Abstract
: Incidence and mortality rates as a result of prostate cancer (PCa) remain high in the world, especially among the population of people living with human immunodeficiency virus (HIV), with high mortality mainly in Africa. Therefore, this study determined the survival rates among HIV-positive compared to HIV-negative PCa patients and factors associated with mortality. This was a retrospective cohort study of PCa patients at Cancer Disease Hospital in Lusaka, Zambia, for the observational period of 5 years. Patients were followed up using mobile phone calls to understand the time contributed from time at diagnosis to death. Patients who were lost to follow-up were censored at the date of last follow-up at the hospital. A total of 662 cases were evaluated. The total person time at risk was 7,548 months. After 5-year follow-up, there were 290 (43.8%) deaths, suggesting crude mortality rate of 430 per 10,000 persons per year. The overall median survival time was 16 months. In an adjusted model, the following variables had a statistically significant effect on the hazard of death: 1-year increase in age, increased the hazard of death by about 3%, AHR: 1.03 (95% CI: 1.02, 1.05, p = 0.001); HIV-positive patients had reduced hazard of death by about 41%, AHR: 0.59 (95% CI: 0.44, 0.79, p = 0.001); Gleason score (GS) less than or equal to 8, the patients with GS greater than 8 had increased hazard of death by about 43%, AHR: 1.43 (95% CI: 1.27, 1.59, p = 0.001); those on hormonal therapy had reduced hazard of death by about 28% AHR: 0.72 (95% CI: 0.54, 0.94, p = 0.018) and those presented with tumour stages I and II had reduced hazard of death by about 82%, AHR: 0.18 (95% CI: 0.04, 0.78), p = 0.021). Survival from death following whether a patient was HIV-positive or not was more in the HIV-positive compared to HIV-negative and this could be due to high GS in the HIV-negatives compared to HIV-positives and late-stage diagnosis of the disease at the hospital especially among the HIV-negative patients as evidenced by the increased hazard of death compared to HIV-positives. Therefore, medical check-up such as screening for PCa which leads to early diagnosis of the cancer must be encouraged in men.
1. Introduction
Prostate cancer (PCa) is the second most common cancer and the sixth leading cause of cancer deaths among men worldwide, and this accounts for over 1.1 million cases and 300,000 deaths (Adeloye et al., Citation2016). In Zambia, cervical cancer, breast cancer and PCa represent 51% of the cancer burden in mortality and morbidity (Kalubula et al., Citation2018) and have increased mortality commonly among human immunodeficiency virus (HIV)-infected people. According to the World Health Organization (Citation2014), PCa deaths have now reached 559 or 0.48% of total deaths and the age-adjusted death rate is 23.03 per 100,000 of population and Zambia ranks 48th in the world, while PCa ranks 12th among leading causes of death in Zambia and it has been the “second most prevalent cancer after cervical cancer and number one cancer in Zambian men with standardised incidence rate of 60.03 per 100,000 males” (Kalubula et al., Citation2018).
However, the influence of HIV-related factors on PCa incidence in HIV-positive men remains poorly defined (Bedimo et al., Citation2009). Some studies reported a higher incidence of PCa in people living with AIDS when compared to the general population. On the other hand, there are also reports of the opposite evidence. Patients with PCa who are HIV-positive have a lower survival rate than HIV-negative people (Facciolà et al., Citation2018). Although this may in part be related to deaths from AIDS-related complications; a recent population-based study found higher cancer-specific mortality among HIV-infected versus uninfected cancer patients (Coghill et al., Citation2017). According to studies comparing cancer prognosis by HIV status, PCa mortality has been linked to HIV infection in numerous investigations (Marcus et al., Citation2014). However, while mortality from AIDS and other causes may result in reduced survival for HIV-infected individuals, few studies have examined PCa mortality (Marcus et al., Citation2015).
Despite having some progress and slightly above average incidence of PCa, Zambia has one of the world’s highest estimated mortality rates from PCa (Bray et al., Citation2018). Compared to developed countries at 72 and 9.7 per 100,000 men (Chiu & Weisenburger, Citation2003), incident and mortality rates of PCa in Zambia were estimated at 21.9 and 18.2 per 100,000 men, respectively (Zyaambo et al., Citation2013). In California, a 5-year survival study of PCa patients showed that the survival was at 83% in HIV-infected men compared to 92% in HIV-uninfected men (Marcus et al., Citation2015), but in Zambia, little is known about the study done.
2. Methods
2.1. Patient selection
The study was a retrospective cohort study, for the observation period from 2014 to 2018, conducted at the Cancer Disease Hospital (CDH), Lusaka, Zambia. Only PCa cases that were diagnosed and treated at CDH were included in the study. Patients or family members were contacted via telephone calls to confirm whether they were still alive. All PCa cases diagnosed, treated and cared for, including deaths or censored (lost to follow-up), recorded at the CDH between January 2014 and December 2018 were included in the study. All PCa patients and cases were referred from other countries. Further, all non-PCa-specific patients (patients who died of other causes) were censored. Data were collected by the researcher from patient records, and a complete enumeration of 662 records of PCa patients was done at CDH for the observation period.
Information on the patient’s age, sex, marital status, whether the patient was on chemotherapy or not, hormonal therapy or not, prostatectomy or not, radiation therapy or not, level of prostate-specific antigen, whether on antiretroviral therapy (ART) or not, radiation dose, whether diabetic or not, history of prostate in the family, smoked or not and drink alcohol or not was collected. Other information collected included body mass index (BMI), cancer stage (I–IV) and HIV status of a patient.
2.2. Statistical methods
The patients were divided into two groups: patients who died at the end of the 5-year period and patients who did not die from PCa at the end of the 5-year period. The numbers of patients in these two groups were 290 and 372, respectively. Descriptive statistics including mean and standard deviation, median and interquartile range, frequencies and percentages were used to describe the two groups. Analysis of the statistical variance between the two groups was also conducted. If the chi-squared test assumptions were met, the chi-squared test was applied for categorical variables; otherwise, Fisher’s exact was used. A Wilcoxon rank-sum test was employed to investigate the connections between continuous variables and the result. On the entire cases initially, then on the imputed data, survival regression was used to derive inferential statistics. The dependent variable (dead or alive) was dichotomous; hence, survival regression was used. Stata/MP version 14.2 (Stata Corporation, Texas, TX, USA) was used to analyze the data. Statistical significance was defined as a p-value of 0.05. After multiple imputation, the multivariable Weibull regression model was the best fit model because it matched all the assumptions, had a hazard function that monotonically increased over time and had a shape that was defined by the estimated parameter p = 1.26. In this study, the hazards ratio for PCa death across all significant predictors from bivariate analyses was monotonically increasing over time; hence, Weibull model was the best model.
2.3. Imputation method
Non-monotone data were missing. We looked at the hard copy records to see if there were any characteristics for those with missing values in order to determine whether the nature of the missing was not random. Using this method, we had no proof that the missing information was not random. All variables from 662 individuals identified had missing values, with the exception of age, prostate-specific antigen, hormone therapy, chemotherapy, family history of PCa, HIV status, ART and radiation dose. Missing values ranged from 6 to 28 values (marital status, prostatectomy, prostate-specific antigen, diabetics, smoked, alcohol, BMI, cancer stage and radiation therapy), with prostate-specific antigen having the lowest percentage of missing values (two values) and radiation therapy having the highest (28 missing values). This may have been caused by 290 patients (or 43.8%) who passed away during the study period. The information of these patients was later categorized as missing. We had missing values for both categorical and continuous variables, and the pattern of the missing values was not monotonic. Therefore, we chose to employ multivariate imputation using chained equations (MICE). A series of regression models were run as part of the MICE technique, and each variable with missing data was modelled conditionally on the other variables in the data. Additionally, this implied that every variable was modelled in accordance with its distribution. For instance, logistic regression was used to model binary variables and linear regression was used to model continuous variables (Adeloye et al., Citation2016; Marcus et al., Citation2014; Bhindi et al., Citation2015). Continuous variables (prostate-specific antigen) were examined for normality to guarantee proper imputation. When non-normally distributed variables are imputed using the assumption of normality, the resulting imputed values diverge from the observed values (Sterne et al., Citation2009; White et al., Citation2011). This is the rationale for how the continuous variables (prostate-specific antigen), which were not normally distributed, were changed using a square root transformation prior to imputation and subsequently transformed back to their initial state prior to analysis. There were 20 sets of imputations produced (Von Hippel, Citation2009; Little & Rubin, Citation2014; Kenward & Carpenter, Citation2007).
3. Results
3.1. Demographic characteristics of the PCa patients
From the time period of 1 January 2014 to 31 December 2018, a total of 662 men were diagnosed with PCa at CDH in Lusaka. The mean age at diagnosis was 69.6 years (SD = 9.3).
Early-onset diagnosis was age less than 55 years which constituted 40 (6.0%), and 622 (94.0%) were 55 years or more. Gleason score (GS) less than or equal to 8 was the dominating tumour grade which comprised 428 (64.7%) followed by GS greater than 8 which constituted 234 (35.3%) of the patients. Most of the participants were HIV-negative (463; 69.9%), and 199 (30.1%) were HIV-positive. All HIV-positive patients were on ART. The majority (501; 76.4%) of the participants were married and 155 (23.6%) were unmarried. A total of 248 (38.3%) participants had either smoked or were currently smoking, 341 (52.5%) either took alcohol or used to take and 132 (22.3%) were diabetic. Ninety-two (13.9%) participants indicated to have history of PCa in the family. For most of the patients, the cancer stage was at stage IV (565; 84.3%), followed by stage III (73; 11.0%), stage II (13; 2%) and stage I (11; 1.70%). About 290 (43.8%) patients died at the time of the study, and 56.2% (372) were either alive or lost to follow-up and were censored. About 135 (20.4%) were on chemotherapy, 296 (44.7%) were on radiotherapy, 388 (58.6%) were on hormonal therapy and 44 (6.7%) were on prostatectomy, as seen in Table .
Table 1. Demographic and clinical characteristics of prostate cancer patients
In this study, the overall survival median time was 16 months and that in HIV and HIV-negative patients was 33 months and 14 months, respectively. The total person time at risk was 7,548 months. Assuming the incident rate to be constant, the incident rate was estimated as 0.04 per month or 40 per 1,000 persons per month, which would correspond to 0.43 per year or 430 per 1,000 persons per year. During the follow-up period, 291 (44.0%) deaths occurred as stated by PCa and the 1-year overall survival rate was 0.65 (65%). The overall survival time for the 5-year survival rate was 0.03 (3%). The overall survival in the HIV uninfected was 0.61 (61%), while it was 0.75 (75%) in the HIV infected.
3.2. Factors associated with PCa patients mortality
There was evidence (p-value = 0.0001) of a difference in the proportions among those who died and those that did not die. The results are presented in Table . Age was significantly associated with mortality as evidenced by the (p-value=0.0001), GS level of a (P = 0.0001). Further results show no significant difference in mortality for those married or not married (p-value = 0.672), a unit increase in body mass (p-value = 0.643), prostate-specific antigen (p-value = 0.477), whether tumour stage I/II or III/IV (p-value = 0.418), whether smoked or did not smoke (p-value = 0.075), whether history of PCa in the family or not (p-value = 0.060), whether took alcohol or not (p-value = 0.180), whether had prostatectomy done or not (p-value = 0.303) and whether on hormonal therapy or not (p-value = 0.528). Results also show that there was no evidence of the difference in mortality among those on radiotherapy or not (p-value = 0.248), whether one was on ART or not (p-value = 0.065) and those on chemotherapy or not (p-value = 0.825).
Table 2. Bivariate analysis of background and clinical characteristics of prostate cancer patients from Zambia Cancer Disease Hospital routinely collected data, 2014–2018
3.3. Key predictors for PCa mortality
A Kaplan–Meier (K-M) estimate in Figure showed that HIV-negative patients of PCa had lower mortality than the patients exposed to HIV. A Wilcoxon (Breslow) test for equality of survival functions showed statistical significance (chi2 = 8.83, P = 0.003) for the observed difference in HIV survival rates between the negative and positive PCa patients. Similarly, K-M estimate in Figure showed that patients with GS less than or equal to 8 had lower mortality compared to those who had GS greater than 8 and less than or equal to 8; a Wilcoxon (Breslow) test for equality of survival functions showed statistical significance (chi2 = 25.38, P = 0.003) for the observed difference in GS. Furthur, K-M estimate in Figure showed that patients on hormonal therapy had lower mortality between those who were on hormonal therapy and those who were not; Wilcoxon (Breslow) test for equality of survival functions showed statistical significance (chi2 = 4.46, P = 0.035).
Figure 1. Kaplan–Meier (K-M) estimate of HIV-negative patients’ prostate cancer had lower mortality than the patients exposed to HIV, with 95% confidence interval.
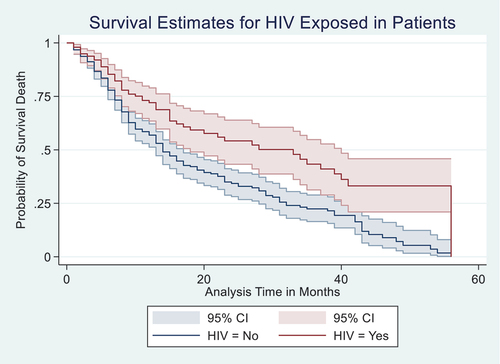
Figure 2. Kaplan–Meier (K-M) estimate of patients with Gleason scores of less than or equal to 8 had lower mortality compared to those who had Gleason scores of greater than 8, with 95% confidence interval.
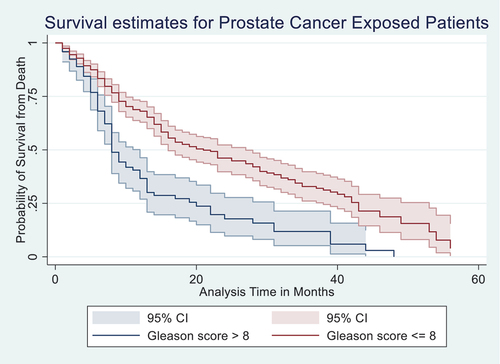
Figure 3. Kaplan–Meier (K-M) estimate of patients on hormonal therapy treatment had lower mortality compared to those who were not on the treatment, with 95% confidence interval.
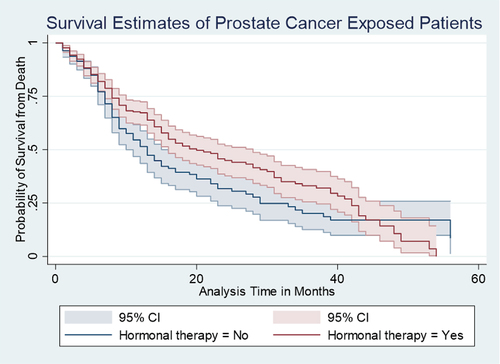
Figure shows the K-M curves (Figure ) for GS in relation to 5-year overall survival rate. The correlation of GS and the 5-year overall survival rate was highly statistically significant at crude and multivariate analysis (Adjusted Hazard Ratio (AHR): 1.43, 95% CI: 1.27, 1.59). Compared with the patients with GS less than or equal to 8, the patients with GS greater than 8 were more likely to die. In other words, patients in the group with GS greater than 8 at any time point during the follow-up period were 43% more likely to die within the 5-year period of the follow-up than those with GS less than or equal to 8. Below 7 months of survival after diagnosis, patients in both groups had almost equal survival.
Figure represents the K-M curves for patients on hormonal therapy and how it correlated with the 5-year overall survival rate. When patients on hormonal therapy and those without therapy were compared for the 5-year overall survival, it was found that those on hormonal therapy were 28% less likely to die within the 5-year period of the follow-up than those not on hormonal therapy. The crude hazard and adjusted mortality rates showed that patients on hormonal therapy were less likely to die (AHR: 0.72, 95% CI: 0.54, 0.94) compared to those not on hormonal therapy. Below 6 months and above 45 months of survival after diagnosis, patients in both groups had almost equal survival. See Figure .
4. Factors associated with mortality of the PCa patients
In unadjusted Weibull model, the following variables had a statistically significant effect on the hazard of death: 1-year increase in age, increased the hazard of death by about 3%, HR: 1.03 (95% CI: 1.02, 1.04, p = 0.0001); patients who were HIV-positive had reduced hazard of death by about 43%, HR: 0.57 (95% CI: 0.43, 0.75, p = 0.0001); patients with GS less than or equal to 8 had reduced hazard of death by about 60%, HR: 0.40 (95% CI: 0.30, 0.52, p = 0.0001); those on hormonal therapy had reduced hazard of death by about 26%, HR: 0.74 (95% CI: 0.59, 0.93, p = 0.011) and a unit increase in the radiotherapy dose reduced the hazard of death by about 2%, HR: 0.98 (95% CI: 0.96, 1.00, p = 0.039). The predictors such as BMI, HR: 1.00 (95% CI: 1.98, 1.03, p = 0.527), prostate-specific antigen, HR: 1.00 (95% CI: 0.99, 1.00, p = 0.645), tumour stage, HR: 1.70 (95% CI: 0.84, 3.43, p = 0.138), marital status, HR: 0.90 (95% CI: 0.0.67, 1.17, p = 0.395), smoking, HR: 0.96 (95% CI: 0.75, 1.23, p = 0.769), alcohol, HR: 1.00 (95% CI: 0.79, 1.26, p = 0.808) and history of PCa in the family, HR: 1.14 (95% CI: 0.79, 1.65, p = 0.473), prostatectomy, HR: 0.75 (95% CI: 0.45, 1.25, p = 0.268), diabetes, HR: 1.00 (95% CI: 0.73, 1.36, p = 0.979) and radiotherapy, HR: 0.84 (95% CI: 0.67, 1.06, p = 0.141) did not have significant effect on mortality, as shown in Table .
Table 3. Crude and adjusted Weibull model of factors associated with mortality of prostate cancer patients
In the adjusted Weibull model, the following variables had a statistically significant effect on the hazard of death: 1-year increase in age, increased the hazard of death by about 3%, AHR: 1.03 (95% CI: 1.02, 1.05, p = 0.0001); patients who were HIV-positive had reduced hazard of death by about 42%, AHR: 0.58 (95% CI: 0.43, 0.78, p = 0.0001); patients with GS less than or equal to 8 had reduced hazard of death by about 55%, AHR: 0.45 (95% CI: 0.33, 0.61, p = 0.0001); those on hormonal therapy had reduced hazard of death by about 28%, AHR: 0.71 (95% CI: 0.54, 0.94, p = 0.015) and patients presented with tumour stage three and four were 2.1 times likely to experience the hazard of death compared to those with cancer stages I and II, AHR: 2.13 (95% CI: 1.02, 4.48, p = 0.044). The predictors such as BMI, AHR: 1.02 (95% CI: 1.00, 1.04, p = 0.116), prostate-specific antigen, AHR: 0.99 (95% CI: 0.99, 1.00, p = 0.645), radiotherapy dose, AHR: 0.99 (95% CI: 0.97, 1.02, p = 0.639), marital status, AHR: 1.03 (95% CI: 0.76, 1.41, p = 0.820), smoking, AHR: 1.08 (95% CI: 0.81, 0.1.43, p = 0.613), alcohol, AHR: 1.03 (95% CI: 0.78, 1.36, p = 0.808) and history of PCa in the family, AHR: 1.33 (95% CI: 0.88, 2.02, p = 0.176), prostatectomy, AHR: 0.77 (95% CI: 0.45, 1.31, p = 0.331), diabetes, AHR: 1.02 (95% CI: 0.75, 1.40, p = 0.880) and radiotherapy, AHR: 1.04 (95% CI: 0.73, 1.47, p = 0.845) had no evidence of any significant effect on mortality.
5. Discussion
The study aimed to determine the survival rates among HIV-positive compared to HIV-negative PCa patients and factors associated with mortality. The results have shown that HIV-negative patients were at higher risk of dying compared to HIV-positive. This higher survival among HIV-positive patients may be associated with better health-seeking behaviours due to the ability to access chronic care and hence get more regular medical check-ups compared to HIV-negatives. As a result, there is an early detection of PCa among HIV-positive patients; hence, treatment starts early before the cancer is advanced and this contributes to reduced mortality. The findings are also in line with the findings of Marcus et al. (Citation2015) who found that when age, race, smoking, alcohol, obesity, GS, diabetes and other risk factors were adjusted for HIV-positive men, their risk of developing PCa was reduced by 27%; this risk was reduced by 45% when only previously PSA-tested individuals were included and when additional testosterone deficiency adjustment was made. Lower-stage malignancies and lower PSA levels were more frequently found in HIV-positive males who underwent testing and were diagnosed (Marcus et al., Citation2014). Because most instances are diagnosed without symptoms, differences in screening must be taken into account when comparing PCa risk based on HIV status. Also, Kimani (Citation2012) found that males with HIV frequently have PSA testing. Men with HIV who are on ART are often examined by their medical team every 3 months, giving them more frequent access to other health exams. Another study found that this could be explained by hypothetic protective role of immunodeficiency, the capacity of HIV to infect, replicate in and damage the proliferation of cancer cells, endocrine effects or direct antineoplastic activity of the ART (Facciolà et al., Citation2018). In contrast with our findings, Mani and Aboulafia (Citation2013) found that PCa in HIV-infected people is burdened with higher mortality rates compared to HIV-negative people and lower rates of PSA screening.
The results have also revealed that increase in age was associated with higher risk of patients dying, and the average age was 69 years, slightly lower than that reported by Adeloye et al. (Citation2016) in which the average age of a man diagnosed with PCa at CDH was 71 years of age. Marcus et al. (Citation2014) also found that for both HIV-positive and HIV-negative men, risk of the cancer increased with age. In contrast to our study, a study done by Bhindi et al. (Citation2015) found that age was not a predictor of the 5-year overall survival. However, a study done by Kahn et al. (Citation2012) reported that patients aged greater than 70 years had poor 5-year overall survival rate compared to patients who were aged less than 70 years. Increased age is usually associated with poor performance of the patients which in turn is more likely to increase mortality of the patients (Yahaya et al., Citation2020). Yahaya et al. (Citation2020) also reveal that age was not a predictor of the 3-year overall survival rate in the study. This is different from other studies which reported that age was the predictor of the overall survival rate (Miyoshi et al., Citation2015).
In this study, mortality rates showed that patients on hormonal therapy were less likely to die compared to those not on hormonal therapy. In line with this study, Lilleby (Citation2013) showed that androgen deprivation therapy improves survival rates in men with locally advanced PCa, but that it was also linked to side effects that impeded daily function, Norwegian SPCG-7 (Widmark et al., Citation2009). A total of 875 patients with locally advanced nonmetastatic illness and a PSA under 70 were randomly assigned to receive continuous flutamide and radiation therapy for 3 months after 3 months of combination androgen blocking. Addition of radiation therapy improved 10-year PCa specific mortality, overall mortality and PSA recurrence. Another study done by (Heidenreich, Citation2012 found that groups Randomized to immediate vs. postponed androgen ablation were 986 TO-4N0-2 patients who rejected or were ineligible for local therapy had an immediate increase in overall survival from 42% to 48%.
Further, in this study, we demonstrated that there was no difference in mortality of patients either on radical prostatectomy or radiation therapy and chemotherapy; other studies show that both patients with HIV infection and those without HIV appear to have similar outcomes following radical prostatectomy and radiation therapy (Cheng et al., Citation2001; Izadmehr et al., Citation2016; MacKintosh et al., Citation2016). According to a study, men with HIV were less likely to undergo surgery for PCa than their HIV-negative counterparts, but they were much more likely to receive radiation (Hentrich & Pfister, Citation2017). In contrast, the 4-year biochemical-failure-free survival rate was 87% in the HIV-infected group compared to 89% in the controls in a matched cohort evaluation of outcomes of definitive radiotherapy for PCa in males with HIV infection (Kahn et al., Citation2012). HIV-positive patients should receive the same treatment for PCa as HIV-negative ones (Hentrich & Pfister, Citation2017).
Our research has shown that there was a substantial difference between the two groups’ GS. In contrast to other prognostic indicators, GS continued to be one of the pathologic prognostic markers that independently predicted the survival of patients. Patients’ survival rates have been found to be extremely dismal in nations where PCa patients are diagnosed with low GS. GS 8 was the primary score used in Spain to make statistically significant predictions about patients’ overall survival (Winter et al., Citation2017; Galego et al., Citation2015). Additionally, we discovered that, unlike PSA, GS 7 was an independent predictor of the disease’s biochemical relapse. Low stage at diagnosis and low tumour grade are typically associated with early PCa detection (Montironi et al., Citation2006). Most PCa patients are discovered at an early stage, and as a result, they have low tumor grade, which is typically determined, in nations where PCa screening is widespread and sustainable (Tindall et al., Citation2014). As a result, they experience better clinical outcomes and live longer.
Our results suggest that the observed lower mortality of PCa among HIV-positive compared to HIV-negative men is attributable to factors other than differences in GS. Therefore, CDH needs to do more screening through healthy campaigns for early detection of the disease for the majority of the patients were in stage IV of the tumour stage. The life expectancy of modern HIV-positive men warrants consideration for treatment for PCa similar to HIV-negative men. Hormonal therapy may be considered as one of the most effective therapy treatments. Although there are limited data on outcomes, some studies have found that HIV-positive men do appear to tolerate surgical or radiotherapeutic interventions with little increased mortality. It has been discovered that the majority of patients are diagnosed at an early stage and, as a result, have low tumor grade, which is often determined for nations where PCa screening is widespread and sustained.
5.1. Limitation
We note potential limitations to our study. Major limitations were missing data from CDH especially on HIV care, CD4 count and information on viral load suppression of HIV-positive patients. The other limitation was that the treatments were non-randomized; it was not known why some were given hormonal therapy vs. surgery or both. Another limitation is the study of PCa screening at the population level is currently not recommended (unlike cervical cancer, breast cancer and colorectal cancer); therefore, the study cannot recommend mass screening at the population level. Another limitation is that the study did not account for what could be early detections.
6. Conclusion
Survival from death following whether a patient was HIV-positive or not was more in the HIV-positive compared to HIV-negative, and this could be due to high GS in the HIV-negatives compared to HIV-positives. Prostate cancer screening must be advocated especially among the HIV-negative patients as evidenced by the increased hazard of death compared to HIV-positives and are presented with high GS.
Author contributions
KM: conceptualization. SC, MM, KM and VM: data curation. KM, VM and PK: investigation and methodology. KM and PM: formal analysis. KM: writing—original draft: KM, VM and PK writing—review and editing. All authors read and approved the final manuscript and contributed to the development of research.
Ethics statement
University of Zambia Biomedical Research Ethics Committee (UNZABREC) which is one of the ethical committee in Zambia gave the approval of this research with reference number 447-2019 before the study was undertaken.
Acknowledgments
We acknowledge Cancer Disease Hospital in Zambia for enabling us to get data by reaching out to the patients or family members of the cancer patient during the follow-up.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data for this article supporting the findings will be made available once requested by the reviewer for supporting the conclusions.
Additional information
Funding
Notes on contributors
Kelvin Mwangilwa
Kelvin Mwangilwa is a Medical Statistician.
Moses Mwale
Moses Mwale is a Data spacialist.
Susan Citonje
Suzan Chitengi is Medical Doctor and spacialist in Oncology.
Michael Vinikool
Micheal Vinikoor is a Medical Doctor/Research and Professor in Public health.
Patrick Musonda
Patrick Musonda is a Professor in Medical Statistics.
References
- Adeloye, D., David, R. A., Aderemi, A. V., Iseolorunkanmi, A., Oyedokun, A., Iweala, E. E., & Ayo, C. K. (2016). An estimate of the incidence of prostate cancer in Africa: A systematic review and meta-analysis. PloS One, 11(4), e0153496. https://doi.org/10.1371/journal.pone.0153496
- Bedimo, R. J., McGinnis, K. A., Dunlap, M., Rodriguez-Barradas, M. C., & Justice, A. C. (2009). Incidence of non-AIDS-defining malignancies in HIV-infected vs. non-infected patients in the HAART era: Impact of immunosuppression. Journal of Acquired Immune Deficiency Syndromes, 52(2), 203. https://doi.org/10.1097/QAI.0b013e3181b033ab
- Bhindi, B., Mamdani, M., Kulkarni, G. S., Finelli, A., Hamilton, R. J., Trachtenberg, J., Zlotta, A.R., Evans, A., van der Kwast, T.H., Fleshner, N.E., & Toi, A. (2015). Impact of the US preventive services task force recommendations against prostate specific antigen screening on prostate biopsy and cancer detection rates. The Journal of Urology, 193(5), 1519–13. https://doi.org/10.1016/j.juro.2014.11.096
- Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., & Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68(6), 394–424. https://doi.org/10.3322/caac.21492
- Cheng, L., Zincke, H., Blute, M. L., Bergstralh, E. J., Scherer, B., & Bostwick, D. G. (2001). Risk of prostate carcinoma death in patients with lymph node metastasis. Cancer, 91(1), 66–73. doi:10.1002/1097-0142(20010101)91:1<66:AID-CNCR9>3.0.CO;2-P
- Chiu, B. C.-H., & Weisenburger, D. D. (2003). An update of the epidemiology of non-Hodgkin’s lymphoma. Clinical Lymphoma, 4(3), 161–168. https://doi.org/10.3816/CLM.2003.n.025
- Coghill, A. E., Pfeiffer, R. M., Shiels, M. S., & Engels, E. A. (2017). Excess mortality among HIV-infected individuals with cancer in the United States. Cancer Epidemiology and Prevention Biomarkers, 26(7), 1027–1033. https://doi.org/10.1158/1055-9965.EPI-16-0964
- Facciolà, A., Ceccarelli, M., Rullo, E. V., d’Aleo, F., Condorelli, F., Visalli, G., & Nunnari, G. (2018). Prostate cancer in HIV-positive patients: A review of the literature. AIDS (PLWHA), 51, 54. https://doi.org/10.32113/wcrj_20189_1136
- Galego, P., Silva, F. C., & Pinheiro, L. C. (2015). Analysis of monotherapy prostate brachytherapy in patients with prostate cancer. Initial PSA and Gleason are important for recurrence? International Braz J Urol, 41(2), 353–359. https://doi.org/10.1590/S1677-5538.IBJU.2015.02.24
- Heidenreich, A. (2012). Management of prostate cancer: EAU guidelines on screening, diagnosis and treatment. Management of Prostate Cancer. Springer Berlin Heidelberg. https://doi.org/10.1007/978-3-642-27597-5_22
- Hentrich, M., & Pfister, D. (2017). HIV-associated urogenital malignancies. Oncology Research and Treatment, 40(3), 106–112. https://doi.org/10.1159/000457130
- Izadmehr, S., Leapman, M., Hobbs, A. R., Katsigeorgis, M., Nabizada-Pace, F., Jazayeri, S. B., & Samadi, D. B. (2016). Clinical characteristics and outcomes of HIV-seropositive men treated with surgery for prostate cancer. International Urology and Nephrology, 48(10), 1639–1645. https://doi.org/10.1007/s11255-016-1338-4
- Kahn, S., Jani, A., Edelman, S., Rossi, P., Godette, K., Landry, J., & Anderson, C. (2012). Matched cohort analysis of outcomes of definitive radiotherapy for prostate cancer in human immunodeficiency virus-positive patients. International Journal of Radiation Oncology* Biology* Physics, 83(1), 16–21. https://doi.org/10.1016/j.ijrobp.2011.05.047
- Kalubula, M., Shen, H., Makasa, M., & Liu, L. (2018). Epidemiology of cancers in Zambia: A significant variation in cancer incidence and prevalence across the nation. bioRxiv [Internet], 402628. PMID: 35233276; PMCID: PMC8843183. https://doi.org/10.4314/mmj.v33i3.6
- Kenward, M. G., & Carpenter, J. (2007). Multiple imputation: Current perspectives. Statistical Methods in Medical Research, 16(3), 199–218. http://dx.doi.org/10.1177/0962280206075304PMID:17621468
- Kimani, F. (2012). Cervical, breast and prostate cancers are a major public health concern in Kenya. They are however easily prevented and controlled through behaviour change, vaccination, screening, early detection and treatment of pre-cancerous lesions and/or at early stage disease thereby leading to better management and outcomes for these diseases.
- Lilleby, W. (2013). Overall-and disease-specific survival in prostata cancer: Too long to wait? In Radiotherapy in prostate cancer (pp. 65–73). Springer. https://doi.org/10.1007/174_2013_941
- Little, R. J., & Rubin, D. B. (2014). Statistical analysis with missing data. John Wiley & Sons.
- MacKintosh, F. R., Sprenkle, P. C., Walter, L. C., Rawson, L., Karnes, R. J., Morrell, C. H., Kattan, M.W., Nawaf, C.B., & Neville, T. B. (2016). Age and prostate-specific antigen level prior to diagnosis predict risk of death from prostate cancer. Frontiers in Oncology, 6, 157. https://doi.org/10.3389/fonc.2016.00157
- Mani, D., & Aboulafia, D. M. (2013). Screening guidelines for non-AIDS defining cancers in HIV-infected individuals. Current Opinion in Oncology, 25(5), 518. https://doi.org/10.1097/CCO.0b013e328363e04a
- Marcus, J. L., Chao, C. R., Leyden, W. A., Xu, L., Klein, D. B., Horberg, M. A., Towner, W.J., Quesenberry, C.P., Abrams, D.I., Silverberg, M.J., & Van Den Eeden, S. K. (2014). Prostate cancer incidence and prostate-specific antigen testing among HIV-positive and HIV-negative men. JAIDS Journal of Acquired Immune Deficiency Syndromes, 66(5), 495–502. https://doi.org/10.1097/QAI.0000000000000202
- Marcus, J. L., Chao, C., Leyden, W. A., Xu, L., Yu, J., Horberg, M. A., Klein, D., Towner, W.J., Quesenberry, C.P., Silverberg, M.J., & Abrams, D. I. (2015). Survival among HIV-infected and HIV-uninfected individuals with common non-AIDS-defining cancers. Cancer Epidemiology and Prevention Biomarkers, 24(8), 1167–1173. https://doi.org/10.1158/1055-9965.EPI-14-1079
- Miyoshi, Y., Noguchi, K., Yanagisawa, M., Taguri, M., Morita, S., Ikeda, I., Fujinami, K., Miura, T., Kobayashi, K., & Uemura, H. (2015). Nomogram for overall survival of Japanese patients with bone-metastatic prostate cancer. BMC Cancer, 15(1), 338. https://doi.org/10.1186/s12885-015-1330-x
- Montironi, R., Mazzucchelli, R., Scarpelli, M., Lopez‐Beltran, A., Mikuz, G., Algaba, F., & Boccon‐Gibod, L. (2006). Prostate carcinoma II: Prognostic factors in prostate needle biopsies. BJU International, 97(3), 492–497. https://doi.org/10.1111/j.1464-410X.2006.05973.x
- Organization, W. HWorld Health Organization. (2014). World health rankings: Live longer live better. WHO.
- Sterne, J. A., White, I. R., Carlin, J. B., Spratt, M., Royston, P., Kenward, M. G., Wood, A. M., & Carpenter, J. R. (2009). Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ, 338(jun29 1), b2393. http://dx.doi.org/10.1136/bmj.b2393PMID:19564179
- Tindall, E. A., Monare, L. R., Petersen, D. C., Van Zyl, S., Hardie, R. A., Segone, A. M., Venter, P.A., Bornman, M.R., & Hayes, V. M. (2014). Clinical presentation of prostate cancer in black South Africans. The Prostate, 74(8), 880–891. https://doi.org/10.1002/pros.22806
- Von Hippel, P. T. (2009). 8. How to Impute interactions, squares, and other transformed variables. Sociological Methodology, 39(1), 265–291. http://dx.doi.org/10.1111/j.1467-9531.2009.01215.x
- White, I. R., Royston, P., & Wood, A. M. (2011). Multiple imputation using chained equations: Issues and guidance for practice. Statistics in Medicine, 30(4), [23] 377–399. https://doi.org/10.1002/sim.4067
- Widmark, A., Klepp, O., Solberg, A., Damber, J.-E., Angelsen, A., Fransson, P., Lund, J.Å., Tasdemir, I., Hoyer, M., Fosså, S.D., & Wiklund, J.-Å. (2009). Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): An open randomised phase III trial. The Lancet, 373(9660), 301–308. https://doi.org/10.1016/S0140-6736(08)61815-2
- Winter, A., Sirri, E., Jansen, L., Wawroschek, F., Kieschke, J., Castro, F. A., Holleczek, B., Emrich, K., Waldmann, A., Brenner, H., & Krilaviciute, A. (2017). Comparison of prostate cancer survival in Germany and the USA: Can differences be attributed to differences in stage distributions? BJU International, 119(4), 550–559. https://doi.org/10.1111/bju.13537
- Yahaya, J. J., Okecha, T., Odida, M., & Wabinga, H. (2020). Prognostic factors for overall survival of patients with prostate cancer in Kyadondo County, Uganda. Prostate Cancer, 2020. https://doi.org/10.1155/2020/8517130
- Zyaambo, C., Nzala, S. H., Babaniyi, O., Songolo, P., Funkhouser, E., & Siziya, S. (2013). Distribution of cancers in Zambia: Evidence from the Zambia National Cancer Registry (1990–2009). Journal of Public Health and Epidemiology, 5(2), 95–100.
