2017).
Recent studies highlight bioethanol as a viable replacement for conventional gasoline, capable of direct use in vehicles or as a blend with gasoline. This substitution has shown promising results, reducing greenhouse gas emissions and gasoline consumption significantly (Hoang et al., Citation2019; Jambo et al., Citation2016; Jeyakumar et al., Citation2022). Despite these advantages, higher production costs compared to petroleum-based fuels limited its potential, especially after World War II. However, the oil crisis of the 1970s sparked renewed interest in bioethanol as an alternative transportation fuel. Since the 1980s, there has been a noticeable resurgence in its consumption, with projections indicating an annual increase of 8% by 2050 (Dave et al., Citation2019; Wu et al., Citation2017). Globally, numerous potential feedstocks can be harnessed for bioethanol production. The quest for a suitable feedstock has resulted in the emergence of three distinct generations: first, second and third generations (Borines et al., Citation2013; Kadimpati et al., Citation2021). The first and second generations are derived from edible and non-edible feedstocks, respectively, whereas the third generation stems from algae feedstocks (micro and macroalgae). The primary focus of current biofuel research studies is on the latter feedstock (third generation) due to its abundant availability and significant potential for commercialisation (Jambo et al., Citation2016; Raheem et al., Citation2015).
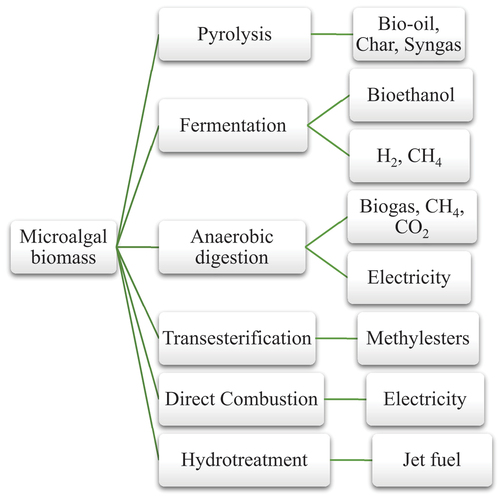
Algae are rapidly growing species with good lipid content, proteins and carbohydrates. These prokaryotic and eukaryotic organisms are majorly grouped into micro and macroalgae (Nigam & Singh, Citation2011; Schenk et al., Citation2008). Microalgae have a unicellular structure with a high lipid content. They serve as eco-friendly, renewable and cost-effective feedstock for biofuels and chemicals. Various species have been explored for their potential as valuable bioproducts. In 2018, Kumar and colleagues discovered that hydrothermal liquefaction (HTL) of microalgae under subcritical water conditions yielded bio-oil, with the most significant output observed using Scenedesmus quadricauda (P. K. Kumar et al., Citation2018). Chiaramonti et al. (Citation2017) also conducted a review of thermochemical conversion routes for microalgae, including HTL and pyrolysis. Their review emphasised the potential of these microalgae for biofuel production. Moreover, HTL of Botryococcus braunii and Dunaliella tertiolecta yielded a substantial amount of bio-oil with diverse chemical components such as carboxylic acids, ketones, esters and phenolic compounds, respectively (Ren et al., Citation2018; Shuping et al., Citation2010). Some challenges revolving around the utilisation of microalgae include limited research in improving growth rate and product synthesis, biomass pretreatment and optimisation of the fermentation process, particularly in the context of bioethanol production (Bahadar & Khan, Citation2013, Khan et al., Citation2018; Peng et al., Citation2019). Some technologies for processing microalgae with their respective end products/fuels are shown in Figure .
Figure 1. Processing technologies and end products from microalgae biomass (source: Peng et al., Citation2019).
The majority of published reviews in the past years have predominantly centred on assessing the sustainability of microalgae as a bioethanol feedstock (De Farias Silva & Bertucco, Citation2016). However, it is essential to recognise that macroalgae although invasive, its distinctive characteristics also harbour vast untapped potential that warrants further exploration. Macroalgae are featured in a recent report by the International Energy Agency (IEA) and the US Department of Energy’s roadmap for algal biofuels. Pilot plant studies for developing macroalgae as a biofuel feedstock are currently in progress in Asia and Europe. Considering that two-thirds of the planet is covered by oceans, seaweeds can be cultivated in the open sea, making it a viable option for biofuel production (Surendhiran & Sirajunnisa, Citation2019).
Ghana is reported to have macroalgae species flooding along its coastal regions, polluting the environment and negatively affecting daily activities in these regions. Distinctly, Sargassum are among the largest species with less development and utilisation (Segbefia et al., Citation2018; Zhao et al., Citation2022). Therefore, the present review highlights the utilisation of macroalgae; Sargassum species in bioethanol production. Gaining a comprehensive understanding of the significance of this renewable feedstock and end product (bioethanol) opens up a versatile utilisation of bioethanol for socio-economic development and environmental benefits in untapped countries like Ghana, enabling the country to achieve its transition policy of a climate-resilient low-carbon energy to contribute to SDG 7 and 13 (Bibi et al., 2016; Anon, Citation2023; Pelizan et al., Citation2019).
2. Bioethanol feedstocks
The economy of ethanol production is heavily dependent on the availability and affordability of feedstocks. At present, three generational distinct feedstocks for bioethanol have emerged. The first-generation feedstocks are derived from sugar and starchy crops which are fermented for bioethanol production. Owing to the competition between food and land use, these feedstocks have become a subject of controversy, sparking disputes concerning their sustainability (Valencia et al., Citation2015; Halima & Archna, Citation2023). Moreover, the economic viability of these feedstocks is compromised by significant cellulose and hemicellulose losses during the bioethanol conversion process (Gómez et al., Citation2011; Jambo et al., Citation2016; Ritslaid et al., Citation2010). Wood residues, straws, sawdust and other lignocellulosic biomass represent the second-generation feedstocks. Although these non-edible feedstocks (second generation) are appealing, they remain a subject of debate due to concerns that these could compete with other food production resources. In addition, the separation of their lignin content poses a significant obstacle that needs to be addressed (Alalwan et al., Citation2019; Limayem & Ricke, Citation2012; Naik et al., Citation2010). To address these glitches evolved the third generational feedstocks: micro- and macroalgae biomass (Gatdula et al., Citation2018). The acceptance of the third-generation feedstocks for bioethanol production is attributed to their high carbohydrate content, ease of cultivation, low lignin, high carbon dioxide (CO2) absorption, among others (Singh & Olsen, Citation2011). Although research studies on algal feedstock for bioethanol production are at their early stages, this feedstock holds immense potential as a promising resource for commercial bioethanol production now and in the future.
3. Macroalgae
The rapid growth rate, abundance carbohydrate content and high level of photosynthetic activity deem macroalgae, also known as seaweeds, a great promising feedstock for bioethanol production (Gatdula et al., Citation2018; Jang et al., Citation2012; Singh & Olsen, Citation2011). In addition, the production of non-food macroalgae with little or no lignin content offers various advantages including high yields, less competition with food crops and land (Azizi et al., Citation2017; Borines et al., Citation2013; Limayem & Ricke, Citation2012; Ravanal et al., Citation2019). Also, this carbon-neutral renewable resource with an abundance of species is renowned for being sulphur-free and highly biodegradable. Consequently, these species which mostly grow on rocky substrates are cultivated and utilised in various countries, notably in East Asia, where China leads with 14 million tonnes, followed by Africa with 140,000 tonnes and America with 15,634 tonnes. Notably, the production of macroalgae tripled in 2018 compared to the year 2000, as reported in multiple studies (Ashokkumar et al., Citation2017; Durbha et al., Citation2016; Godvin et al., Citation2021; Zhao et al., Citation2022). Moreover, their cultivation is noted to reduce pollution and consume large amounts of CO2, thereby contributing significantly to carbon sequestration and environmental protection. Also, unlike microalgae cultured in bioreactors, the cultivation of macroalgae can easily be done in large quantities in marine environments, which will reduce both cost and energy input for nutrient supply (Hebbale et al., Citation2017; Kadimpati et al., Citation2021; Kraan, Citation2010; Zeng et al., Citation2019). Macroalgae are rich in polysaccharides; nonetheless, the type and concentration of carbohydrates typically differ among different classes of macroalgae. They can be categorised into three groups: Phaeophyceae (brown), Rhodophyceae (red) and Chlorophyceae (green) with major compositions of carbohydrates, proteins and lipids. Recently, there has been a gradual surge in seaweed studies, and distinctly their carbohydrate composition potential, which plays a vital role in bioethanol conversion. Figure summarises the sugar profile of macroalgae and Table outlines the dry weight average composition of a typical macroalgae species.
Figure 2. Sugar profile of macroalgae (source: Ardalan et al., Citation2018; Ramachandra & Hebbale, Citation2020; Stiger-Pouvreau et al., Citation2016).
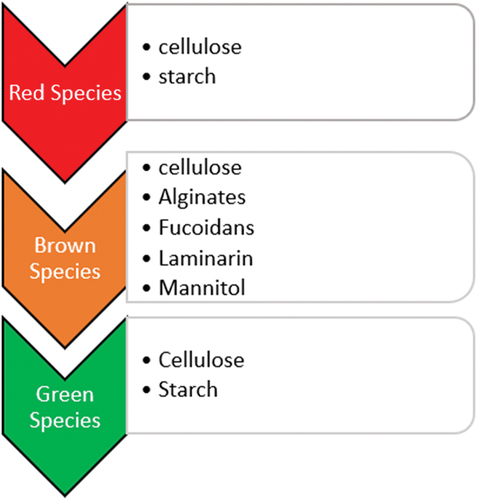
Table 1. Average composition of typical macroalgae species (% dry weight)
The red species have an abundance of red pigments and mostly thrive in greater depths. Their cell wall is made of polysaccharides such as cellulose, xylene, mannan, etc. Green species, on the other hand, thrive in shallow and freshwater zones and require plenty of light due to their possession of photosynthetic chlorophyll pigments. Cellulose, xylene and mannose are the main components of their wall structure. The polysaccharides found in the cell walls of brown seaweeds species are cellulose, alginate and fucoidan. Cellulose remains a large fraction component of these feedstocks, and as such an essential ingredient for bioethanol production (Hong et al., Citation2014, Valencia et al., Citation2015; Jambo et al., Citation2016; Hebbale et al., Citation2017; Orozco-González et al., Citation2022). Table shows some common macroalgae species employed in bioethanol production. Among the three classifications of macroalgae, seaweeds categorised under class Phaeophyceae are particularly remarkable raw materials for ethanol fermentation, of which Sargassum species are among the most abundant species in West Africa-Ghana (Addico & de-Graft Johnson, Citation2016; Amador-Castro et al., Citation2021; Davis et al., Citation2020; Lamptey et al., Citation2022).
Table 2. Macroalgae species exploited in bioethanol production
Summary of the importance of macroalgae
Macroalgae thrive in salty water using only sunlight and nutrients from seawater, eliminating the need for chemical fertilisers. This not only saves substantial energy and money but also promotes the sustainability of macroalgae-based bioethanol production.
Selection of macroalgal biomass for ethanol production instead of using terrestrial plant biomass brings several significant advantages, notably avoiding any adverse effects on food security. Moreover, the macroalgae’s relatively high sugar content and lower lignin content compared to lignocelluloses make it conducive to achieving high-volume production.
Macroalgae supplies oxygen to the ocean, which aids in the reduction of carbon dioxide accumulation in the atmosphere. These species have the capability to cleanse water by removing heavy metals, providing environmental benefits. Additionally, specific algal species possess the unique trait of producing substantial amounts of carbohydrates rather than lipids as preserved polymers (Rajkumar et al., Citation2013; Chaldun et al., Citation2022).
4. Sargassum species
A well-known brown seaweed species that has been increasingly inundating coastal areas worldwide in recent times is Sargassum (Milledge & Harvey, Citation2016; Offei et al., Citation2019; Thompson et al., Citation2020). The primary components of the cell walls in these species are cellulose, alginate and fucoidans (Ravanal et al., Citation2019). It is predominantly found on beaches or in shallow waters. The main constituents of Sargassum are water, trace metals and organic compounds. The cell walls also contain non-toxic, biodegradable polysaccharides such as alginate, laminarin, mannitol, fucoidan and cellulose (Hasnain et al., Citation2020; Monroy-Velázquez et al., Citation2019; Saravanan et al., Citation2018). The blooms and overabundance of Sargassum pose a threat to both humans and marine organisms, impacting fishing and tourism industries significantly (Davis et al., Citation2021; Orozco-González et al., Citation2022). Researchers have been exploring effective and efficient ways to utilise these species or feedstocks to produce valuable chemicals and products, contrary to their conventional usage as fertilisers and animal feeds (Milledge & Harvey, Citation2016). This will not only achieve economic benefits but environmental benefits as well. One of the prominent applications found in the literature is the production of clean energy, fuels and pharmaceuticals, which aligns with SDG 7 (Amador-Castro et al., Citation2021). In particular, the fuels derived from these renewable feedstocks, Sargassum species, offer a sustainable and green alternative to fossil fuels. Due to their relatively low levels of lignin and hemicellulose compared to lignocellulosic biomass, they are considered highly suitable for bioethanol production (Del Río et al., Citation2021; Khuong et al., Citation2017; López-Sosa et al., Citation2020; Rajkumar et al., Citation2013; Widyaningrum et al., Citation2016). A study by Zeng et al. (Citation2019), supported this statement.
Sargassum species are known to be made of essential ingredients for the production of valuable chemicals, energy and fuels. Alginates are a significant and major cell wall component of brown algae, constituting up to 40% of their dry weight. They consist of linear binary copolymers made up of (1,4)-linked β-D-mannuronic acid (M) and α-L-guluronic acid (G) monomers. These non-toxic bio-polysaccharide components are essential biomaterials with wide-ranging applications in food, cosmetics, textiles and biomedical fields. This shows extracting them from Sargassum can serve as a significant source of value-added products, enhancing the economy (Chen et al., Citation2021; Orozco-González et al., Citation2022). The global annual alginate production is estimated at 30,000 tonnes, and their uses have been extensively reviewed by various researchers (Hasnain et al., Citation2020; Milledge & Harvey, Citation2016; Nayak et al., Citation2019; Widyaningrum et al., Citation2016). Literature studies by Ardalan et al. (Citation2018) revealed that alginate extraction served as a beneficial pretreatment for bioethanol production, yielding superior outcomes compared to direct fermentation of raw biomass. Moreover, alginate extraction highlighted a 28% yield and 92% purity for a valuable hydrocolloid; sodium alginate extracted from Sargassum seaweed in the Caribbean (Mohammed et al., Citation2020). In addition, Flórez-Fernández et al. (Citation2021) backed this statement by concluding a better susceptibility of alginate-free Sargassum in anaerobic digestion. Brown algae generally contain a relatively small amount of lipids, typically below 5% of their dry biomass. However, various Sargassum species have been found to possess over 40% polyunsaturated fatty acids relative to their total lipid content (Amador-Castro et al., Citation2021; Nomura et al., Citation2012; Saini & Keum, Citation2018; Stiger-Pouvreau et al., Citation2016). Additionally, the protein content of brown algae species is low. Sargassum is mostly known to have averagely 7–11% dry weight basis (Amador-Castro et al., Citation2021). However, this may differ with reference to seasons and type of species (Pangestuti & Kim, Citation2015). Table shows the chemical composition of some selected Sargassum species on dry weight basis.
Table 3. Chemical composition of some sargassum species (% dry weight)
5. Sargassum influx
Sargassum species, despite their abundance, have been underutilised and less developed (Offei et al., Citation2018; Segbefia et al., Citation2018; Zhao et al., Citation2022). Their invasion in Ghana in 2009 is believed to have originated from the West Africa Coastline, specifically Senegal and Cote d’Ivoire. These species (Sargassum vulgare, Sargassum fluitans III, Sargassum natans I and Sargassum natans VIII) are reported to be invading various coastal areas of the country, Greater Accra and Western region. They thrive in shallow depth; for instance, Sargassum vulgare in Ghana is well within the depth range of 11–25 meters, temperature range between 15–25 degree Celsius, free from pollution and strong winds (Addico & de-Graft Johnson, Citation2016; Segbefia et al., Citation2018). Lamptey et al. (Citation2022), highlight the persistence of these algae blooms in the country with not yet found usefulness. This was backed with a survey study from the inhabitants in these coastal areas.
5.1 Fishing industry
The continuous influx of Sargassum in coastal regions has caused a state of emergency in the fisheries sector. Fisher-folks have reported severely of reduced visibility, increased occurrences of fishing net clogs by these species and decreased fish capture during periods of inundation, which is affecting the livelihood of local inhabitants (Oyesiku & Egunyomi, Citation2014; Thompson et al., Citation2020). In Ghana for instance, this industry contributes 4.5% to the annual gross domestic product (GDP) of the country; as such, a decrease in operation affects livelihoods, threatening national food and economic security (van der Plank et al., Citation2023).
5.2 Tourist industry
This industry is a major sector of most countries. In Ghana, it accounts for 5.5% of the total GDP. The growth and expansion of this industry primarily depends on the attractiveness, hospitality and atmosphere of beach-front locations. The invasion of Sargassum had a severe impact on tourist sites and businesses, hindering various swimming activities on festive occasions in recent years (Anon, Citation2022; Segbefia et al., Citation2018).
6. Bioethanol production
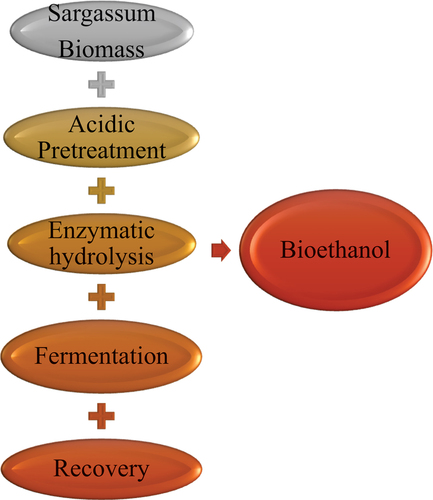
Bioethanol, a renewable biofuel derived from macroalgae biomass and known for its eco-friendly nature, serves as an excellent alternative and blending component for gasoline engines. Its high-octane number helps reduce emissions, safeguard the environment and contribute to Sustainable Development Goal 13 (Ayyanna et al., Citation2023; Bušić et al., Citation2018). The increasing demand for this green fuel can be attributed to global concerns over industrial development, energy and transportation, all focusing on environmental well-being. Currently, bioethanol accounts for approximately 75% of the overall biofuel consumption (Rastogi & Shrivastava, Citation2018), with billions of litres produced annually at an impressive growth rate of 7%, confirming its viability as a sustainable fuel choice (Edeh, Citation2020; Sakharkar, Citation2018). Seaweeds present numerous advantages for bioethanol production, including cost-effectiveness, low lignin content, carbon neutrality and high photosynthetic efficiency, all contributing to its potential as an eco-friendly and sustainable fuel source (Fischer et al., Citation2017; Offei et al., Citation2018). However, despite these benefits, utilisation projects are limited in sub-Sahara Ghana (Ameka et al., Citation2019). Ghana’s energy policy recorded significant growth in primary energy output, exceeding 6.2 million tons of oil equivalent in 2000 and reaching 7.6 million tons in 2004. Despite relying on biomass, hydropower and solar power as major energy sources, the country faced increasing demands, leading to a substantial surge in energy imports from 2000 to 2004. Notably, around 80% of the net energy imports were crude oil, and 20% were petroleum products. For instance, in 2017, the complete fuel supply, including gasoline; a primary consumed fuel in the country valued at 695 million dollars, was imported, making up 1.3% of GDP and comprising 6% of all imports. Also, a major consumer of ethanol; Kasapreko Company Limited was known to rely heavily on imports until 2016 when a domestic bioethanol venture was established in the Volta region, producing 3.6 million litres of first-generation ethanol annually, signifying untapped and less attention to these potential algae bioresource which presents a viable route for the country’s equitable shift towards carbon-neutrality for efficient energy and economic growth, as highlighted in the national energy transition policy (Amo-Aidoo et al., Citation2021; Anon, Citation2023; Asumadu-Sarkodie & Owusu, Citation2016; Pelizan et al., Citation2019).
The production of bioethanol involves a multi-step process that begins with the pre-treatment of the biomass feedstocks. This can be achieved by either reducing their size or utilising chemicals and microorganisms to degrade the biomass, thus releasing sugars. The next step is the integration of these sugars into simpler forms through acid or enzyme-catalysed hydrolysis, using concentrated or dilute acids or enzymes. Subsequently, these simpler sugars undergo fermentation by yeast, bacteria, or fungi, converting them into ethanol (Alfonsín et al., Citation2019; Borines et al., Citation2013). During the bioethanol production from algae biomass, special attention should be given to critical stages in the process: pre-treatment, hydrolysis, fermentation and recovery. The ethanol yields heavily depend on the available conditions and the ability of microbes (enzymes and yeasts) to process these sugars (Offei et al., Citation2018; Sebayang et al., Citation2017; Zhao et al., Citation2022).
6.1. Pretreatment
The pretreatment process is well-known for its ability to break down biomass structure, releasing cellulose and enabling efficient enzymatic hydrolysis (M. D. Kumar et al., Citation2021; Sankaran et al., Citation2019). Moreover, it helps minimise the formation of inhibitors that could otherwise reduce the yield of hydrolysis and sugar fermentation to ethanol (Ceballos, Citation2018; Edeh, Citation2020). However, this process can be costly and is influenced by factors like temperature, pH level and treatment duration (Jeyakumar et al., Citation2022; H. M. Zabed et al., Citation2019). To improve cellulose reactivity and maximise reducing sugar yields, various pre-treatment techniques are used, categorised as conventional or advanced methods. Conventional methods include physical, chemical, physicochemical and biological approaches, with chemical methods being predominantly used due to their high efficiency (Godvin et al., Citation2021; Lugani et al., Citation2020). Advanced techniques may involve ionic liquid-based fractionation (ILF) and acid-based fractionation, which fall under the chemical method.
Physical pre-treatment involves milling and grinding biomass to increase surface area and facilitate enzymatic hydrolysis. It is often a necessary step before other pre-treatment methods and can be combined with chemical pre-treatment to enhance biomass deconstruction (Gruduls et al., Citation20181; Oliveira et al., Citation2015; Yuhendra et al., Citation2021). The chemical process, on the other hand, employs acids, alkali, or organic solvents to selectively break down the biomass matrix, enabling further processing in the hydrolysis stage. Dilute acids are commonly used due to lower toxicity and corrosion concerns (Sambusiti et al., Citation2015). Various studies have demonstrated successful bioethanol production using acid pre-treatment with different brown algae species (Ashokkumar et al., Citation2017; Borines et al., Citation2013; Ravanal et al., Citation2016; Yazdani et al., Citation2015). In physicochemical pre-treatment, a combination of both chemical and physical methods to disintegrate biomasses is employed and mostly considered feasible and cost-effective, utilising techniques like microwave irradiation and steam explosion (Zhao et al., Citation2022). Biological pre-treatment employs microorganisms, such as fungi and bacteria, to break down biomass for enzymatic hydrolysis. This eco-friendly method has low energy requirements and avoids the use of chemical agents (Ferdeș et al., Citation2020; Hassan et al., Citation2018).
6.2. Hydrolysis
In bioethanol production, the hydrolysis stage primarily involves converting cellulose into simple sugars. Key factors influencing hydrolysis include lignin content, accessible surface area and cellulose content. This process can be accomplished using either enzymes or acids. Enzymatic hydrolysis utilises enzymes to break down complex carbohydrates into sugar monomers under gentle conditions. This highly efficient method ensures significant sugar recovery while preventing the formation of inhibitors and corrosion risks. Achieving optimal performance requires specific incubation temperatures, pH levels and residence times, with enzyme selection tailored to the chemical composition of the treated biomass (Aparicio et al., Citation2021). The process offers two pathways: separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SSF). To lower costs, researchers are exploring the use of natural and engineered microorganisms capable of secreting cellulolytic enzymes, including Clostridium, Bacillus, Streptomyces, Trichoderma, Penicillium and Schizophillum, Dunaliella salina and others (Edeh, Citation2020; Sirajunnisa & Surendhiran, Citation2016; Surendhiran & Sirajunnisa, Citation2019). Notably, in a study by Aparicio et al. (Citation2021), the simultaneous saccharification and fermentation (PSSF) approach achieved a conversion yield of 76.23 ± 4.68% for glucose to bioethanol from Sargassum sp. Additionally, separate hydrolysis and fermentation showed promising outcomes with Sargassum muticum and Sargassum angustifolium, resulting in glucose conversion rates of 74% and remarkable bioethanol yields of 96% and 73%, respectively (Ardalan et al., Citation2018; Del Río et al., Citation2021).
Acid-catalysed methods, utilising either concentrated or dilute acids like H2SO4 and HCl, are commonly employed in hydrolysis. Concentrated acid hydrolysis ensures rapid sugar recovery at lower temperatures and higher acid concentrations, but it comes with higher production costs due to acid recovery, disposal and recycling challenges. Additionally, it carries the risk of sugar degradation, particularly for macroalgae biomass, which can impact the fermentation process (Azizi et al., Citation2017; Jeyakumar et al., Citation2022; Kim et al., Citation2015). On the other hand, dilute acid hydrolysis requires higher temperatures and lower acid concentrations, with dilute acid being the preferred choice. This method produces fewer inhibitors compared to concentrated acid hydrolysis (Edeh, Citation2020).
6.3. Fermentation
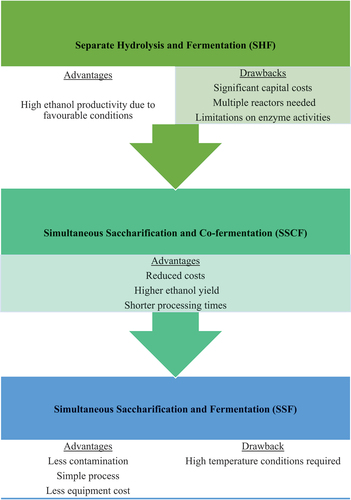
A biological process that converts monomeric sugars (glucose, fructose, or mannose) from hydrolysis into ethanol and other products, using microorganisms like yeast, fungi, or bacteria, is known as fermentation (Ghadiryanfar et al., Citation2016; Senatore et al., Citation2019). Temperature plays a crucial role during fermentation, as increasing it can boost the fermentation rate, but excessive temperatures may deactivate the microorganisms (Miyuranga et al., Citation2022). Saccharomyces cerevisiae, commonly known as brewer’s yeast, is widely preferred in this process due to its high ethanol yield and tolerance limits (Miyuranga et al., Citation2022; Surendhiran & Sirajunnisa, Citation2019). Various technologies can be employed at this stage of production, including separate hydrolysis and fermentation (SHF), simultaneous saccharification and fermentation (SSF) and simultaneous saccharification and co-fermentation (SSCF), as well as other batch, continuous and fed-batch methods (Mohd Azhar et al., Citation2017; Ravanal et al., Citation2019). These fermentation pathways are summarised in Figure .
Figure 3. Comparison of common fermentation pathways (Rastogi & Shrivastava, Citation2018; Ravanal et al., Citation2019; Tavva et al., Citation2016; Vohra et al., Citation2014).
6.4. Ethanol recovery
Following the fermentation of monomeric sugars, the next stage involves recovering ethanol from the fermented broth. To achieve this, the water content of the broth is reduced to produce anhydrous ethanol. However, challenges arise due to the azeotropic nature of the ethanol-water solution. Distillation techniques, exploiting the difference in boiling points of solution components, can be employed to overcome this limitation. The azeotropic solution problem can be resolved by introducing a separating agent that modifies the relative volatility of the key component. Various techniques are utilized for recovering pure ethanol from the fermentation broth, including adsorption distillation, extractive distillation, vacuum distillation, membrane distillation and chemical dehydration. Among the conventional methods, liquid–liquid extraction, extractive distillation and azeotropic distillation are commonly used (Nitsche & Gbadamosi, Citation2017). Extractive distillation is particularly prominent for large-scale operations, although emerging techniques like pervaporation and salt distillation are gaining attention for their potential in future applications, notably due to their lower energy requirements (Edeh, Citation2020). Studies on Sargassum species in bioethanol production and adopted pathway are summarised in Table and Figure respectively.
Table 4. Literature studies on Sargassum species in bioethanol production
7. Challenges and prospectives
Bioethanol production from Sargassum species in Ghana holds great promise as a sustainable source of energy. However, limited infrastructure for the cultivation, harvesting and processing (pretreatment, hydrolysis and fermentation) of seaweed, supportive policies in research and scaleup production still have challenges which need to be resolved. Nevertheless, its prospects remain greater compared with production from edible crops and lignocellulosic materials, contributing to sustainable energy production, waste management and economic development.
8. Conclusion
Algal bioethanol in recent times presents as an attractive and sustainable alternative to fossil fuels. Distinctly, cultivation of macroalgae emerges as a superior alternative feedstock for the production of this fuel due to their land independence, rich carbohydrate content and rapid growth. Particularly, the growth of macroalgae not only reduces the necessity for additional cost but also harnesses excessive nutrients present in coastal areas through photosynthesis. This, in turn, contributes to mitigating the pressures of global warming. Exploring Sargassum species as bioethanol feedstocks presents a potentially sustainable and environmentally friendly solution to fuel imports, tackling energy security concerns, emissions, development and job opportunities in Ghana. This can contribute to achieving SDG 7, SDG 13, the nation’s energy transition target and initiative of 10% ethanol blend in petroleum products by 2030. However, consideration should be given to the various pretreatment, hydrolysis and fermentation stages of the production, as they are critical in determining the bioethanol yield from this feedstock. The present summarises the production pathway and opportunities Sargassum species hold for underutilised countries such as Ghana.
9. Recommendation
Continuous research and development with respect to the utilisation of Sargassum seaweeds for biofuel (bioethanol) production should be encouraged to discover new technologies, enhance the production process and improve efficiency. In addition, the environmental sustainability as far as cultivating, harvesting and processing Sargassum is concerned should be clear-cut to help commend impacts on the ecosystem. Also, Sargassum-based ethanol production with other valuable products should receive domestic and international policy attention and support for its usage as a renewable fuel and energy source, especially in inundated regions where there is little or no use.
Author contributions
Winnie A. Owusu: conceptualisation, writing-original draft and editing. Solomon A. Marfo: conceptualisation and writing-review. Harrison Osei: conceptualisation and writing-review.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
No underlying data was collected or produced in this study.
Additional information
Funding
References
- Addico, G. N. D., & de Graft Johnson, K. A. (2016). Preliminary investigation into chemical composition of the invasive brown seaweed sargassum along the West Coast of Ghana. African Journal of Biotechnology, 15, 2184–16. .
- Agboola, A. A., Ishola, N. B., & Betiku, E. (2023). Bioethanol production via fermentation: Microbes, modelling and optimization. In I. I. A. I. In: Betiku & I. I. A. I. M. Ishola (Eds.), Bioethanol: A Green Energy Substitute for fossil fuels. Green Energy and technology. Springer. https://doi.org/10.1007/978-3-031-36542-3_8
- Alalwan, H. A., Alminshid, A. H., & Aljaafari, H. A. S. (2019). Promising evolution of biofuel generations. Renewable Energy Focus, 28, 127–139. https://doi.org/10.1016/j.ref.2018.12.006
- Alfonsín, V., Maceiras, R., & Gutiérrez, C. (2019). Bioethanol production from industrial algae waste. Waste Management, 87, 791–797. https://doi.org/10.1016/j.wasman.2019.03.019
- Amador-Castro, F., García-Cayuela, T., Alper, H. S., Rodriguez-Martinez, V., & Carrillo-Nieves, D. (2021). Valorization of Pelagic Sargassum Biomass into sustainable applications: Current trends and challenges. Journal of Environmental Management, 283, 112013. https://doi.org/10.1016/j.jenvman.2021.112013
- Ameka, G. K., Doamekpor, L. K., Amadu, A. A., & Amamoo, A. P. (2019). Production of biodiesel from Marine macroalgae occurring in the Gulf of Guinea, off the coast of Ghana. Ghana Journal of Science, 60, 50–58. https://doi.org/10.4314/gjs.v60i1.5
- Amo-Aidoo, A., Hensel, O., Korese, J. K., Abunde Neba, F., & Sturm, B. (2021). A framework for optimization of energy efficiency and integration of hybridized-solar energy in agro-industrial plants: Bioethanol production from cassava in Ghana. Energy Reports, 7, 1501–1519. https://doi.org/10.1016/j.egyr.2021.03.008
- Anon. (2022). Nzema youth express concerns over invasion of seaweeds. Retrieved October 6, 2022. www.newsghana.com.gh
- Anon. (2023). National energy transition framework (2022-2070). Retrieved August 7, 2023. www.energymin.gov.gh
- Aparicio, E., Rodríguez-Jasso, R. M., Pinales-Márquez, C. D., Loredo-Treviño, A., Robledo-Olivo, A., Aguilar, C. N. … Ruiz, H. A. (2021). High-pressure technology for sargassum spp biomass pretreatment and fractionation in the third generation of bioethanol production. Bioresource Technology, 329, 124935. https://doi.org/10.1016/j.biortech.2021.124935
- Ardalan, Y., Jazini, M., & Karimi, K. (2018). Sargassum angustifolium Brown Macroalga as a high potential substrate for alginate and ethanol production with minimal nutrient requirement. Algal Research, 36, 29–36. https://doi.org/10.1016/j.algal.2018.10.010
- Ashokkumar, V., Salim, M. R., Salam, Z., Sivakumar, P., Chong, C. T., Elumalai, S., Suresh, V., & Ani, F. N. (2017). Production of liquid Biofuels (biodiesel and Bioethanol) from brown Marine Macroalgae Padina Tetrastromatica. Energy Conversion and Management, 135, 351–361. https://doi.org/10.1016/j.enconman.2016.12.054
- Asumadu-Sarkodie, S., & Owusu, P. A. (2016). A review of ghana’s energy sector national energy statistics and policy framework. Cogent Engineering, 3(1), 1155274. https://doi.org/10.1080/23311916.2016.1155274
- Ayyanna, C., Sujatha, K., Mohanthy, S. K., Rajangam, J., Sudha, B. N., & Raghavendra, H. G. (2023). Bioethanol production. IntechOpen, 1–11. https://doi.org/10.72/intechopen.109097
- Azizi, N., Najafpour, G., & Younesi, H. (2017). Acid pretreatment and enzymatic saccharification of brown seaweed for polyhydroxybutyrate (PHB) production using Cupriavidus necator. International Journal of Biological Macromolecules, 101, 1029–1040. https://doi.org/10.1016/j.ijbiomac.2017.03.184
- Bahadar, A., & Khan, M. B. (2013). Progress in energy from microalgae: A review. Renewable and Sustainable Energy Reviews, 27, 128–148. https://doi.org/10.1016/j.rser.2013.06.029
- Balboa, E. M., Gallego-Fábrega, C., Moure, A., & Domínguez, H. (2015). Study of the seasonal variation on proximate composition of oven-dried sargassum muticum biomass collected in Vigo Ria, Spain. Journal of Applied Phycology, 28(3), 1943–1953. https://doi.org/10.1007/s10811-015-0727-x
- Balwan, W. K., & Kour, S. (2021). A systematic review of biofuels: The cleaner energy for cleaner environment. Indian Journal of Scientific Research, 12(1), 135–142. https://doi.org/10.32606/IJSR.V12.I1.00025
- Bambase, M. E. J., Demafelis, R. B., Borines, M. G., Gatdula, K. M., Alquiros, A. J. A., & Atienza, G. E. A. (2015). Ethanol production from Mannitol of Sargassum using Saccharomyces cerevisiae 2055. Philippine Journal of Crop Science, 40, 76–85.
- Borines, M. G., de Leon, R. L., & Cuello, J. L. (2013). Bioethanol production from the macroalgae sargassum spp. Bioresource Technology, 138, 22–29. https://doi.org/10.1016/j.biortech.2013.03.108
- Bušić, A., Marđetko, N., Kundas, S., Morzak, G., Belskaya, H., Šantek, M. I., Komes, D., Novak, S., & Šantek, B. (2018). Bioethanol production from renewable raw materials and its separation and purification: A review. Food Technology and Biotechnology, 56(3), 289–311. https://doi.org/10.17113/ftb.56.03.18.5546
- Ceballos, R. M. (2018). Bioethanol and natural resources: Substrates, chemistry and engineered systems. Taylor & Francis Group. https://doi.org/10.1201/9781315154299
- Chaldun, E. R., Andayani, D. G. S., & Handayani, T. (2022). Physicochemical Properties of Sodium Alginate from Brown Algae Sargassum Aquifolium and Sargassum cinereum. IOP Conference Series: Earth and Environmental Science, 1201(1), 012097. https://doi.org/10.1088/1755-1315/1201/1/012097
- Chen, J., Wu, A., Yang, M., Ge, Y., Pristijono, P., Li, J., & Mi, H. (2021). Characterization of Sodium Alginate-Based Films Incorporated with thymol for fresh-cut apple packaging. Food Control, 126, 108063. https://doi.org/10.1016/j.foodcont.2021.108063
- Chiaramonti, D., Prussi, M., Buffi, M., Rizzo, A. M., & Pari, L. (2017). “Review and experimental study on pyrolysis and hydrothermal liquefaction of microalgae for biofuel production. Applied Energy, 185, 963–972. https://doi.org/10.1016/j.apenergy.2015.12.001
- Dave, N., Selvaraj, R., Varadavenkatesan, T., & Vinayagam, R. (2019). A critical review on production of bioethanol from Macroalgal Biomass. Algal Research, 42, 101606. https://doi.org/10.1016/j.algal.2019.101606
- Davis, D., Simister, R., Campbell, S., Marston, M., Bose, S., McQueen-Mason, S. J., Gomez, L. D., Gallimore, W. A. & Tonon, T. (2021). Biomass composition of the golden tide pelagic seaweeds sargassum fluitans and S. natans (morphotypes I and VIII) to inform valorisation pathways. Science of the Total Environment, 762 143134 https://doi.org/10.1016/j.scitotenv.2020.143134
- Davis, D., Simister, R., Campbell, S., Marston, M., Bose, S., McQueen-Mason, S. J., & Tonon, T. (2020). Biomass composition of the golden tide pelagic seaweeds sargassum fluitans and S. natans (morphotypes I and VIII) to inform valorisation pathways. Science of the Total Environment, 762, 143134. https://doi.org/10.1016/j.scitotenv.2020.143134
- De Farias Silva, C. E., & Bertucco, A. (2016). Bioethanol from microalgae and cyanobacteria: A review and technological outlook. Process Biochemistry, 51(11), 1833–1842. https://doi.org/10.1016/j.procbio.2016.02.016
- Del Río, P. G., Gullón, B., Pérez-Pérez, A., Romaní, A., & Garrote, G. (2021). Microwave hydrothermal processing of the invasive macroalgae sargassum muticum within a green biorefinery scheme. Bioresources Technology, 340, 125733. https://doi.org/10.1016/j.biortech.2021.125733
- Durbha, S. R., Tavva, S. S. M. D., Guntuku, G., Tadimalla, P., Yechuri, V. R., Nittala, S. R., & Muktinutalapati, V. S. R. (2016). Ethanol production from the Biomass of Two Marine Algae, padina tetrastromatica and Sargassum vulgare. Columbia International Publishing American Journal of Biomass and Bioenergy, 5(1), 31–42. https://doi.org/10.7726/ajbb.2016.1003
- Edeh, I. (2020). Bioethanol production: An overview. IntechOpen, 1–24. https://doi.org/10.5772/intechopen.94895
- Ferdeș, M., Dincă, M. N., Moiceanu, G., Zăbavă, B. Ș., & Paraschiv, G. (2020). Microorganisms and enzymes used in the biological pretreatment of the substrate to enhance biogas production: A review. Sustainability, 12(17), 7205. https://doi.org/10.3390/su12177205
- Fischer, J., Lopes, V. S., Cardoso, S. L., Coutinho Filho, U., & Cardoso, V. L. (2017). Machine Learning Techniques Applied to Lignocellulosic Ethanol in Simultaneous Hydrolysis and Fermentation. Brazilian Journal of Chemical Engineering, 34(1), 53–63. https://doi.org/10.1590/0104-6632.20170341s20150475
- Flórez-Fernández, N., Illera, M., Sánchez, M., Lodeiro, P., Dolores Torres, M., López-Mosquera, E. M., de Vicente, M. S., & Domínguez, H. (2021), Integral Valorization of Sargassum muticum in Biorefineries. Chemical Engineering Journal, 404. . https://doi.org/10.1016/j.cej.2020.125635
- Gatdula, K. M., Rex, B., Demafelis, J. L., Movillon, D. E. S., Sanchez, K. A. H., & Richard, V. M. J. (2018). Energetics and environmental assessment of a commercial scale bioethanol processing plant using Sargassum spp. Philippine Journal of Crop Science, PJCS, 10–20.
- Gengiah, K., Rajendran, N., Al-Ghanim, K. A., Govindarajan, M., & Gurunathan, B. (2023). Process and technoeconomic analysis of bioethanol production from residual biomass of marine macroalgae ulva lactuca. Science of the Total Environment, 868, 1–8. https://doi.org/10.1016/j.scitotenv.2023.161661
- Ghadiryanfar, M., Rosentrater, K. A., Keyhani, A., & Omid, M. (2016). A review of macroalgae production, with potential applications in biofuels and bioenergy. Renewable and Sustainable Energy Reviews, 54, 473–481. https://doi.org/10.1016/j.rser.2015.10.022
- Godvin, S. V., Dinesh, K. M., Arulazhagan, P., Amit, K. B., Poornachander, G., & Rajesh, B. (2021). Biofuel production from Macroalgae: Present scenario and future scope. Bioengineered, 12(2), 9216–9238. https://doi.org/10.1080/21655979.2021.1996019
- Gómez, A., Rodrigues, M., Montañés, C., Dopazo, C., & Fueyo, N. (2011). The technical potential of first-generation Biofuels obtained from energy crops in Spain. Biomass and Bioenergy, 35(5), 2143–2155. https://doi.org/10.1016/j.biombioe.2011.02.009
- Gruduls, A., Maurers, R., & Romagnoli, F. (2018). Baltic Sea seaweed biomass pretreatment: Effect of combined CO2 and thermal treatment on biomethane potential. Energy Procedia, 147, 607–613. https://doi.org/10.1016/j.egypro.2018.07.078
- Guo, M., Song, W., & Buhain, J. (2015). Bioenergy and biofuels: History, status, and perspective. Renewable and Sustainable Energy Reviews, 42, 712–725. https://doi.org/10.1016/j.rser.2014.10.013
- Halima, R., & Archna, N. (2023). Nano-Enzymatic Hydrolysis and fermentation of waste starch sources for bioethanol production: An optimization study. Journal of Mines, Metals and Fuels, 71, 439–445. https://doi.org/10.18311/jmmf/2023/33756
- Hasan-Tuaputty. (2020). Yeast concentration, pH, and fermentation time on the production and concentration of bioethanol made from sargassum crassifolium as a renewable energy source. Biosel Biology Science and Education, 9(1), 50. https://doi.org/10.33477/bs.v9i1.1317
- Hasnain, M. S., Jameel, E., Mohanta, B., Dhara, A. K., Alkahtani, S., & Nayak, A. K. (2020). Alginates: Sources, structure, and properties. Alginates in Drug Delivery, 1–17. https://doi.org/10.1016/b978-0-12-817640-5.00001-7
- Hassan, S. S., Williams, G. A., & Jaiswal, A. K. (2018). Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresource Technology, 262, 310–318. https://doi.org/10.1016/j.biortech.2018.04.099
- Hebbale, D., Chandran, M. D. S., Joshi, N. V., & Ramachandra, T. V. (2017). Energy and food security from Macroalgae. Journal of Biodiversity, 8(1), 1–11. https://doi.org/10.1080/09766901.2017.1351511
- Hoang, A. T., & Pham, V. V. (2021). 2-methylfuran (MF) as a potential biofuel: A thorough review on the production pathway from biomass, combustion progress, and application in engines. Renewable and Sustainable Energy Reviews, 148, 111265. https://doi.org/10.1016/j.rser.2021.111265
- Hoang, A. T., Tran, Q. V., Al-Tawaha, A. R. M. S., Pham, V. V., & Nguyen, X. P. (2019). Comparative analysis on performance and emission characteristics of an In-Vietnam popular 4-stroke motorcycle engine running on biogasoline and mineral gasoline. Renewable Energy Focus, 28, 47–55. https://doi.org/10.1016/j.ref.2018.11.001
- Hong, I. K., Jeon, H., & Lee, S. B. (2014). Comparison of red, Brown and green seaweeds on Enzymatic Saccharification Process. Journal of Industrial and Engineering Chemistry, 20(5), 2687–2691. https://doi.org/10.1016/j.jiec.2013.10.056
- Hoppe, F., Burke, U., Thewes, M., Heufer, A., Kremer, F., & Pischinger, S. (2016). Tailor-made fuels from biomass: Potentials of 2-butanone and 2-methylfuran in direct injection spark ignition engines. Fuel, 167, 106–117. https://doi.org/10.1016/j.fuel.2015.11.039
- Jambo, S. A., Abdulla, R., Mohd Azhar, S. H., Marbawi, H., Gansau, J. A., & Ravindra, P. (2016). A review on third generation bioethanol feedstock. Renewable and Sustainable Energy Reviews, 65, 756–769. https://doi.org/10.1016/j.rser.2016.07.064
- Jang, J., Cho, Y., Jeong, G., & Kim, S. (2012). Optimization of Saccharification and ethanol production by simultaneous Saccharification and fermentation (SSF) from seaweed saccharina japonica. Bioprocess Biosystems and Engineering, 35(1–2), 11–18. https://doi.org/10.1007/s00449-011-0611-2
- Jeyakumar, N., Hoang, A. T., Niˇzeti, S., Balasubramanian, D., Kamaraj, S., Pandian, P. L., Sirohi, R., Nguyen, P. Q. P., & Nguyen, X. P. (2022). Experimental investigation on simultaneous production of bioethanol and biodiesel from macro-algae. Fuel, 329, 125362. https://doi.org/10.1016/j.fuel.2022.125362
- Jężak, S., Dzida, M., & Zorębski, M. (2016). High pressure physicochemical properties of 2-methylfuran and 2,5-dimethylfuran – second generation biofuels. Fuel, 184, 334–343. https://doi.org/10.1016/j.fuel.2016.07.025
- Kadimpati, K. K., Thadikamala, S., Devarapalli, K., Banoth, L., & Uppuluri, K. B. (2021). Characterization and Hydrolysis Optimization of Sargassum Cinereum for the fermentative production of 3G bioethanol. Biomass Conversion and Biorefinery, 13(3), 1831–1841. https://doi.org/10.1007/s13399-020-01270-3
- Khan, M. I., Shin, J. H., & Kim, J. D. (2018). The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microbial cell factories, 17(1), 36. https://doi.org/10.1186/s12934-018-0879-x
- Khuong, L. S., Masjuki, H. H., Zulkifli, N. W. M., Niza Mohamad, E., Kalam, M. A., Abdullah, A., Arslan, A., Mosarof, M. H., Syahira, A. Z., & Jamshaid, M. (2017). Effect of gasoline–bioethanol blends on the properties and lubrication characteristics of commercial engine oil. RSC Advances, 7(25), 15005–15019. https://doi.org/10.1039/C7RA00357A
- Kim, S. W., Hong, C.-H., Jeon, S.-W., & Shin, H.-J. (2015). High-yield production of biosugars from Gracilaria verrucosa by acid and enzymatic hydrolysis processes. Bioresource Technology, 196, 634–641. https://doi.org/10.1016/j.biortech.2015.08.016
- Kraan, S. (2010). Mass-cultivation of Carbohydrate Rich Macroalgae, a possible solution for sustainable biofuel production. Mitigation and Adaptation Strategies for Global Change, 18(1), 27–46. https://doi.org/10.1007/s11027-010-9275-5
- Kumar, M. D., Kavitha, S., Tyagi, V. K., Rajkumar, M., Bhatia, S. K., Kumar, G., & Banu, J. R. (2021). Macroalgae-Derived Biohydrogen Production: Biorefinery and Circular Bioeconomy. Biomass Conversion and Biorefinery, 12(3), 769–791. https://doi.org/10.1007/s13399-020-01187-x
- Kumar, P. K., Krishna, S. V., Verma, K., Pooja, K., Bhagawan, D., Srilatha, K., & Himabindu, V. (2018). Bio oil production from microalgae via hydrothermal liquefaction technology under subcritical water conditions. Journal of Microbiological Methods, 153, 108–117. https://doi.org/10.1016/j.mimet.2018.09.014
- Kusuma Wardani, A., & Herrani, R. (2019). Bioethanol from Sargassum sp using acid hydrolysis and fermentation method using microbial association. Journal of Physics. Conference Series, 1241(1), 012008. https://doi.org/10.1088/1742-6596/1241/1/012008
- Lamptey, B. L., Sackey, A. D., & Kpabitey, M. K. (2022). Outlining the causes and effects of algae blooms on Ghana’s West Coast. Journal of Education and Psychological Research, 4(2), 350–360.
- Limayem, A., & Ricke, S. C. (2012). Lignocellulosic Biomass for Bioethanol Production: Current perspectives, potential issues and future prospects. Progress in Energy and Combustion Science, 38(4), 449–467. https://doi.org/10.1016/j.pecs.2012.03.002
- López-Sosa, L. B., Alvarado-Flores, J. J., Corral-Huacuz, J. C., Aguilera-Mandujano, A., Rodríguez-Martínez, R. E., Guevara-Martínez, S. J., Ávalos-Rodríguez, M. L., Morales-Máximo, M., Zárate-Medina, J., Ávalos-Rodríguez, M. L., & Morales-Máximo, M. (2020). A prospective study of the exploitation of pelagic sargassum spp. As a Solid Biofuel Energy source. Applied Sciences, 10(23), 8706. https://doi.org/10.3390/app10238706
- Lugani, Y., Rai, R., Prabhu, A. A., Maan, P., Hans, M., Kumar, V., Kumar, S., Chandel, A. K., & Sengar, R. S. (2020). Recent advances in bioethanol production from Lignocelluloses: A comprehensive review with a Focus on Enzyme Engineering and Designer biocatalysts. Biofuel Research Journal, 7(4), 1267–1295. https://doi.org/10.18331/BRJ2020.7.4.5
- Marinho-Soriano, E., Fonseca, P. C., Carneiro, M. A. A., & Moreira, W. S. C. (2006). Seasonal variation in the chemical composition of two tropical seaweeds. Bioresource Technology, 97(18), 2402–2406. https://doi.org/10.1016/j.biortech.2005.10.014
- Matanjun, P., Mohamed, S., Mustapha, N. M., & Muhammad, K. (2009). Nutrient content of tropical edible seaweeds, eucheuma cottonii, caulerpa lentillifera and Sargassum polycystum. Journal of Applied Phycology, 21(1), 75–80. https://doi.org/10.1007/s10811-008-9326-4
- Milledge, J., & Harvey, P. (2016). Golden tides: Problem or golden opportunity? The valorisation of Sargassum from beach inundations. Journal of Marine Science and Engineering, 4(3), 60. https://doi.org/10.3390/jmse4030060
- Mishra, S., Kumari, N., Singh, V. K., & Sinha, R. P. (2023). Cyanobacterial Biofuel: A platform for Green Energy “. Advances in Environmental and Engineering Research, 4(3), 1–25. https://doi.org/10.21926/aeer.2303041
- Miyuranga, K. V. A., De Silva, D. J., Arachchige, U. S. P. R., Jayasinghe, R. A., & Weerasekara, N. A. (2022). Comparison of the properties of biodiesel-bioethanol-diesel blended fuel. Asian Journal of Chemistry, 34(7), 1809–181. https://doi.org/10.14233/ajchem.2022.23767
- Mohammed, A., Rivers, A., Stuckey, D. C., & Ward, K. (2020). Alginate extraction from sargassum seaweed in the Caribbean Region: Optimization using response surface methodology. Carbohydrate Polymers, 245, 116419. https://doi.org/10.1016/j.carbpol.2020.116419
- Mohapatra, S., Mishra, C., Behera, S. S., & Thatoi, H. (2017). Application of pretreatment, fermentation and molecular techniques for enhancing bioethanol production from Grass Biomass – a review. Renewable and Sustainable Energy Reviews, 78, 1007–1032. https://doi.org/10.1016/j.rser.2017.05.026
- Mohd Azhar, S. H., Abdulla, R., Jambo, S. A., Marbawi, H., Gansau, J. A., Mohd Faik, A. A., & Rodrigues, K. F. (2017). Yeasts in sustainable bioethanol production: A review. Biochemistry and Biophysics Reports, 10, 52–61. https://doi.org/10.1016/j.bbrep.2017.03.003
- Monroy-Velázquez, L. V., Rodríguez-Martínez, R. E., van Tussenbroek, B. I., Aguiar, T., Solís-Weiss, V., & Briones-Fourzán, P. (2019). Motile macrofauna Associated with Pelagic Sargassum in a Mexican Reef Lagoon. Journal of Environmental Management, 252, 109650. https://doi.org/10.1016/j.jenvman.2019.109650
- Naik, S. N., Goud, V. V., Rout, P. K., & Dalai, A. K. (2010). Production of first and second generation biofuels: A comprehensive review. Renewable and Sustainable Energy Reviews, 14(2), 578–597. https://doi.org/10.1016/j.rser.2009.10.003
- Nayak, A. K., Ahmed, S. A., Tabish, M., & Hasnain, M. S. (2019). Natural polysaccharides in tissue engineering applications. Natural Polysaccharides in Drug Delivery and Biomedical Applications, 531–548. https://doi.org/10.1016/b978-0-12-817055-7.00023-6
- Nigam, P. S., & Singh, A. (2011). Production of Liquid Biofuels from Renewable Resources. Progress in Energy and Combustion Science, 37(1), 52–68. https://doi.org/10.1016/j.pecs.2010.01.003
- Niphadkar, S., Bagade, P., & Ahmed, S. (2017). Bioethanol production: Insight into past, present and future perspectives. Biofuels, 9(2), 229–238. https://doi.org/10.1080/17597269.2017.1334338
- Nitsche, M., & Gbadamosi, R. (2017). Extractive and azeotropic distillation. Practical Column Design Guide, 153–164. https://doi.org/10.1007/978-3-319-51688-2_5
- Nomura, M., Kamogawa, H., Susanto, E., Kawagoe, C., Yasui, H., Saga, N., Hosokawa, M., & Miyashita, K. (2012). Seasonal variations of total lipids, fatty acid composition, and fucoxanthin contents of sargassum horneri (Turner) and cystoseira hakodatensis (Yendo) from the Northern Seashore of Japan. Journal of Applied Phycology, 25(4), 1159–1169. https://doi.org/10.1007/s10811-012-9934-x
- Offei, F., Mensah, M., & Kemausuor, F. (2019). Cellulase and acid-catalysed hydrolysis of Ulva Fasciata, Hydropuntia Dentata and sargassum vulgare for bioethanol production. Applied Sciences, 1(11). https://doi.org/10.1007/s42452-019-1501-5
- Offei, F., Mensah, M., Thygesen, A., & Kemausuor, F. (2018). Seaweed bioethanol production: A process selection review on hydrolysis and fermentation. Fermentation, 4(4), 1–18. https://doi.org/10.3390/fermentation4040099
- Oliveira, J. V., Alves, M. M., & Costa, J. C. (2015). Optimization of biogas production from sargassum sp. Using a design of experiments to assess the codigestion with glycerol and waste frying oil. Bioresources Technology, 175, 480–485. https://doi.org/10.1016/j.biortech.2014.10.121
- Oo, N. M. M., & Kywe, T. T. (2019). Preparation of bioethanol from brown seaweed (sargassum sp.). International Journal of Trend in Scientific Research and Development, 3, 2273–2278. https://doi.org/10.31142/ijtsrd28011
- Orozco-González, J. G., Amador-Castro, F., Gordillo-Sierra, A. R., García-Cayuela, T., Alper, H. S., & Carrillo-Nieves, D. (2022). Opportunities surrounding the use of sargassum biomass as precursor of biogas, bioethanol, and biodiesel production. Frontiers in Marine Science, 8, 1–11. https://doi.org/10.3389/fmars.2021.791054
- Owusu, W. A., Marfo, S. A., & Andiappan, V. (2023). Artificial intelligence application in bioethanol production. International Journal of Energy Research, 2023, 1–8. https://doi.org/10.1155/2023/7844835
- Oyesiku, O., & Egunyomi, A. (2014). Identification and chemical studies of pelagic masses of sargassum natans (Linnaeus) Gaillon and S. Fluitans (Borgessen) Borgesen (Brown Algae), found offshore in Ondo State Nigeria. African Journal of Biotechnology, 13(10), 1188–1193. https://doi.org/10.5897/AJB2013.12335
- Pangestuti, R., & Kim, S.-K. (2015). Seaweed proteins, peptides, and amino acids. Seaweed Sustainability, 125–140. https://doi.org/10.1016/b978-0-12-418697-2.00006-4
- Pelizan, L., Lickteig, L., & Rahnema, A. (2019). Biofuel production in Ghana: Exploring the opportunity. IESE OP-320-E, 1–28. https://doi.org/10.15581/018
- Peng, L., Fu, D., Chu, H., Wang, Z., & Qi, H. (2019). Biofuel Production from Microalgae: A Review. Environmental Chemistry Letters, 18(2), 285–297. https://doi.org/10.1007/s10311-019-00939-0
- Qian, Y., Zhu, L., Wang, Y., & Lu, X. (2015). Recent progress in the development of biofuel 2,5-dimethylfuran. Renewable and Sustainable Energy Reviews, 41, 633–646. https://doi.org/10.1016/j.rser.2014.08.085
- Rachmayanti, A., Putri, A. M. S., & Fadli, A. I. (2019). Separate saccharification and Fermentation for Bioethanol Production from raw seaweed sargassum sp. Marinade, 2(01), 19–28. https://doi.org/10.31629/marinade.v2i01.1253
- Raheem, A., Wan Azlina, W. A. K. G., Taufiq Yap, Y. H., Danquah, M. K., & Harun, R. (2015). Thermochemical Conversion of Microalgal Biomass for Biofuel Production. Renewable and Sustainable Energy Reviews, 49, 990–999. https://doi.org/10.1016/j.rser.2015.04.186
- Rajkumar, R., Yaakob, Z., & Takriff, M. S. (2013). Potential of micro and macro algae for biofuel production: A brief review. BioResources, 9(1). https://doi.org/10.15376/biores.9.1.1606-1633
- Ramachandra, T. V., & Hebbale, D. (2020). Bioethanol from Macroalgae: Prospects and challenges. Renewable and Sustainable Energy Reviews, 117, 109479. https://doi.org/10.1016/j.rser.2019.109479
- Rastogi, M., & Shrivastava, S.(2018). Current methodologies and advances in bioethanol production. Journal of Biotechnology & Bioresearch, 1, 1–8.
- Ravanal, M. C., Camus, C., Buschmann, A. H., Gimpel, J., Olivera-Nappa, Á., Salazar, O., & Lienqueo, M. E. (2019). Production of bioethanol from brown algae. Advances in Feedstock Conversion Technologies for Alternative Fuels and Bioproducts, 69–88. r. https://doi.org/10.1016/b978-0-12-817937-6.00004-7
- Ravanal, M. C., Pezoa-Conte, R., von Schoultz, S., Hemming, J., Salazar, O., Anugwom, I., Jogunola, O., Mäki-Arvela, P., Willför, S., Mikkola, J. P., & Lienqueo, M. E.(2016). Comparison of different types of pretreatment and Enzymatic Saccharification of Macrocystis pyrifera for the Production of Biofuel. Algal Research, 13, 141–147.
- Ren, R., Han, X., Zhang, H., Lin, H., Zhao, J., Zheng, Y., & Wang, H. (2018). High yield bio-oil production by hydrothermal liquefaction of a hydrocarbon-rich microalgae and biocrude upgrading. Carbon Resources Conversion, 1(2), 153–159. https://doi.org/10.1016/j.crcon.2018.07.008
- Ritslaid, K., Kuut, A., & Olt, J. (2010). State of the art in bioethanol production. Agronomy Research, 8(1), 236–254.
- Rodrigues, D., Freitas, A. C., Pereira, L., Rocha-Santos, T. A. P., Vasconcelos, M. W., Roriz, M., Gomes, A. M. P., & Duarte, A. C. (2015). Chemical Composition of Red, Brown and green macroalgae from Buarcos Bay in Central West Coast of Portugal. Food Chemistry, 183, 197–207. https://doi.org/10.1016/j.foodchem.2015.03.057
- Saini, R. K., & Keum, Y.-S. (2018). Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance — a review. Life Sciences, 203, 255–267. https://doi.org/10.1016/j.lfs.2018.04.049
- Sakharkar, P. A. (2018). Treatment of Food Waste for Bioethanol Production using pervaporation separation technology. International Journal for Research in Applied Science and Engineering Technology, 6(1), 2568–2572. https://doi.org/10.22214/ijraset.2018.1353
- Sambusiti, C., Bellucci, M., Zabaniotou, A., Beneduce, L., & Monlau, F. (2015). Algae as promising feedstocks for fermentative biohydrogen production according to a biorefinery approach: A comprehensive review. Renewable and Sustainable Energy Reviews, 44, 20–36. https://doi.org/10.1016/j.rser.2014.12.013
- Sankaran, R., Andres Parra Cruz, R., Pakalapati, H., Loke Show, P., Chuan Ling, T., Wei-Hsin, C., & Ao, Y. (2019). Recent Advances in the Pretreatment of Microalgal and Lignocellulosic Biomass: A Comprehensive Review. Bioresource Technology, 298, 122476. https://doi.org/10.1016/j.biortech.2019.122476
- Saravanan, K., Duraisamy, S., Ramasamy, G., Kumarasamy, A., & Balakrishnan, S. (2018). Evaluation of the saccharification and fermentation process of two different seaweeds for an ecofriendly bioethanol production. Biocatalysis and Agricultural Biotechnology, 14, 444–449. https://doi.org/10.1016/j.bcab.2018.03.017
- Schenk, P., Thomas-Hall, S., Stephens, E., Marx, U., Mussgnug, J., & Posten, C. (2008). Second generation biofuels: High efficiency microalgae for biodiesel production. BioEnergy Research, 1(1), 20–43. https://doi.org/10.1007/s12155-008-9008-8
- Sebayang, A. H., Masjuki, H. H., Ong, H. C., Dharmaa, S., Silitongaa, A. S., Kusumoa, F., & Milano, J. (2017). Optimization of bioethanol production from sorghum grains using artificial neural networks integrated with ant colony. Industrial Crops and Products, 97, 146–155. https://doi.org/10.1016/j.indcrop.2016.11.064
- Segbefia, A. Y., Barnes, V. R., Akpalu, L. A., & Mensah, M. (2018). Environmental Location Assessment for Seaweed Cultivation in Ghana. International Journal of Applied Geospatial Research, 9(1), 51–64. https://doi.org/10.4018/ijagr.2018010104
- Senatore, A., Dalena, F., & Basile, A. (2019). Novel bioethanol production processes and purification technology using membranes. Catalysis, Green Chemistry and Sustainable Energy, 359–384. https://doi.org/10.1016/b978-0-444-64337-7.00019-7
- Shuping, Z., Yulong, W., Mingde, Y., Kaleem, I., Chun, L., & Tong, J. (2010). Production and characterization of bio-oil from hydrothermal liquefaction of microalgae dunaliella tertiolecta cake. Energy, 35(12), 5406–5411. https://doi.org/10.1016/j.energy.2010.07.013
- Silalertruksa, T., Gheewala, S. H., Hünecke, K., & Fritsche, U. R. (2012). Biofuels and employment effects: Implications for Socioeconomic Development in Thailand. Biomass & bioenergy, 46, 409–418. https://doi.org/10.1016/j.biombioe.2012.07.019
- Singh, A., & Olsen, S. I. (2011). A critical review of biochemical conversion, sustainability and life cycle assessment of algal biofuels. Applied Energy, 88(10), 3548–3555. https://doi.org/10.1016/j.apenergy.2010.12.012
- Sirajunnisa, A. R., & Surendhiran, D. (2016). Algae – a quintessential and positive resource of bioethanol production: A comprehensive review. Renewable and Sustainable Energy Reviews, 66, 248–267. https://doi.org/10.1016/j.rser.2016.07.024
- Soliman, R. M., Younis, S. A., El-Gendy, N. S., Mostafa, S. S. M., El-Temtamy, S. A., & Hashim, A. I. (2018). Batch bioethanol production via the biological and chemical saccharification of some Egyptian Marine macroalgae. Journal of Applied Microbiology, 125(2), 422–440. https://doi.org/10.1111/jam.13886
- Stiger-Pouvreau, V., Bourgougnon, N., & Deslandes, E. (2016). Carbohydrates from seaweeds. Seaweed in Health and Disease Prevention, 223–274. https://doi.org/10.1016/b978-0-12-802772-1.00008-7
- Surendhiran, D., & Sirajunnisa, A. R. (2019). Role of genetic engineering in bioethanol production from algae. Bioethanol Production from Food Crops, 361–381. https://doi.org/10.1016/b978-0-12-813766-6.00018-7
- Tamayo, J. P., & Del Rosario, E. J. (2014). Chemical Analysis and utilisation of sargassum sp. As substrate for ethanol production. Iranica Journal of Energy & Environment, 5(2), 202–208. https://doi.org/10.5829/idosi.ijee.2014.05.02.12
- Tavva, S. S. M. D., Deshpande, A., Durbha, S. R., Palakollu, V. A. R., Goparaju, A., Yechuri, U. R. V., & Muktinutalapati, V. S. R.(2016). Bioethanol production through separate hydrolysis and fermentation of parthenium hysterophorus biomass. Renewable Energy, 86, 1317–1323. https://doi.org/10.1016/j.renene.2015.09.074
- Thompson, T. M., Young, B. R., & Baroutian, S. (2020). Pelagic sargassum for Energy and fertiliser production in the Caribbean: A case study on Barbados. Renewable and Sustainable Energy Reviews, 118, 109564. https://doi.org/10.1016/j.rser.2019.109564
- Valencia, J. M. T., Demafelis, R. B., Borines, M. G., & Gatdula, K. M. (2015). Bioethanol potential of brown macroalgae sargassum spp. Philippine Journal of Crop Science, 40, 1–11.
- van der Plank, S., Appeaning, K. A., Jayson-Quashigah, P.-N., & Sowah, W. N. A. (2023). Ghana’s fishing industry has a golden seaweed problem-how citizen science can help. Retrieved May 23, 2023. Www.Theconversation.com
- Vohra, M., Manwar, J., Manmode, R., Padgilwar, S., & Patil, S.(2014). Bioethanol production: Feedstock and Current technologies. Journal of Environmental Chemical Engineering, 2, 573–584. https://doi.org/10.1016/j.jece.2013.10.013
- Wei, N., Quarterman, J., & Jin, Y.-S. (2013). Marine macroalgae: An untapped resource for producing fuels and chemicals. Trends in Biotechnology, 31(2), 70–77. https://doi.org/10.1016/j.tibtech.2012.10.009
- Widyaningrum, T., Prastowo, I., Parahadi, M., & Prasetyo, A. D. (2016). Production of Bioethanol from the hydrolysate of brown seaweed (sargassum crassifolium) using a naturally β-glucosidase producing yeast saccharomyces cereviceae JCM 3012. Biosciences, Biotechnology Research Asia, 13(3), 1333–1340. https://doi.org/10.13005/bbra/2274
- Wu, C., Jiang, P., Guo, Y., Liu, J., Zhao, J., & Fu, H. (2017). Isolation and characterization of ulva prolifera Actin1 gene and function verification of the 5′ flanking region as a strong promoter. Bioengineered, 9(1), 124–133. https://doi.org/10.1080/21655979.2017.1325041
- Yazdani, P., Zamani, A., Karimi, K., & Taherzadeh, M. J. (2015). Characterization of nizimuddinia zanardini Macroalgae Biomass Composition and its potential for biofuel production. Bioresource Technology, 176, 196–202. https://doi.org/10.1016/j.biortech.2014.10.141
- Yeon, J. H., Lee, S. E., Choi, W. Y., Kang, D. H., Lee, H. Y., & Jung, K. H. (2011). Repeated-Batch Operation of Surface-Aerated Fermenter for bioethanol production from the hydrolysate of seaweed sargassum sagamianum. Journal of Microbiology and Biotechnology, 21(3), 323–331. https://doi.org/10.4014/jmb.1010.10057
- Yuhendra, A. P., Farghali, M., Mohamed, I. M. A., Iwasaki, M., Tangtaweewipat, S., Ihara, I., Sakai, R., & Umetsu, K. (2021). Potential of biogas production from the anaerobic digestion of sargassum fulvellum macroalgae: Influences of mechanical, Chemical, and biological pretreatments. Biochemical Engineering Journal, 175, 108140. https://doi.org/10.1016/j.bej.2021.108140
- Zabed, H. M., Akter, S., Yun, J., Zhang, G., Awad, F. N., Qi, X., & Sahu, J. N. (2019). Recent Advances in Biological Pretreatment of Microalgae and Lignocellulosic Biomass for Biofuel Production. Renewable and Sustainable Energy Reviews, 105, 105–128. https://doi.org/10.1016/j.rser.2019.01.048
- Zabed, H., Sahu, J. N., Suely, A., Boyce, A. N., & Faruq, G. (2017). Bioethanol production from renewable sources: Current perspectives and technological progress. Renewable and Sustainable Energy Reviews, 71, 475–501. https://doi.org/10.1016/j.rser.2016.12.076
- Zeng, G., You, H., Wang, K., Jiang, Y., Bao, H., Du, M., Chen, B., Ai, N., & Gu, Z. (2019). Semi‑simultaneous saccharification and Fermentation of Ethanol Production from Sargassum horneri and biosorbent Production from Fermentation residues. Waste and Biomass Valorization, 11(9), 4743–4755. https://doi.org/10.1007/s12649-019-00748-0
- Zhao, Y., Bourgougnon, N., Lanoisellé, J.-L., & Lendormi, T. (2022). Biofuel production from seaweeds: A comprehensive review. Energies, 15(24), 9395. https://doi.org/10.3390/en15249395