Abstract
Objective: Garlic can help humans ensure healthy lives and promote well-being for all at all ages by sufficient zinc and magnesium intake.Method: Serpentine treated it by microwaving and sintering to enhance its crystallinity as well as its magnesium and zinc ion release rates. Furthermore, an enriched garlic enzyme extract had an approximately 8-fold increase in alliinase activity. Results: Strong bonding was observed for the microwaved and sintered powders, but also facilitated zinc ion reactions and reduced lattice defects. Accordingly, used for the garlic growth and enzyme experiments.Conclusions: (1) The sintered powder excellent magnesium and zinc ion release capability. (2) The enriched garlic enzymes had high alliinase activity, likely increasing the health benefits of the garlic.
Introduction
Types and applications of garlic vinegar
Garlic vinegar is a seasoned additive with garlic as its main ingredient. It has a distinctive flavor and numerous health benefits. Several common types of garlic vinegar are as follows (Citation1):
Conventional garlic vinegar: This is the most prevalent type of garlic vinegar. It is produced by chopping fresh garlic and fermenting it in vinegar. The end product has a rich garlic taste with a slight acidity. It is used as a condiment.
Garlic honey vinegar: Produced by fermenting a mixture of garlic and honey, this vinegar has both the spiciness of garlic and the sweetness of honey. It has a milder flavor and is often used to make salad dressings or flavored sauces.
Chili garlic vinegar: This vinegar is produced by adding chili peppers to conventional garlic vinegar. It is used as a seasoning or in the preparation of chili sauces.
Citrus garlic vinegar: This vinegar is produced by fermenting garlic mixed with citrus fruit (e.g., lemons or oranges); it features both garlic flavors and a tangy sweetness.
Types and characteristics of enzymes in garlic
Garlic contains various enzymes. Alliinase is one of the main active enzymes in garlic (Citation2). Upon cutting or crushing garlic, alliinase is activated, converting alliin into other bioactive compounds, such as diallyl disulfide, also known as allicin; it has a potent odor and antioxidant properties.
In addition to alliinase and the allicin produced from enzymatic degradation, garlic also contains other key enzymes:
Esterase: This enzyme can break down the fatty esters in garlic, producing volatile thiol compounds, which further intensify the garlic aroma.
Oxidase: Oxidases can catalyze certain chemical reactions. For example, they can transform allicin into other sulfur-containing compounds, enhancing the garlic flavor.
The enzymatic activity of garlic is the reason for its bioactivity and health benefits, such as its antibacterial, antioxidant, and anti-inflammatory properties.
Differences between garlic vinegar and garlic enzyme extract
Garlic vinegar and garlic enzyme are two distinct products derived from garlic. Their differences are as follows (Citation1, Citation2):
Composition: Garlic vinegar is a liquid mixture of garlic and vinegar, whereas garlic enzyme extract refers to enzymatic substances extracted from garlic.
Production process: Garlic vinegar is produced by chopping garlic, soaking it in vinegar and, typically, fermenting it for a specific time. By contrast, garlic enzyme extracts are produced by activating garlic components through methods such as fermentation or enzyme extraction, followed by filtering and purification to obtain the enzyme product.
Applications: Garlic vinegar is primarily used as a seasoning and food additive and is commonly used in cooking, for flavor, and to prepare sauces. By contrast, garlic enzyme extract is typically an ingredient in health foods and is ascribed certain health benefits, such as promoting digestion and enhancing immune function.
Efficacy: Garlic vinegar, which comprises a combination of garlic and vinegar, inherits the respective benefits of both components. Garlic possesses antibacterial properties and aids in reducing lipid levels, whereas vinegar promotes digestion, reduces blood sugar, and softens blood vessels. Garlic enzymes extract primarily comprise enzymes found in garlic and are known to offer health benefits (Citation2).
Effects of garlic enzymes on human health
The primary impacts of garlic enzymes on the human body are as follows (Citation3, Citation4):
Antimicrobial properties: Garlic enzymes have considerable antimicrobial properties; they are effective against various bacteria, viruses, and fungal infections. Specifically, they can interfere with bacterial growth and reproduction, thereby aiding in the prevention of infectious diseases.
Cardiovascular health: Garlic enzymes can enhance blood circulation and reduce cholesterol and triglycerides in the blood, the risk of cardiovascular diseases, blood pressure, and thrombosis risk.
Antioxidant properties: Garlic enzymes are rich in antioxidants, such as sulfur compounds and polyphenols. These antioxidants neutralize free radicals, thereby protecting cells from damage and aging.
Immune regulation: Garlic enzymes can enhance immune system function, immune cell activity, and antibody production, thereby increasing the body’s resistance to infectious diseases (Citation4).
Anti-inflammatory properties: Garlic enzymes exhibit anti-inflammatory effects; they both alleviate inflammatory responses and promote wound healing (Citation4).
Production of garlic enzyme extract
Garlic enzyme extract can be produced as follows (Citation5):
Ingredients: Fresh garlic and chlorine-free water.
Cleaning: Break the garlic bulb and wash the garlic cloves thoroughly.
Crushing: Crush or chop the garlic cloves to release alliinase.
Mixing: Place the crushed garlic in a container and cover it with chlorine-free water.
Fermentation: Allow the garlic to ferment in a container in a cool place for 1–2 weeks.
Stirring: Stir the fermenting garlic mixture daily.
Filtering and storage: Once fermentation is complete, filter out the remaining solids to obtain the garlic enzyme solution.
Effects of magnesium on human health
Bone health: Magnesium facilitates calcium absorption and fixation and maintains bone structure and density, thereby preventing osteoporosis and fractures (Citation6).
Cardiovascular health: Magnesium helps regulate the contraction and relaxation of heart muscles and thus aids in maintaining a regular heartbeat, stable blood pressure, and overall cardiovascular health.
Nervous system functioning: Magnesium plays a role in neural transmission, ensuring the accurate relay of nerve impulses and supporting normal neuromuscular function and coordination (i.e., preventing muscle spasms and tension).
Blood glucose control: Magnesium helps regulate the release and use of insulin and stabilize blood glucose levels.
Energy metabolism: Magnesium is involved in the body’s energy metabolism processes, including the metabolism of carbohydrates, fats, and proteins; hence, it assists in providing the energy required for bodily functions.
Immune function: Magnesium participates in cell-mediated immunity and antibody production, enhancing the activity of immune cells and aiding in resisting diseases and infections.
Effects of zinc and human health
Zinc has numerous positive effects on human health, as follows (Citation7):
Immune system: Zinc supports the development and function of immune cells. It aids in combating viruses, bacteria, and other pathogens and can promote the growth and activity of white blood cells.
Cellular growth and repair: Zinc is involved in protein synthesis and is vital for cell proliferation, repair, and regeneration. It facilitates wound healing and tissue repair, maintaining the health of the skin, bones, and internal organs.
Antioxidant properties: Zinc possesses antioxidant properties, neutralizing free radicals and reducing cellular oxidation. This contributes to cellular health and delaying aging. Zinc is also a component of certain antioxidant enzymes.
Hormonal regulation: Zinc participates in the synthesis and regulation of various hormones, including testosterone and other sex hormones, insulin, and thyroid hormones. It has numerous benefits for the development of the gonads.
Magnesium and zinc requirements
Magnesium is an essential nutrient of the human body, participating in more than 300 chemical reactions, including protein production, bone synthesis, and DNA replication (Citation6–8). The Health Promotion Administration of the Taiwan Ministry of Health and Welfare recommends that adults consume approximately 400 mg of magnesium daily (Citation7). The body requires a substantial amount of magnesium for protein digestion and vitamin D metabolism.
Magnesium deficiency can lead to several symptoms, including (1) muscle twitching, cramping, or spasms; (2) feelings of fatigue, irritability, and depression; (3) frequent headaches and body aches; and (4) premature aging (magnesium deficiency is an initial sign of aging, and the risk of deficiency increases with age) (Citation8). Notably, calcium and magnesium have mutually antagonistic effects. Many people prioritize calcium intake, but excessive calcium can deplete magnesium levels, leading to health complications such as kidney stones, cardiovascular diseases, and cancer.
Zinc is an essential trace element that is necessary for the human body. It assists in maintaining metabolic regularity and plays a key role in cell replication, tissue repair, and regeneration. The human body can neither produce nor store zinc naturally; zinc must be obtained through food or supplements to meet daily needs. Mild to moderate zinc deficiencies are relatively common. Most people obtain zinc from red meat, chicken, grains, and nuts. However, the phytic acid in these foods can bind with zinc and hinder absorption. Although magnesium tablets, magnesium effervescent tablets, zinc sulfate, and zinc gluconate supplements are commercially available, many of these supplements are chemically synthesized, which could lead to limited zinc absorption.
If the magnesium content garlic is increased, it forms magnesium sulfide compounds. These stabilize allicin; it is less susceptible to decomposition or release. Furthermore, the zinc content in garlic is low (approximately 0.88 mg of zinc per 100 g of garlic, intake of zinc per day is 12–15 mg). Providing adequate zinc promotes the development of garlic roots and the growth of the plant and garlic cloves. Zinc can also enhance the antioxidant properties of garlic, which facilitates subsequent fermentation processes. Accordingly, providing magnesium and zinc during growth can improve the chemical composition of garlic, stabilizing the fermentation reaction and resulting in increased synthesis of alliinase, allicin, and magnesium and zinc enzymes (Citation9).
Characteristics of natural serpentinite
Serpentinite is a type of metamorphic rock that comprises hydrated magnesium-rich silicate minerals. The main characteristics of natural serpentine are as follows (Citation10, Citation11):
Surface appearance: Serpentinite is deep green in color, has snakeskin patterns on its surface, and has a hard texture with a sheen. It is widely used in construction materials.
Chemical composition: It contains minerals such as plagioclase, hornblende, and olivine as well as compounds such as silicon dioxide and iron oxide.
Material characteristics: It contains some metallic elements and is therefore conductive. When immersed in water, it can release magnesium ions; hence, it is suitable for growing crops rich in magnesium minerals. In Japan, serpentine rice has been cultivated, and this rice has been used to produce sake.
Overview of the garlic cultivation experiment
Magnesium-rich serpentine powder was immersed in food-grade zinc citrate solution and sintered to yield serpentine powder containing magnesium and zinc (Citation12).
The magnesium–zinc serpentine powder was mixed into soil, and Chinese red garlic was cultivated in the soil. The characteristics of the resulting magnesium- and zinc-rich garlic (i.e., the enriched garlic) were then examined.
An extraction from the fresh garlic was prepared to analyze the magnesium and zinc content of the enriched garlic as well as its other chemical components.
The shelf life of the enriched garlic was explored.
Analysis was performed on the allicin, sulfides, magnesium, and zinc contents of the enriched garlic after drying.
Upon the completion of the fermentation process, analysis was performed on the composition of the fermented solution, including alliinase, allicin, magnesium mineral, and zinc enzyme contents.
According to the study results, novel garlic enzyme extraction and application techniques were proposed.
Experimental procedures and methods
Powder experiment and analysis
This experiment involved two stages. In stage 1, the serpentine powder was first prepared and then analyzed. To prepare the powder, raw serpentine ore was cleaned in an ultrasonic cleaner, dried, and then crushed. The crushed material was then ground into powder with grains of 10–200 µm diameter by using a ball mill. Next, 10 g of this powder was mixed with 100 mL of 60 °C distilled water and allowed to stand for 60 minutes. Subsequently, the magnesium ion release rate was measured; this value can be used to determine the specific surface area of the raw powder. A room-temperature zinc citrate solution was produced by adding 5 g of zinc citrate to 100 mL distilled water, and 10 g of the serpentine powder was added to this solution. After 60 minutes, the powder was removed from the solution and underwent a zinc-enrichment experiment, which involved heating the powder through a microwave at 1000 W twice for 5 minutes each (a total of 10 minutes). The produced zinc-enriched powder was then sintered in a furnace at 400 °C for 60 minutes to crystallize it. After sintering, the powder was mixed with 60 °C distilled water and left to stand for 60 minutes. Subsequently, the magnesium and zinc ion release rates were measured, and a magnesium–zinc ion solution was obtained (Citation13).
In stage 2, the powder was examined to discern its composition using scanning electron microscopy–energy dispersive spectroscopy (SEM-EDS; HITACHI SU-7000). Additionally, the phase structure was analyzed using X-ray (XRD, Bruker AXS GmbH) diffraction (Cu-Kα radiation at 30 Kv, 20 mA, 3°(2θ)/min), and its crystallinity was calculated using Origin 9.0 software. The bond characteristics of the powder were measured using Fourier-transform infrared spectroscopy (FTIR), and the structures of the powder compounds were evaluated through Raman spectroscopy. The concentrations of ions released from the powder were calculated, in addition to determining the surface areas of the powder by performing inductively coupled plasma (ICP) spectroscopy. To verify that both zinc enrichment and zinc crystallization had occurred, a photoluminescence (PL) (Citation14) analysis was conducted. Comparing the spectra revealed the structural differences (lattice defects) in the powder; this information can be used for powder surface modification.
Ion release experiment
An ion release experiment was performed to measure the ability of the powder to release ions in a liquid medium. Such experiments are typically used to evaluate the release performance of materials, drug release systems, and biomaterials (Citation15, Citation16). In the ion release experiment, 5 g of powder was immersed in 100 mL of distilled water at 60 °C and let stand for 1 hour, producing a magnesium–zinc solution (used filter paper for secondary filtration). Solution extraction was then performed to determine the concentrations of magnesium and zinc ions by using ICP. In the subsequent experiment, the experiment group (enriched garlic) and control group (unenriched garlic) comprised garlic samples obtained from cultivation through the prepared magnesium–zinc solution and distilled water, respectively.
Garlic cultivation experiment and composition analysis
Garlic was cultivated either using the magnesium–zinc solution or distilled water. The magnesium–zinc solution was prepared by soaking 5 g of the enriched powder in 100 mL of distilled water for 1 hour at 60 °C; this was then diluted to a volume of 1000 mL to obtain a magnesium- and zinc-rich stock solution prepared without using synthesized substances. For each group, 25 garlic shoots were planted approximately 10 cm apart in potting soil with a soil depth of approximately 5 cm. The garlic was watered every other day with either the magnesium–zinc solution (experimental group) or distilled water (control group) in a measured amount sufficient to maintain a moisture content >50%. The garlic was grown at ambient temperature and under partial sunlight for 10 weeks. Except for the use of the magnesium–zinc solution or distilled water as the cultivation solution, the remaining cultivation conditions were identical for the experimental and control groups.
After harvesting, the composition of the garlic was analyzed. Some of the fresh garlic (referred to as “wet garlic”) was air-dried for 7 days (referred to as “dry garlic”). The allicin and sulfide content per unit weight were determined for (1) enriched wet garlic, (2) unenriched wet garlic, (3) enriched dry garlic, and (4) unenriched dry garlic (Citation17).
Garlic enzyme extract production and analysis
Enzyme extract was produced from cloves of the enriched and unenriched wet garlic. The garlic enzyme extract for each group was made by mixing 100 g of minced garlic cloves from with 300 mL of distilled water and placing the mixtures in containers. The mixture was stored in a cool, shaded area and stirred daily. After fermenting for 14 days, the mixture was filtered to remove the solid residues. Accordingly, enriched and unenriched garlic enzyme solutions were obtained.
Enzyme activity (units/100 mL) and enzyme concentration were determined using the spectrophotometric method and liquid chromatography, respectively. The activity and concentration of alliinase, esterase, and oxidase enzymes were measured in gelatin digesting units (GDU) and GDU/g, respectively (Citation18).
Experimental results and discussion
Characteristics of silicate powder and the zinc-enrichment mechanism
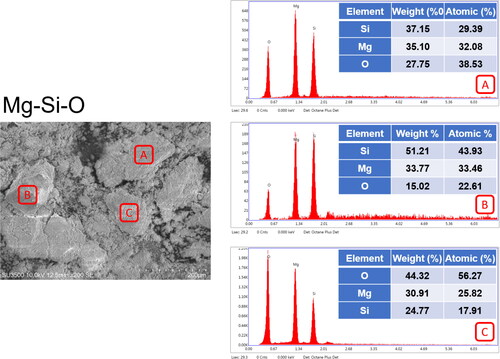
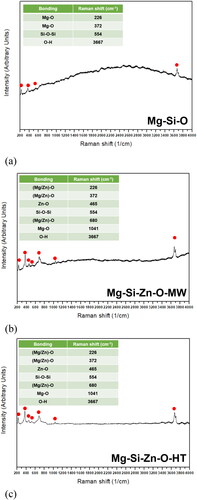
Serpentine was acquired from Hualien, Taiwan. The rocks were crushed and then milled using a grinding machine to obtain a lime-green serpentine powder (). The raw serpentine powder was characterized and its composition was analyzed with SEM and EDX (). The results revealed that the rock powder had a fragmented granular morphology with a mean particle size of 10–200 μm. A semiquantitative EDX analysis at multiple points on the particle surface confirmed that the main components of the raw mineral powder were Mg (30.92–35.10 wt.%), Si (24.77–51.21 wt.%), and O (15.02–44.32 wt.%). This powder was either used raw (raw powder: Mg-Si-O) or enriched with zinc. The enriched powder was either microwaved (microwaved powder: Mg-Si-Zn-O-MW) or microwaved and sintered (sintered powder: Mg-Si-Zn-O-HT).
Natural serpentine rock primarily comprises magnesium silicate; thus, it tends to release magnesium ions but not zinc ions. To further enrich serpentine rock with zinc, 10 g of the raw natural serpentine powder was soaked for 60 minutes in a room-temperature zinc citrate solution produced by adding 5 g of zinc citrate to 100 mL of distilled water. The enriched serpentine powder was then removed from the solution and underwent microwaving because sintering may cause the ions to combust, resulting in ion runaway. This can be avoided by enriching the powder by subjecting it to microwaves to cause the zinc ions to nucleate and grow. Accordingly, the powder was microwaved at 1000 W in 2 sessions of 5 minutes each (a total of 10 minutes). Both the microwaved and sintered powders were sintered in a furnace at 400 °C for 60 minutes to stabilize and crystallize the zinc. Previous experiments and the literature (Citation6) suggest that sintering at 400–600 °C is optimal for enhancing the properties of a powder. At temperatures higher than 600 °C, the structure of the powder tends to collapse, affecting the ion release rate.
presents the surface characterizations of the raw, microwaved, and sintered powders. The surface of the sintered powder exhibited a zinc-rich crystalline lattice. In a previous study, such structures were not observed in sintered powder that did not undergo microwaving (Citation6). This result indicates that the microwave heating did facilitate zinc ion nucleation on the powder surface. Moreover, the subsequent heat treatment served 3 main purposes: (1) promoting zinc crystallization, (2) removing impurities in the mineral rock, and (3) stabilizing the structural properties of the serpentine powder (maintaining interatomic layer distances).
Figure 3. Morphological characteristics of the powders: (a) Mg-Si-O, (b) Mg-Si-Zn-O-MW, (c) Mg-Si-Zn-O-HT.
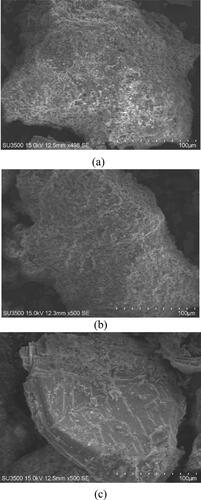
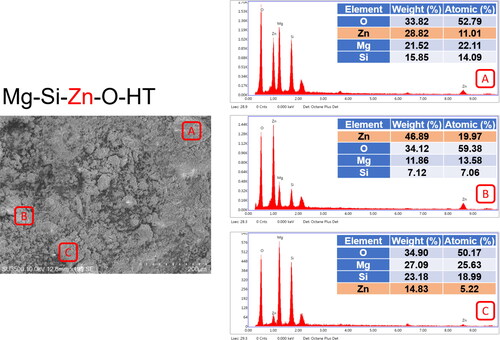
presents the EDX results for the sintered powder. The composition of the powder surface included magnesium, silicon, zinc, and oxygen; the zinc content was approximately 14.83–46.89 wt.%. This suggests that the powder effectively adsorbed zinc ions, which then migrated to the silicate lattice surface. The oxygen content of the enriched powder (33.82–34.90 wt.%) was not substantially different from that of the raw powder (). However, both the magnesium and silicate content were both lower, suggesting that the enrichment treatment caused zinc to either replace magnesium and silicate in the lattice or fill defects in the silicate lattice (such as by migrating into interatomic spacings).
Ten grams of the 3 powders (i.e., the raw, microwaved, and sintered powders) was individually mixed with 100 mL of distilled water at 60 °C and left to stand for 60 minutes, thereby yielding 3 solutions. The magnesium and zinc ion release rates and specific surface area were measured for each solution ( and Citation2). The results revealed that the microwaved powder had higher magnesium and zinc ion release rates than the raw powder. The magnesium ion release rate of the sintered powder also increased, but its zinc ion release rate decreased (). Moreover, the sintered powder had a higher specific surface area () than the raw powder, suggesting that sintering increased zinc crystallization and reduced the zinc ion release rate. The total ion release rate (i.e., Mg + Zn) of the sintered powder was greater than that of the microwaved powder. Sintering stabilized the powder structure, reduced impurities, and increased the specific surface area. Accordingly, raw and sintered powders were used for the garlic growth and enzyme experiments.
Table 1. Ion release rates of the raw, microwaved, and sintered powders (water temperature: 60 °C).
Table 2. Specific surface area (SSA) of the raw, microwaved, and sintered powders.
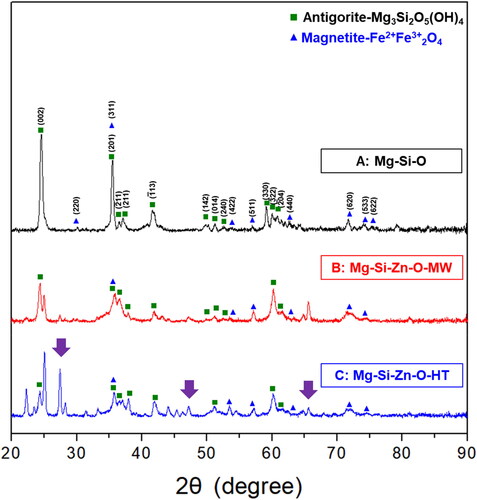
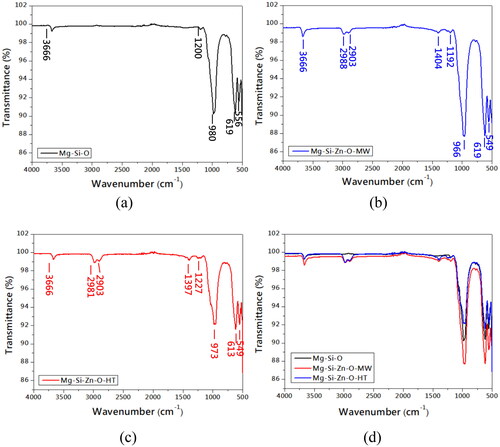
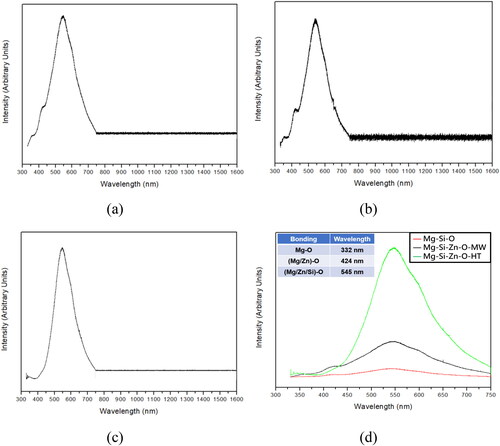
presents the X-ray diffraction (XRD) patterns of the powders. Both the microwaving and sintering processes increased phase crystallinity (fewer impurities). Moreover, the enrichment process led to the formation of a zinc-rich compound phase (indicated by arrows). The diffraction intensity of the sintered powder was higher than that of the microwaved powder, suggesting that sintering resulted in a greater volume ratio of zinc-rich compounds and higher crystallinity than did microwaving. displays the FTIR spectra of the powders, which indicate that the structure of the serpentine powder did not changes substantially after sintering. After zinc enrichment, new peaks were observed at the frequencies of 2903 and 2981 cm−1, and the intensity of the spectrum in the low-frequency region (500–1000 cm−1) decreased. The Raman spectra of the powders () were generated with a 532 nm laser. The spectra of the raw and enriched powders differed somewhat. This was attributed to two reasons. First, sintering stabilizing the compound phases of the powders. Second, the content of magnesium–zinc compounds on the powder surfaces were notably higher for the sintered powder than for other powders. presents the PL spectra of the powders (obtained with a 325 nm laser for 330–750 nm and 532 nm laser for 750–1600 nm). The results revealed that the raw powder had low intensity, whereas strong bonding was observed for the microwaved and sintered powders. Notably, the sintered powder had fewer crystal defects than the other powders, confirming that sintering not only reduced impurities but also facilitated zinc ion reactions and reduced lattice defects.
Magnesium–zinc garlic cultivation and growth patterns
and Citation2 reveal that the powder surfaces had zinc ions that were adsorbed through immersion plating. After sintering, these surface zinc ions underwent ion runaway and were lost due to combustion, reducing the zinc content on the powder surfaces and therefore the zinc ion release rate. Ion release capability is closely related to the specific surface area of powders. reveals that the sintered powder, which had largest specific surface area, exhibited the highest total ion release capability for both magnesium and zinc ions. Therefore, this powder was used to produce the magnesium–zinc solution for garlic cultivation.
The magnesium–zinc solution was prepared by placing 5 g of sintered powder in 100 mL of distilled water at 60 °C and allowing it to soak for 1 hour. This solution was then diluted to 1000 mL. To objectively evaluate the ion transport effects, tests were conducted with organic potting soil. This potting soil was characterized: It had a neutral pH of 7.3 and contained substantial amounts of potassium and calcium but had extremely low magnesium ion content (0.4 mg/g); zinc was not detected ().
Table 3. Organic soil composition analysis (by ICP) and pH values.
Garlic was cultivated with either the magnesium–zinc water (enriched garlic) or distilled water (unenriched garlic). depicts raw garlic cloves harvested upon maturity from both groups. The enriched garlic cloves had greater volume. As aforementioned, some cloves were dried for further analysis. The moisture content of both the wet and dry garlic were measured, and liquid-chromatography mass-spectrometry was used to analyze the content of allicin and sulfides in the enriched wet garlic, unenriched wet garlic, enriched dry garlic, and unenriched dry garlic ().
Figure 9. Fresh garlic cloves (left: unenriched garlic, right: enriched garlic). (a) Unprocessed and (b) after peeling.
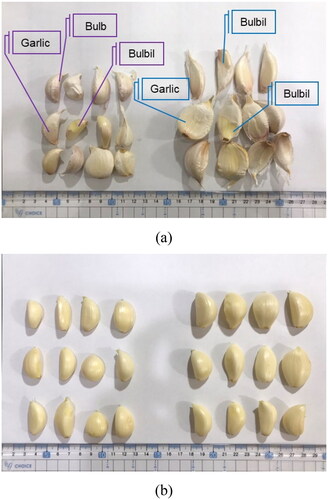
Table 4. Magnesium and zinc ion concentration and composition of the garlic extracts.
The enriched garlic contained magnesium (32–41 mg/L) and zinc (10–15 mg/L) and had more garlic glands and higher sulfide content than other garlic. Moreover, the garlic glands of the enriched dry garlic (11.24 mg/g) were less prone to decomposition than that of the wet garlic (13.20 mg/g). The enriched garlic also had a higher moisture content than unenriched garlic, indicating that the enriched garlic had superior antioxidant properties.
Enzyme analysis of the magnesium–zinc garlic
Enzyme analysis was performed for the enriched and unenriched wet garlic. A total of 100 g of cloves from each type were chopped (); these were then mixed separately with 300 mL of distilled water and placed in containers. After fermenting in a cool, dark place for 14 days (), the mixture was filtered to remove the remaining solids. Enriched and unenriched garlic enzyme solutions were thus obtained () (Citation17, Citation18).
Figure 12. Filtered garlic fermentation solution and remaining garlic cloves (left: unenriched garlic, right: enriched garlic.

Enzyme activity and concentration tests were performed for alliinase, esterase, and oxidase. As shown in , the alliinase activity was 8 times higher for the enriched than unenriched garlic enzyme extract; however, the activity levels of esterase and oxidase were similar. These findings confirm that the enriched garlic enzyme extract had more magnesium and zinc ions and alliinase activity than the unenriched extract.
Table 5. Enzyme activity and concentration analysis.
Conclusions
In this study, serpentine from natural sources was powdered, sintered, and used to produce a magnesium–zinc ion solution. This solution was used to grow magnesium–zinc enriched garlic with health benefits. Subsequently, the growth of the enriched garlic and its enzyme activity were investigated. The key findings of this study are as follows:
Zinc enrichment in combination with microwave heating and sintering promoted zinc crystallization in serpentine powder and reduced impurities.
The sintered powder had a stable structure, a high specific surface area, stable interatomic layer distances, and excellent magnesium and zinc ion release capability.
Garlic grown with the magnesium–zinc water had superior antioxidant properties and contained more garlic glands and sulfides than unenriched garlic.
The enriched garlic enzymes had high alliinase activity, likely increasing the health benefits of the garlic.
Future prospects
This study spans multiple domains, including mineral resources, powder engineering, agriculture, and biotechnology. Enriched and unenriched garlic enzymes were obtained from the proposed cultivation method for growing garlic, which has anticancer properties. Future studies could apply these enzymes in health foods or as enzyme capsules.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ali Z, Ma H, Ayim I, Wali A. Efficacy of new beverage made of dates vinegar and garlic juice in improving serum lipid profile parameters and inflammatory biomarkers of mildly hyperlipidemic adults: a double-blinded, randomized, placebo-controlled study. J Food Biochem. 2018;42(5):e12545. doi:10.1111/jfbc.12545.
- Rahman MS. Allicin and other functional active components in garlic: health benefits and bioavailability. Int J Food Prop. 2007;10(2):245–68. doi:10.1080/10942910601113327.
- Hirsch K, Danilenko M, Giat J, Miron T, Rabinkov A, Wilchek M, Mirelman D, Levy J, Sharoni Y. Effect of Purified Allicin, the Major Ingredient of Freshly Crushed Garlic, on Cancer Cell Proliferation. Nutr Cancer. 2000;38(2):245–54. doi:10.1207/S15327914NC382_14.
- Oommen S, Anto RJ, Srinivas G, Karunagaran D. Allicin (From Garlic) induces caspase-mediated apoptosis in cancer cells. Eur J Pharmacol. 2004;485(1-3):97–103. doi:10.1016/j.ejphar.2003.11.059.
- Miron T, Bercovici T, Rabinkov A, Wilchek M, Mirelman D. [3H] Allicin: preparation and Applications. Anal Biochem. 2004;331(2):364–9. doi:10.1016/j.ab.2004.03.054.
- Hung FS. Growth characteristics of shallots and absorption of Mg ions dissolved from serpentinites, Journal of. Emerging Mater Res. 2022;11(4):472–7. doi:10.1680/jemmr.22.00101.
- Sugihara S, Honda T, Fujii K. Does soil chemical characteristics affect the brewer’s rice quality? A case study in Hyogo prefecture. The International Journal of Tourism Science. 2016;9:125–9.
- Wegmüller R, Tay F, Zeder C, Brnic M, Hurrell RF. Absorption by young adults from supplemental zinc citrate is comparable with that from zinc gluconate and higher than from zinc oxide. J Nutr. 2014;144(2):132–6. doi:10.3945/jn.113.181487.
- Seelig MS. Auto-immune complications of D-penicillamine a possible result of zinc and magnesium depletion and of pyridoxine inactivation. J Am Coll Nutr. 1982;1(2):207–14. doi:10.1080/07315724.1982.10718989.
- Taubert L. Hydrochloric attack of serpentinites: mg2+ leaching from serpentinites. Magnes Res. 2000;13(3):167–73.
- Perrora D. A report on serpentinites in the context of heritage stone resources. Episodes. 2012;35(4):478–80.
- Rivlin RS. Historical perspective on the use of garlic. J Nutr. 2001;131(3s):951S–4S. doi:10.1093/jn/131.3.951S.
- Spencer H, Norris C, Williams D. Inhibitory effects of zinc on magnesium balance and magnesium absorption in man. J Am Coll Nutr. 2013;13(5):479–84. doi:10.1080/07315724.1994.10718438.
- Pascual-Esco A, Alonso-Chamarro J, Puyol M. Rapid warning microanalyzer for heavy metals monitoring in natural waters. Sens Actuators, B. 2022;368:132180. doi:10.1016/j.snb.2022.132180.
- Wang Z, Xu C, Lu Y, Chen X, Yuan H, Wei G, Ye G, Chen J. Fluorescence sensor array based on amino acid derived carbon dots for pattern-based detection of toxic metal ions. Sens Actuators, B. 2017;241:1324–30. doi:10.1016/j.snb.2016.09.186.
- Simpson A, Pandey RR, Chusuei CC, Ghosh K, Patel R, Wanekaya AK. Fabrication characterization and potential applications of carbon nanoparticles in the detection of heavy metal ions in aqueous media. Carbon. 2018;127:122–30. doi:10.1016/j.carbon.2017.10.086.
- Xiu Y, Li X, Sun X, Xiao D, Miao R, Zhao H, Liu S. Simultaneous determination and difference evaluation of 14 ginsenosides in Panax ginseng roots cultivated in different areas and ages by high-performance liquid chromatography coupled with triple quadrupole mass spectrometer in the multiple reaction–monitoring mode combined with multivariate statistical analysis. J Ginseng Res. 2019;43(4):508–16. doi:10.1016/j.jgr.2017.12.001.
- Porgalı E, Büyüktuncel E. Determination of phenolic composition and antioxidant capacity of native red wines by high performance liquid chromatography and spectrophotometric methods. Food Res Int. 2012;45(1):145–54. doi:10.1016/j.foodres.2011.10.025.