?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The study survey was done to select three desalination intake sampling sites to be used for the National Nuclear Power Plants (NNPs) desalination units. Different physicochemical analysis was done for the collected samples. Study results showed that there is an increment of the mean average concentrations of the measured TDS, Chlorides, Hardness, and sulfates values of the selected Sidi Kirir desalintion intake site than other Al-Alamin and Sidi Abdelrahaman sites, which are leading for consume large amounts of anti-scaling chemicals (as polyphosphates) at this site than the others. It was observed that the ecological risks which resulted from the use of polyphosphates as anti-scale formation were more higher in chemical risks than the use both of the hydrazine and benzatriazole anticorrosion chemicals. Study results showed that the using of benzatriazole will produce lesser ecological risks on marine living organisms than the using of hydrazine anticorrosion chemical. On the other hand the study recommended the using of Quater phenyl as non oxidizing biocide where its results showed lesser ecological effects (LC50 & EC50), than which being resulted from the using of chlorine dioxide (oxidizing biocide). The using of the economical risk code (DEEP) showed that the nuclear plant with Reverse osmosis (RO) desalination unit had the lowest water cost value than the other nuclear power plants, which are using both of the Multiple Effects Desalination (MED) and Multiple Stage Flash (MSF) desalination types, while the analytical physicochemical and corrosion index results showed that the RO desalination unit will be exposed to more corrosive and scale formation impacts than the using of both of MED and MSF desalination types. The study recommend the using of both MED & MSF desalination units, on the other hand, study showed that both MED & MSF units had less corrosive and scale formation impacts on the studied nuclear desalination power plant than the using of RO desalination unit.
1. Introduction
Seawater cooling systems in nuclear power plants are being used as intake source of the demineralization units, based on the different economical and environmental impact assessments studies (EIA) studies. It was reported that desalination plants have a high corrosion risk, where sea water are causing different corrosion shapes, such as pitting, crevice, galvanic and stress corrosion. In addition biological fouling and mineral scaling which may be formed at the coastal desalination unit may alter the equipment surface performance and induce corrosion. Studies showed that there are three main processes used in desalination plants, the oldest of which is multi flash (MSF), where the water is essentially boiled at low temperature and the steam that flashes off is condensed for drinking water, the second process is multi-effect desalination (MED), in which low pressure is used to force evaporation of sea water and the vapor is then condensed for drinking water. Although actual MSF and MED plants are land based, it was observed that some small-scale units have been fitted to large ships to generate fresh water. The third process is sweater reverse osmosis (SWRO), where chloride is selectively removed by forcing it at a high pressure through a special membrane. This method involves no heat transfer but requires electricity to power the high pressure pumps that are required. As for energy requirements for each process reverse osmosis, currently has the lowest energy requirements to construct itmes consumed in the process. Production costs appear to increase in proportion to the capacity of the plants, in many applications, distillation provides the best way for achieving waters of high purity for industrial use; for volume less than 4000 m3/day m the RO process is likely to be most effective; above that range, the MSF process will probably be preferable.[Citation1] There are many challenges associated with seawater desalination in the Mediterranean region include the impacts associated with the intakes, outfalls and energy consumption. Larvae and different marine organisms are most vulnerable to disappearing often due to poorly designed desalination plant intakes causing damage to the near-shore marine ecosystems.[Citation2] Different technologies have been developed for desalination, which include reverse osmosis, electro dialysis, vacuum freezing etc., the common element in all of these desalination processes is the removal of dissolved minerals from seawater. The main environmental, chemical and economical risks were identified depending on the review of many EIA studies for using RO in desolation plants.[Citation3] It was reported that the using of Reverse Osmosis (RO) for desalination plants showing their chemical, ecological and economical impacts on the environment. For example during the planning phase of the new desalination unit, the possible negative environmental impacts need to be evaluated.[Citation4] Reverse Osmosis (RO) is becoming the most common desalination technology being used in the Mediterranean region where the second-most common desalination technology in use in the region is Multiple Stage Flash (MSF) followed by Multiple Effects Desalination (MED).[Citation5] Nuclear power plant uses nuclear reaction in fission and fusion to utilize the heat for steam generation this may affect the distribution percentage of thermal desalination units than nuclear desalination units.[Citation6] Many studies are encouraging the applying of different nuclear desalination economical risk codes such as Desalination Economic Evaluation Program (DEEP-30) and Desalination Thermodynamic Optimization Program (DETOP).[Citation7,Citation8] Based on the modeled configurations in DEEP, it has been observed that membrane processes, such as RO, show lower cost as compared to thermal process (MED or MSF) but produced water quality of thermal processes is always higher than membrane process. There are variations of chemicals types and additives are being used in desalination, to control the formation of mineral scale and biological growth that would otherwise interfere with the process. Most chemicals are mainly used as biocides, anti scaling, anti fouling and antifoaming agents and ultimately affect the seawater concentration and composition complying with Mansouri & Ghoniem.[Citation8] The intake pipe would inevitably foul without anti fouling procedures. Periodic shock chlorination will use a far higher and highly toxic dose of chlorine to kill organisms living along the pipe work.[Citation9] The corrosive agents are generally oxygen, hydrogen sulfide, and carbon dioxide, Oxygen is generally removed by reductive inhibitors such as amines and hydrazine’s. Some corrosion inhibitors form a coating on the surface such as Benzotriazole which is one of such species used to protect copper as most of desalination plants and cooling water materials of thermal and nuclear power plants are from copper nickel alloys. In scale forming water, a precipitate or coating of calcium or magnesium carbonate forms on the inside of the piping. This coating can inhibit the corrosion of the pipe because it acts as a barrier, but it can also cause the pipe to clog as recoded by Mirzabeygi et al.[Citation10] In general, the corrosion rate depends on both the type of the metals and the physicochemical parameter of water. The most common factors affecting the corrosion rates of water industrial systems include abnormal water of total dissolved solids, pH, chloride, high temperature and water flow rate, and bacterial load as well as iron and steel specifications of pipelines as recorded by Lin et al.[Citation11] Biocides, generally divided into oxidizing and non-oxidizing biocide. The oxidizing biocide used commonly includes chlorine, hypochlorite such as sodium hypochlorite and calcium hypochlorite etc., chlorine dioxide, ozone, bromine and bromide. The non-oxidizing biocide used commonly includes chlorine phenol such as double chlorophenol, trichlorophenol and pentachlorophenol, quaternary ammonium salt such as benzalkonium chloride [geramine], organic amine such as rosin amine, etc., organic sulfur compounds such as methylene sulfur cyano, etc., copper salts such as copper sulfate and quaternary phosphorus salt. Toxicity is generally observed to be greatest when the carbon chain equals sixteen which is described by Hogan & Al-Bloushi et al.[Citation12,Citation13] The size of the hydrophobic component, usually a linear alkyl carbon chain, can be estimated by simply counting the number of carbons in the hydrophobic alkyl chain.[Citation13,Citation14] The acute toxicity of a chemical to fish (both fresh and saltwater], water fleas (daphnids), and green algae has been the focus of the development of QSARs, although for some chemical classes QSARs are available for other effects (e.g, chronic toxicity and bio concentration factor) and organisms (e.g., earthworms).[Citation13,Citation15]
2. Material and methods
2.1. Site description and sampling sites
Previous studies showed that the existing desalination units at the Egyptian coastal study region producing about 24,000 m /day at the Egyptian Masra Matrouh region, were 150,000 m/day where others are under construction in various regions.[Citation16] A number of studies have been performed on nuclear desalination under the guidelines of the International Atomic Energy Agency (IAEA).[Citation17] Therefore the study survey was on done on the Egyptian Mediterranean coastal zone in order to select three sampling sites to be used as a feeding source for nuclear desalination plants, the three selected sampling sites were Alamin, Sidi Abdel rahman, and Sidi krir, where their longitudes and latiitudes were as follows: Alamin its latitude was 30.8301 and its longitude was 28.9550, while Sidi Abdel rahman latitude was 30.963659 and its longitude 28.72908, and Sidi kirir latitude was 31.0966721 and its longitude was 29.6223164.
2.2. Physicochemical analysis
Seawater samples were taken monthly from the three sites during the period 2/10/2021-12/3/2022. Different parameters include planned Dissolved major ions for which concentrations determined in water from samples comprised bicarbonate (HCO3), chloride (Cl−), calcium (Ca++), magnesium (Mg), sodium (Na) and potassium (K) were being done by using different gravimetric and volumetric analytical methods which were done for the different collected sea water samples, all the types of samples preservation, types of both gravimetric and volumetric techniques being shown in . In case of determination of heavy metal in the collected sea water samples the samples were digested using the microwave acid digestion method. For trace element analysis, drops of concentrated Nitric acid were added to the samples to decrease the pH value (<2 pH unit) in order to mitigate the metal precipitation. The bottles were closed firmly to prevent evaporation and contamination, and they were kept in a refrigerator (at ~ 4°C), and in a short period transported to the laboratory to conduct the required analyses The trace element concentrations Fe, Co, Cu, Zn in water samples were measured using Inductively Coupled Argon Plasma [ICAP 6500 Duo, Thermo Scientific instrument).[Citation18] On the other hand the study methods didn’t used ion chromatography analytical method where the ion chromatographic cycling column switching system was developed for the determination of low level anions (fluorides, nitrate, bromide, sulfates and phosphates] in sea water samples. This method showed good linearity in the range of 0.05-25.0 mg/l, and there limits of detection were in the range of 2-23 µg/l, on the hand high salinity percentage of marine water samples will lead to the results interference, (chlorides inference), when using Ion chromatography for such analysis.[Citation19] All the preservation tools of the collected samples and type of the chemical analysis used were shown in , according to the Standard Methods for Examination of Water and wastewater described by Baired et al.[Citation18]
Table 1. Using standard methods for preservation and analysis of different collected samples of the sea water.
2.3. Quality assurance
EquationEquation 1(1)
(1) showed the trace elements concentrations which were measured with a measurement uncertainty in the range of 1–10%. The concentrations of these metals were determined in mg/kg.
Where: V = Final digestion volume, M = Initial weight (0.3 g) of measured sample.
for the all measured physicochemical parameters such as dissolved major ions in water samples for which concentrations determined in water comprised bicarbonate (HCO3), chloride (Cl−), Sulfate, calcium (Ca++), magnesium (Mg), sodium (Na) and potassium (K). The calculation balance of (Anions-cations) was used to indicate that the concentrations of major ions in the sample were accurately analyzed. The principle of electrical neutrality requires that the equivalent weight of positively charged ions (cations) equal that of negatively charged ions (anions). Because major ions usually represent most of the dissolved ions in water, equivalent amounts of major cations and anions are typically found. In an accurate analysis, the sum of the mill-equivalents of major and anions should be nearly equal.[Citation18,Citation20]
2.4. Using the anions cations balance
The anions – cations balance method presented to determine the reliability of major ions analysis of fresh or saline water in desalination process assumes that major ions comprise most of the dissolved solid in water sample, and requires that all major ion concentrations be measured, where the average difference in mill equivalents of anions and cations can be estimated as follows:
If the average difference exceeds 15 percent, one should have serious reservations about the accuracy of the analysis.[Citation20]
3. Statistical analysis
Statistical analysis is the process of collecting and analyzing large volume of data to identify trends and develop valuable insights. Different statistical analysis were being done using different parameters such as average mean, range, standard deviation, t-test for correlation.
3.1. Evaluation of the corrosion in the studied desalination plants
Rate of corrosion in desalination plants is influenced by several factors: a] water composition as the corrosion rates are greatly influenced by chemical makeup of the water, which includes the quantity of chlorides, pH, dissolved gases and contaminates, b) temperature where the elevated temperature can influence the corrosion process and make metal parts ready to corrosion, c) velocity and flow conditions, by encouraging erosion-corrosion, high flow rates of turbulent flow conditions can raise corrosion rates, d) choosing right materials for the desalination systems components is essential. High corrosion resistance materials are frequently chosen such as stainless steel or alloys resistance to corrosion (Cu-Ni) alloys. The use of corrosion inhibitors in desalination plants is essential for quarantining the safety and durability of the infrastructure and machinery.
3.2. Using corrosion saturation indexes as monitoring tool for the evaluation of chemical risks
The LSI used for low TDS ranges (<10,000 ppm) and the Stiff -Davis (S&DSI) is used for high TDS ranges (>10,000 ppm). In general, the Langelier Saturation Index (LSI) is used for brackish waters and the Stiff & Davis Stability Index (S&DSI) for seawater. LSI approaches the concept of saturation using pH as a main variable. Bicarbonates [mg/l) concentration was used to express the water alkalinity as follows: LSI = pH-pHs, where pHs = A + B-log [Ca]-log [Alkalinity], and if: LSI < −0.5 → Corrosion, LSI < −2 high corrosion, −2< LSI< −0.5 serious corrosion, −0.5< LSI<0 slight corrosion, LSI = 0 Balance 0 < LSI< 0.5 slight scaling 0.5 < LSI< 2 → High.[Citation20] The RSI indicates the degree of saturation of CaCO3 in water and was calculated by using pH, alkalinity [bicarbonate], calcium concentration, total dissolved solids. As follows; RSI = 2pHs – pH where; RSI< 6 scaling, 7< RSI<8 Low corrosion, RSI >8 High corrosion. In case of Stiff and Davis Index is used to overcome the high total dissolved solids’ waters and the impact of “common ion” effects on the driving force for scale formation. Stiff-Davis index has its basis in the concept of saturation level reported by Amjad & Demadis.[Citation21] Stiff-Davis indices predicting scales at the same LSI water conditions. As LSI = pH – pHs, (TDS <10,000 mg/l], S&DSI = pH – pHs (TDS >10,000 mg/l). Puckorious stability index [PSI) provided the most accurate values of the three methods. As follows; PSI = 2 pHs – pHeq. Where: pHs is the pH at saturation in calcite or calcium carbonate, pHeq = 1.465 x log10 [Total Alkalinity] where [Alkalinity] = [HCO3−] + 2 [CO32-] + [OH−, The PSI index is calculated in a manner similar to the Ryznar stability index where PSI < 5.5 high scaling, 5.8 ≤ PSI ≤ 6.5 scaling, 6.5≤ PSI ≤ 7.5 low scaling, 7.5 ≤ PSI ≤ 8.5 corrosion, PSI > 8.5 corrosion showed by Altman et al.[Citation20]
3.3. Using DEEP code as monitoring tool for the economical risk evaluations
DEEP code estimates a comparison of a number of design alternatives, which helps in the identification of the lowest cost options for water and power production for any specific location reported by IAEA.[Citation7]
3.4. Using ECOSAR code as monitoring tool for the evaluation of marine ecological risks
The Ecological Structure Activity Relationship (ECOSAR) code is a computerized version of the eco-toxicity analysis procedures which practiced by the Office of Pollution Prevention and Toxics (OPPT) when data are lacking for risk assessment development. Most QSAR calculations in the ECOSAR code are based upon the Octane/water partition coefficient (Kow). Different EC50 and LC50 were being calculated, where EC50 was the Median Effect Concentration. It is usually expressed as milligrams (mg) of substance per liter (L) water and LC50 was the Median Lethal Concentration which is usually expressed as milligrams (mg) of substance per liter [L) water, study showed that, all the previous calculations will be done on different marine living organisms such as Fish, Daphnide and Green algae in order to determine the ecological impacts of using Different ionizing and non ionizing biocide in the intake inlets of different Nuclear and thermal desalination types which reported by Fuentes-Bargues & Mayo-Bean et al.[Citation22,Citation23] As a result the study used ECOSAR code as one of the non destructive tools, where didn’t use the vivo tool as it is a destructive analytical technique, lead to damage many marine living organisms (algae & fishes] during the biological experiments might be done, it was observed that the using of ecological modeling codes are more safe for evaluation ecological and toxicological parameters of marine living organisms than the other vivo techniques.
4. Results and discussion
4.1. Determination of the different chemical risks resulting from of the selection of the intake of nuclear desalination plant
indicated that the highest TDS mean average concentration value was showed in Sidi Kirir region with 42266.42 ± 32.656 in (mg/l), while its lower mean average concentration value was 42111.42 ± 22.558 in (mg/l), showed at Sidi Abdel rahaman region, this little increment in TDS’ concentration values in Sidi Kirir region is related to the presence of some industrial activity, which may be found at this region such as Sidi kirir Steam power plant. In case of Chlorides concentrations indicated that the highest mean average concentration value of Chlorides was showed in Sidi Kirir region with 32297.92 ± 5.09 measured in [mg/l), while its lowest mean average value was 32233.72 ± 4.29 mg/l, being showed in Sidi Abdel rahaman region, it was observed that the high increament in the mean average concentrations of chloride could increase the tendency of water to cause corrosion in different water disribution and desalination system, as water with high levels of sodium, chloride, or other ions will promote the corrosion process as complying with Lin et al.[Citation11] showed that the highest mean average value of sulfates was observed in Sidi Kirir region with mean average value 3184.16 ± 16.09 in mg/l while its lowest value was 3166.12 ± 11.06 mg/l at Sidi Abdel rahaman region. It was repored that the presence of the metallic materials in water distribution channels is easily exposed to major corrosion risks due to the conversion of sulfate in water to corrosive sulfides by anaerobic sulfate-reducing bacteria. Where the increment in the number of sulfur-producing bacteria increases the amount of hydrogen sulfide gas that reacts with metallic surfaces of the distribution system to cause corrosion as observed by Skoczko & Szatyłowicz.[Citation24] The corrosion products often include harmful heavy metals such as nickel (Ni], copper (Cu) and molybdenum (Mo) and less toxic metals such as iron (Fe) and zinc (Zn). This will be explained in which showed that the mean average concentration value of Cu in the selected site of Sidi Abdelrahaman was 0.058 ± 0.0156 µg/l, while the measured mean average value was 0.050 ± 0.016 µg/l at Alamin site and 0.075 ± 0.0125 µg/l in the Sidi kirir site inlet, where all these mean average values were being compared with the natural background concentrations in seawater which was 0.12 ppb for the Mediterranean as reported by Lin et al.[Citation11] It was reported that copper concentrations in seawater cover a wide range of values: the range of concentrations for open sea is 0.04-0.70 ppb, while for coastal waters the range is 0.01-50 ppb. Assuming 20 ppb copper in the brine of a desalination plant with a capacity of 50,000 m3 per day with water conversion of 10% then more than 10 kg of copper will be discharged with the 500,000 m3 brine, as complying with the report recorded by IAEA.[7] showed that the highest mean average concentration value of calcium hardness was showed in Sidi Kirir region with mean average concentration value was 1164.66 ± 20.69 mg/l, while its lowest mean average concentration value was 1154.66 ± 19.69 mg/l, at Sidi Abdel Rahaman region. In case of Mg-hardness, indicated that the highest mean average value of Magnesium hardness was showed in Sidi Kirir region with mean average concentration value 5405.58 ± 108.08 mg/l, while its lowest value was 5311.58 ± 100.08 mg/l, showed at Sidi Abdel rahaman region, this means that the probability of using the selected site of Sidi Kirir as desalination unit will be more exposed to logging by scale formation than the other selected inlet sites for desalination pipes, therefore using Sidi Kirir as nuclear desalination intake site will be required the using of more amounts of anti scaling chemicals than the other two selected intake sites.
Table 2. Measured physicochemical parameters of marine water samples of three intake sites during the period 2/10/2021-12/3/2022.
4.2. Using scale saturation indexes
and showed that there are an ascending arrangement in the degree of scale formation in the selected sampling sites as follows: Sidi Kirir ˃ Al-Alamine ˃ Sidi Adel rahman, where indicated that there are ascending arrangement in the degree of scale formation and the saturation index type as follows: Ryzanar Index ˃ Puckorus index ˃ Langlier Saturation Index (LS) ˃ Stiff-Davis-stability index, there are different t-paired correlations between the different saturation indexes were done, where there is a significance +ve t-paired correlation between Langlier Saturation Index (LS) and Stiff-Davis-stability index (SDI) with value 0.999, while there is a – ve t-paired correlation between LS and Ryzanar Index (RI) with value −0.1, finally there is a – ve t-paired correlation between LS and Puckorus Index with value −0.53. These previous correlations were used as helpful chemical tool in determining the suitable amounts of anti scale formation chemicals which should be applied in each selected site, which may be selected for the desalination process of the nuclear coastal power plant. The using of Puckorious stability index showed that there will be a high degree of scale formation in the all three selected regions where all the obtained results were with PSI values < 5.5 as shown in and where the highest scale formation value was found in Sidi Kirir site and lowest one was found in Sidi Abdelrahan region. As the result the lowest amount of ant scale chemicals were expected to be used in Sidi Abdelrahan’s region while the large amounts was expected to be used in Sidi Kirir’s region. In case of using corrosion stability indexes it was shown that the Sidi Kirir was the site most exposed to the corrosion risk, compared to the other selected sites, where its Stiff Stability Index was −0.28 as shown in which means that, its value was −0.5< LSI and SDI <0, this will lead to a slight degree of corrosion as being recommended in Stiff Davis stability index (SDI) limits which were used in case of using sea water. These results will support the consuming of more amounts of anticorrosion chemicals in case of using Sidi Kirir as intake site than the other selected intake sites.
Figure 1. Determination of corrosion risks in the different selected desalination intake sites where Sidi Abdelrahaman in blue and Alamin in red while Sidi Kirir in green series.
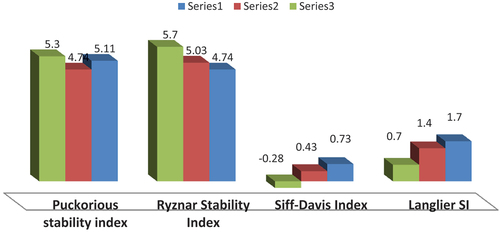
Table 3. Determination of corrosion risks in the different selected desalination intake sites.
4.3. Determination of the toxicological and ecological risks which being resulted from the using of different biocides in the three desalination intake sites
Chlorine dioxide ClO2 is considered as one of the most recommended biocide being used in both cooling water and desalinations systems, because of its lowest cost and its high biocide efficiency. Using chlorine dioxide as oxidizing biocides had different degrees of toxicity on marine living organisms found in the selected intake inlet sites. showed that in case of using ECOSAR software the ClO2 values will have Log Kow = −3.22, with water solubility 1E+006 mg/l, where its LC50 value of Fish with 2.71E+006 mg/l at 96 hr, and its LC50 of Daphnia at 48 hr was 9.49E+005 mg/l, where its EC50 value of green algae was 9635.99 mg/l, these effective levels will not exceed the solubility of chlorine dioxide which was 1E+006 by 10x, so that an ecological effects will be occurred when using chlorine dioxide in the three suggested intake sites. On the hand, showed that the using of Quaterphenyl as non oxidizing biocides [c1c(cccc1c2cccc(c2)c3ccccc3)c4ccccc4] had different degrees of toxicity on marine living organisms which were being found in the selected intake inlet sites of the suggested desalination units, as the using of ECOSAR software showed that the Quaterphenyl C24H28 will have Log Kow value 7.28, where the Quaterphenyl LC50 value of Fish was 0.005 mg/l at 96 hr, and the LC50 value of Daphnia at 48 hr was 0.004, where its EC50 value of green algae was 0.023, these effective levels not exceed the solubility of Quaterphenyl which was 0.0067 by 10x so there will be an ecological effects being occurred from the using Quaterphenyl on both Fish, Daphnia and green marine living organisms at the suggested inlet intake sites which may be suggested. showed that the ecological effects which are resulting from the using of chlorine dioxide (oxidizing biocide) are more higher than the ecological effects resulting from the using of Quaterphenyl (non oxidizing biocide), this will be very helpful tool for the decision maker to take decision of using non oxidizing biocide Quaterphenyl in the three selected intakes sides as mitigation result of the EIA Study.
Figure 2. Relation between ecological and toxicological risks of both oxidizing and non oxidizing biocide by using ECOSAR software.
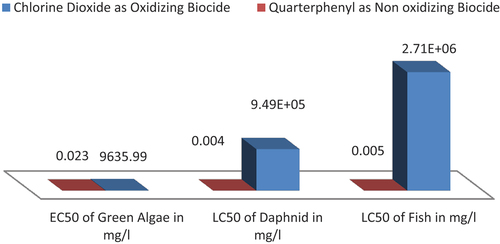
Table 4. Using ECOSAR model to calculate different ecological risks resulting from the using of chlorine dioxide as biocide in the selected cooling water sites.
Table 5. Using ECOSAR model to calculate different ecological risks resulting from the using of Quater-phenyl as biocide in the selected cooling water sites.
4.4. Determination of the toxicological and ecological risks resulted from the using of different anti-scales formation in the suggested three intake sites
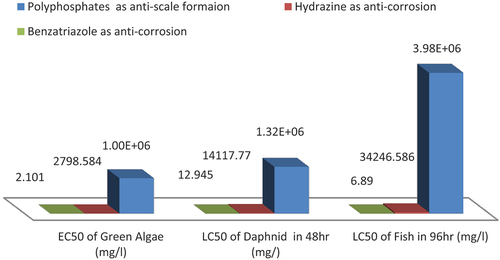
showed that the using of polyphosphates in the suggested intake inlets as ant scale formation chemical had different degrees of toxicity on marine living organisms being found in the selected intake inlet sites, as described , where using ECOSAR software showed that polyphosphates has Log Kow = −2.708, and water solubility was 1E+006 mg/l, where its LC50 value of Fish was 3.58E+006 mg/l at 96 hr, and its LC50 of Daphnia at 48 hr was 1.32E+006 mg/l, while its EC50 value of green algae was1.630E+005 mg/l, these effective levels not exceed the solubility of polyphosphates which was 1E+006 mg/l by 10x, so that there will be an biological effects resulting from the using polyphosphates as one of the effective anti scale formation which being recommended to be used in inlet intake sites for both thermal nuclear desalination plants.
Table 6. Determination of different ecological risks which are resulting from the using of polyphosphates as anti-scales formation by using ECOSAR code.
4.5. Determination of the toxicological and ecological risks resulting from the using of anticorrosion in the suggested three intake sites
The using of hydrazine H4N2 in the suggested intake inlets as one of the effective anticorrosion chemicals which has different degrees of toxicity on marine living organisms, which being found at the selected intake inlet sites. showed that in case of the using ECOSAR software it showed that hydrazine has Log Kow = −1.468, with water solubility 1E+006 mg/l, where its LC50 value of Fish was 34246.586 mg/l at 96 hr, and its LC50 value of Daphnia at 48 hr was 14117.779 mg/l, where its EC50 value of green algae was 2798.584 mg/l, these effective levels not exceed the solubility of polyphosphates which was 1E+006 by 10x, so that there will be an biological effects resulted from the using of hydrazine as one of the effective anticorrosion chemicals which recommended to be used in the inlet intake sites of thermal and nuclear desalination plants. The using of benzatriazole in the suggested intake inlets as one of the effective anticorrosion chemicals and is recommended as it makes an protective layer for copper alloy which are widely being used in both cooling water systems and desalination plants for both nuclear and thermal power plants, on the other hand both hydrazine and benzariazole chemicals had different degrees of toxicity on marine living organisms found in the selected intake inlet sites, showed that in case of using ECOSAR software, it showed that benzatriazole has Log Kow = 2.926, and water solubility was 85.98 mg/l where its LC50 value of Fish was 6.89 mg/l at 96 hr, and its LC50 value of Daphnia at 48 hr was 12.945, where its EC50 value of green algae was 2.101 mg/l, these effective levels are not exceed the solubility of polyphosphates which was 85.98 by 10x so that there will be an biological effects of using benzatriazole as one of the effective anticorrosion chemicals which being recommended to be used in the intake sites of both thermal and nuclear desalination plants.
Table 7. Determination of different ecological risks resulting from the using of hydrazine as anticorrosion by using ECOSAR code.
Table 8. Determination of different ecological risks resulting from the using of benzatrizol as anticorrosion by using ECOSAR code.
showed that the ecological effects resulted from the using of polyphosphates (anti scale formation) is more higher than the ecological effects resulted from the using of both hydrazine and benzariazole chemicals (anticorrosion chemicals), this helps the decision maker to take a decision by diluting the outlet discharge of different amounts of polyphosphates with cooling water in case of thermal power plants or brine salted water to mitigates is ecological impacts on marine organisms. While showed that the using of benzariazole anticorrosion in the three selected intakes sites as mitigation measure of the EIA Study, will produce less ecological impacts on marine organisms than the using of hydrazine anticorrosion chemical.
4.6. Determination of the economical impacts of the suggested regions for nuclear and thermal desalination plants by using DEEP code
4.6.1. In case of nuclear desalination units
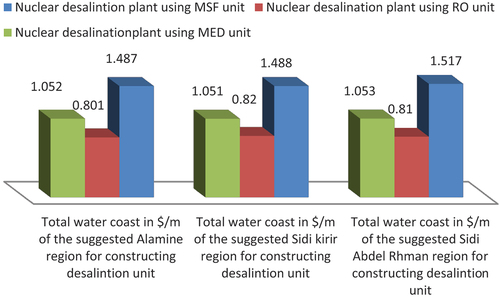
showed that most of the different water cost values of the selecting sites are seemed to be similar, as the most of the operating conditions were being used in DEEP code were similar at these three selected regions. There is significant difference in the data especially in Alamin site. Where showed that Alamin site was the lowest water desalination cost by using the RO for nuclear desalination process than the other three selected intake sites. On the other hand the RO will have more bio-fouling and corrosion problems than the other MSF and MED desalinations units, as being discussed in previous figures. As the result the RO desalination unit need to inhibit the corrosion of membranes by using different corrosion inhibitors, which should be added to the reverse osmosis feed water unit. Where polymeric protective coating, rubber membranes should be used also for RO units as shielding for desalination contents and all of clay and epoxy nano-composite are being also used to prevent corrosion, where the stainless steel components may be coated with poly aniline/Zn – Porphyrin composite coatings.[Citation25] Therefore the ideal solution for new nuclear desalination unit was by using of combined desalination system including both MSF and MED types, as both of the two thermal desalination types (MED & MSF) are more resistance for scale formation and corrosion risks than RO systems, this recommendation will be supported by using results obtained from the different corrosion index & marine toxicity ().
5. Conclusions and recommendations
The study showed that that there is an increment of mean average concentrations values (mg/l) of the measured TDS, Chlorides, Hardness, and sulfates of the selected Sidi Kirir desalination intake site than other Alamin and Sidi Abdelrahaman sites, which will increase the probability of scale formation and corrosion impacts on desalination tubes of the selected intake site of Sidi Kirir than the other two selected sites, therefore Sidi Kirir desalination intake site will be need to use more amount of anti scaling chemicals (as polyphosphates) than the other two selected intake sites. while the ecological effects which resulted from the using of polyphosphates as anti scale formation were more higher than the ecological effects resulted from the using of both hydrazine and benzariazole anticorrosion chemicals, as this will help the decision maker to dilute the discharge of using polyphosphates with cooling water or brine salt water in case of using desalination intake sites, as this will mitigate the ecological impacts on different marine organisms. On the other hand the using of benzariazole in the three selected desalination intakes sites was recommended as anticorrosion mitigation measure of this study, as this will produce less ecological impacts on marine organisms than the using of hydrazine anticorrosion chemical. The ECOSAR results showed that the ecological effects (LC50 & EC50) which resulted from the using of chlorine dioxide (oxidizing biocide) were more higher than the ecological effects resulted from the using of Quarter phenyl (non oxidizing biocide). The using of the economical risk code (DEEP), showed that the nuclear plant with Reverse osmosis (RO) desalination unit had the lowest water cost value than the other nuclear power plants, which are using both of the Multiple Effects Desalination (MED) and Multiple Stage Flash (MSF) desalination types, while the analytical physicochemical and corrosion index results showed that the RO desalination unit will be exposed to more corrosive and scale formation impacts than the using of both of MED and MSF desalination types. The study recommend the using of both MED & MSF desalination units, on the other hand, study showed that both MED & MSF units had less corrosive and scale formation impacts on the studied nuclear desalination power plant than the using of RO desalination unit.
Ethics approval
All of the experimental protocols were not need to get ethic approval as there were no experimental animals used.
Consent to publish
All of authors consent that this manuscript was published in this journal.
Author contribution
Dr. Mohamed Safwat is corresponding author and he is responsible for all physiochemical analysis and using and evaluation of different corrosion indexes, ECOSAR and DEEP software, as he is also responsible for explaining all the chemical and ecological results while Dr. Nadia is responsible for using GIS code and making different ecological maps from the results being obtained.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Funding
The authors have no funding to report.
Data availability
All data generated or analyzed during this study are included in this published article
References
- W, M. S.; Valdez Sales, B.; Ocampo Diaz, J.; Eliezer, A. Corrosion Control in the Desalination Industry. In Desalination, Trends and Technologies; Schorr, M., Ed.; InTech, 2011, February; pp 71–86.
- Khordagui, H. Assessment of Potential Cumulative Environmental Impacts of Desalination Plants around the Mediterranean Sea Sustainable Water Integrated Management (SWIM) - Project funded by the European Union, 2013.
- Shames, H. A.; Ashur, M. M.; Shames, A. A. Social, Environmental and Economical Impacts Assessment for Seawater Desalination in Libya. 1st National Conference on Marine and Groundwater Pollution (1stNCMGP-2017), Tripoli, Libya, 2017; p 49–59.
- Dawoud, M. A.; Al Mulla, M. M. Environmental Impacts of Seawater Desalination: Arabian Gulf Case Study. Int. J. Environ. Sustainability. 2012, 3, 22–37.
- Cuanca, J. C. Report on Water Desalination Status in the Mediterranean Countries; Institute of Murciano (Murcia): Spain, 2012.
- World Nuclear Association. World Nuclear Performance Report; World Nuclear Association: London, UK, 2020.
- IAEA. Toolkit on Nuclear Desalination: DE-TOP User Manual Version 2.0 Beta; IAEA: Vienna, Austria, 2013.
- Mansouri, N. Y.; Ghoniem, A. F. Does Nuclear Desalination Make Sense for Saudi Arabia? Desalination. 2017, 406, 37–43. DOI: 10.1016/j.desal.2016.07.009.
- MEDRC. Environmental Planning, Prediction and Management of Brine Discharges from Desalination Plants. Final report by Tobias Bleninger & G.H. Jirka; Middle East Desalination Research Center Muscat: Sultanate of Oman, 2010.
- Mirzabeygi, M.; Naji, M.; Yousefi, N.; Shams, M.; Biglari, H.; Mahvi, A. H. Evaluation of Corrosion and Scaling Tendency Indices in Water Distribution System: A Case Study in Iran. Desalin. Water Treat. 2016, 5757(5454), 25918–25926. DOI: 10.1080/19443994.2016.1162206.
- Lin, L.; Jiang, W.; Xu, X.; Xu, P. A Critical Review of the Application of Electromagnetic Fields for Scaling Control in Water Systems: Mechanisms, Characterization, and Operation. NPJ Clean Water. 2020, 3(11), 25. DOI: 10.1038/s41545-020-0071-9.
- Hogan, M. Mediterranean Sea. In Encyclopedia of Earth; Cutler, J., Ed.; Environmental Information Coalition, National Council for Science and the Environment: Cleveland Washington, D.C, 2011.
- Al-Bloushi, M.; Saththasivam, J.; Al-Sayeghc, S.; Jeong, S.; Ng, K. C.; Amy, G. L.; Leiknes, T. Performance Assessment of Oxidants as a Biocide for Biofouling Control in Industrial Seawater Cooling Towers. J. Ind. Eng. Chem. 2017a, 59, 127–133.
- Al-Bloushi, M.; Saththasivam, J.; Jeong, S.; Amy, G. L.; Leiknes, T. Effect of Organic on Chemical Oxidation for Biofouling Control in Pilot-Scale Seawater Cooling Towers. J. Water Process. Eng. 2017b, 20, 1–7. DOI: 10.1016/j.jwpe.2017.09.002.
- Al-Karaghouli, A.; Kazmerski, L. L. Energy Consumption and Water Production Cost of Conventional and renewable-energy-powered Desalination Processes. Renew. Sustain. Energy Rev. 2013, 24, 343–356. DOI: 10.1016/j.rser.2012.12.064.
- Egypt Nuclear Power Plant Authority (NPPA). http://www.nppa.gov.eg (accessed October 19, 2020).
- IAEA. Toolkit on Nuclear Desalination: DEEP 5 User Manual; IAEA: Vienna, Austria, 2013.
- Baired, R. B.; Eaton, A. D.; Rice, E. W. Standard Methods for the Examinations of Water and Wastewater, 23rd.;. APHA, AWAWA, Water Environment Federation, USA, 2020.
- Wang, R.; Wang, N.; Mingli, Y.; Zhu, Y. Determination of Low Level Anions in Seawater by Ion Chromatography with Cycling Column Switching. J. Chromatogr. A. 2012, 1265, 186–190. DOI: 10.1016/j.chroma.2012.09.086.
- Altman, S. J.; Jensen, R. P.; Cappelle, M. A.; Sanchez, A. L.; Everett, R. L.; Anderson, H. L.; McGrath, L. K. Membrane Treatment of side-stream Cooling Tower Water for Reduction of Water Usage. Desalination. 2012, 285, 177–183. DOI: 10.1016/j.desal.2011.09.052.
- Amjad, Z.; Demadis, K. Mineral Scales and Deposits: Scientific and Technological Approaches, 1st ed. Science Direct: Elsevier, 2015.
- Lis, J. L.; Fuentes-Bargues. Analysis of the Process of Environmental Impact Assessment: For Seawater Desalination Plants in Spain. Desalination. 2014, 347, 166–174. DOI: 10.1016/j.desal.2014.05.032.
- Mayo-Bean, K.; Moran-Bruce, K.; Vince Nabholza, J.; Meylan, W. M.; Philip Kim, H. S.; No, H. C. Thermal Coupling of HTGRs and MED Desalination Plants, and Its Performance and Cost Analysis for Nuclear Desalination. Desalination. 2012, 303, 17–22. DOI: 10.1016/j.desal.2012.07.004.
- Skoczko, I.; Szatyłowicz, E. Treatment Method Assessment of the Impact on the Corrosivity and Aggressiveness for the Boiler Feed Water. J. Water. 2019, 11, 1–14.
- Kamees Thabet, H. K.; El Shamy, O. A. A.; Ashmawy, A. M.; Deyab, M. A. The Impact of Corrosion Inhibitors in Desalination Systems. ACS Omega. 2023, 8(48), 45224–45231. DOI: 10.1021/acsomega.3c07129.