ABSTRACT
Hydrogels are a series of soft and wet materials with three dimensions of crosslinked networks. Hydrogels have attracted great attention due to their diverse functional properties, and their wide range of applications, such as in soft robots and actuators, stretched electronic devices, tissue engineering materials, controlled-release drug delivery vehicles, biomedicine materials, food science, and (bio)sensors. In general, there are four core concerns in hydrogel science, including the polymer source, structure fabrication, gel function, and gel applications. According to the logic that the “structure determines function”, it is believed that rational design of structures can effectively regulate the functions and applications of hydrogels. Hence, in the current review, “structure” as the core topic will be highly regarded, and the crosslinking mechanisms and structural diversity of hydrogels are comprehensively summarized. Additionally, hydrogels also show their great application potential in food science. Hence, the current review also pays more attention to the application of hydrogels in food nutrition and health, food engineering and processing, and food safety. It is whished that this review not only serves as a reference for improving the comprehensive understanding of the structural design of hydrogels but also provides a forward-looking idea for hydrogel applications in food science.
Graphical abstract
Graphical Abstract
Introduction
Hydrogels, also referred to as aqua gels, are a series kind of soft and wet materials that have low-volume-fraction, three-dimensional porous networks of polymer molecules, fibers or particles, in which the water or aqueous phase acts as the dispersion medium.[Citation1-3] Depending on the hydrophilicity endowed to the hydrogel from some hydrophilic residues of the polymer(s), as well as on the nature and density of the network joints, such network structures can absorb up to several thousand times their dry weight to form chemically stable or biodegradable gels in the swollen state.[Citation2,Citation4] Hydrogels differ in size, architecture, and function. Depending on the source of the polymers, method of preparation, ionic charge, size, physical and structural features, [Citation2,Citation5–10] etc., hydrogels can be classified into several categories, as detailed in . It is hard to determine whether a certain hydrogel definitely belongs to a particular type.
Table 1. Classification of hydrogels
Hydrogels, especially some hydrogels with special properties, such as responsive hydrogels, [Citation11–16] self-healing/self-recovering hydrogels, [Citation17–25] injectable hydrogels,[Citation26–29] strong adhesive hydrogels,[Citation30–34] and superabsorbent hydrogels,[Citation23,Citation35] etc. have been widely applied in many fields, such as soft robots and actuators, [Citation36–41] stretched electronic devices, [Citation42–51] tissue engineering materials, [Citation4,Citation17,Citation52–57] controlled-release drug delivery vehicles, [Citation50,Citation58–60] biomedicine materials, [Citation61–72] (bio)sensor,[Citation73–77] and even neutron shielding.[Citation78] Moreover, molecular synthesis, copolymerization, and the blending of synthetic polymers can be used to create a variety of hydrogels containing both synthetic and biomolecule moieties, such as topological hydrogels,[Citation79–81] nanocomposite hydrogels,[Citation82–85] double-network hydrogels,[Citation86–90] and hybrid hydrogels, and these hydrogels may exhibit more comprehensive performances (not just a simple sum of the properties of the two gel materials).[Citation91–96]
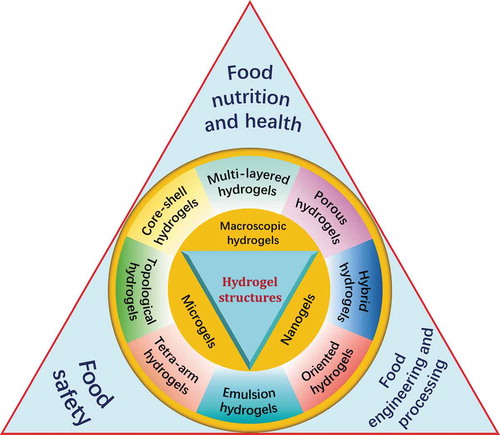
In general, there are four core concerns involved in hydrogel science, [Citation97] including the polymer source, structure fabrication, gel function, and gel applications. Then, “structure fabrication” can be divided into structure types and crosslinking approaches. There is no doubt that every aspect can prove its importance and indispensability with enough research and cases. However, according to the logic that the “structure determines function”, we are more willing to believe that the rational design of the structure is the premise of effectively regulating the hydrogel functions and is also a more targeted guarantee of the applications. Hence, we regard structure as the “core of core” in hydrogel science. Moreover, the crosslinking approach acts as the “bridge” between the gel sources and gel structures, while the structure type acts as the “bridge” between the gel structures and gel functions. However, few existing reviews can comprehensively offer the summary of structural diversity of hydrogels. Most of the existing hydrogel reviews focus on the certain applications of hydrogel and make a deep statement, or only focus on certain hydrogel structure. Fewer reviews can be summarized from the angle that structural richness of hydrogels.
The rapid developments in hydrogel applications require complex hydrogel structures. The challenge is that hydrogels have diverse structures. The main difficulty for researchers is how to determine what kind of functional properties a structure has and what kind of gelling method it needs. Most of the existing hydrogel reviews focus on the performance of a certain application of hydrogel and make a deep statement. Fewer reviews summarize the structural diversity of hydrogels. Therefore, the original intention of this review is not to focus on a specific function and application of hydrogels, for instance, smart hydrogels, super-strong hydrogels, etc., which are most often described in review papers, but to focus on summarizing the structural diversity and fabrication of hydrogels (whether it is by mature, conventional, or current technologies). Hence, in the current review, the crosslinking mechanisms and structural diversity of hydrogels will be discussed, respectively.
It is believed that the field of food science is an area where hydrogels can do their best, especially in the areas of food quality improvement, nutrient-modification, sensory perception optimization, targeted nutrient delivery and protection, calorie control, risk monitoring for food safety, and food packaging. Although compared with some areas of high concerned (such as biomedicine and tissue engineering), the applications of hydrogels in the field of food science are still very narrow. This status also shows that there are broad spaces to promote for the applications of hydrogels in the food science. Meanwhile, as the outer edges of “food” become more and more broad (not limited to edible), as well as the rapid development of hydrogel science brings more and more new technologies and new materials to the development of hydrogel science, causing cross integration to be an inevitable trend. Hence, it is expected that the rational design of the hydrogel structure will bring more functional applications to continuously meet the development of new foods (materials). In the current review, the application potential and process of structured hydrogels in food nutrition and health, food engineering and processing, and food safety will be also well discussed.
Crosslinking types and mechanisms
The formation of hydrogels requires two necessary conditions. First, the polymer molecules (synthetic or natural) must have hydrophilic groups on the main chain or side chain. Second, a certain crosslinking strength must be created between the molecules to form gel network structures. As shown in , hydrogels can be divided into four categories based on the crosslinking mechanisms of gelling, and these categories include physical hydrogels, chemical hydrogels, enzymatic hydrogels, and multi-crosslinked hydrogels.[Citation98–100]
Physical crosslinking
Physical hydrogels are formed via noncovalent interactions (intermolecular interactions), such as (a) electrostatic interactions, (b) hydrogen bonds, (c) crystallization, (d) metal-ligand coordination, (e) stereocomplex crystallization, (f) hydrophobic interactions, (g) conformation transformations, (h) host-guest interactions, (i) molecular-specific binding, and (j) π-π stacking. Physical gels can be sub-categorized into strong physical hydrogels and weak hydrogels. Strong physical hydrogels (for example, containing lamellar microcrystals, glassy nodules or double and triple helices, etc.) have strong physical bonds between the polymer chains, which are effectively permanent at a given set of experimental conditions. Hence, strong physical hydrogels are somewhat analogous to chemical hydrogels. Meanwhile, weak physical hydrogels have reversible crosslinks formed from temporary associations between the chains (for example, formed by hydrogen bonds, block copolymer micelles, hydrophobic interactions, and ionic associations, etc.). These associations have finite lifetimes and are constantly broken and reshaped, making them suitable for the preparation of self-healing hydrogels.[Citation101–108] There are many research reports involving the physical crosslinking, and this review tried to offer at least 10 types to provide readers with sufficient clues.
Electrostatic interactions
Electrostatic interactions generally take place between cations and anions, e.g., between paired anionic and cationic amino acid side chains in proteins, and between proteins, charged polysaccharides and polyampholytes,[Citation109–111] as well as between some charged polymers that mediated by oppositely charged ions (ionic bridges).[Citation76,Citation112–115] For instance, γ-polyglutamic acid (γ-PGA), a kind of polyanionic electrolyte, can undergo electrostatic interactions with polycations, such as chitosan (CS), to form hydrogels. In Tsao’s research, [Citation116] polyelectrolyte complex hydrogels comprising CS as the cationic polyelectrolyte and γ-PGA as the anionic polyelectrolyte were formed. Gong et al. [Citation109] fabricated one kind of tough and viscoelastic polyampholyte hydrogel with multiple mechanical properties via cationic-anionic bonds. Their results showed that randomness produces ionic bonds with a wide distribution of strengths. The strong bonds served as permanent crosslinking, imparting elasticity, whereas the weak bonds reversibly break and re-form, dissipating energy. In Yan’s research,[Citation117] anti-freezing hydrogels were designed and synthesized based on a zwitterionic poly(ionic liquid) (PIL). These zwitterionic PIL hydrogels exhibited potential in artificial skin with super-stretchability (approximately 900%), self-healing ability, and high conductivity (−1.1 S m−1) even at low temperature (−20°C).
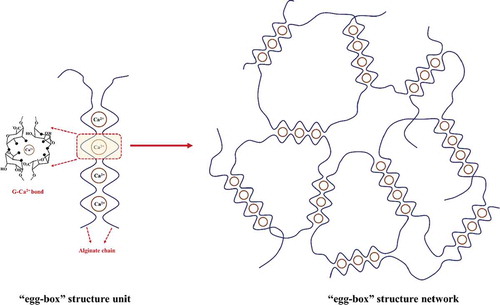
Sodium alginate (a highly charged anionic polyelectrolyte and a widely used gel-forming matrix) can form hydrogels by crosslinking with divalent cations, such as calcium ions (Ca2+), [Citation98,Citation118] due to the formation of cationic bridges between the α-L-guluronic acid (G) residues of alginate polymer chains, hydrogel with “egg-box” structure network can be fabricated [Citation119,Citation120] (). Similarly, propylene glycol alginate (PGA, high molecular weight linear polysaccharides) hydrogels can be formed via extensive hydrogen bonding and electrostatic point associations with Ca2+ .[Citation121] Although hydrogels formed via electrostatic interactions are simply manufactured by one-step-mixing procedures, their inelasticity and brittleness are still major drawbacks for further applications. One solution to overcome the challenges of ionically crosslinked hydrogels is to blend them with a covalently cross-linkable polymer to yield interpenetrating networks (see interpenetrating network hydrogels in Hybrid hydrogels Section) or double-network hydrogels (see double-network hydrogels in Hybrid hydrogels Section).[Citation76,Citation122,Citation123]
Figure 1. ‘Egg-box’ structure of hydrogel formed between G residues of alginate and CaCitation2+. Dimensions are not drawn to scale
Hydrogen bond
Hydrogen bond is one of the most common noncovalent interactions found in nature and plays a very important role in maintaining the stability of biomolecular systems. For instance, hydrogen bonds keep complementary strands of DNA together in their unique helix structure and play a determining role in the secondary and tertiary structure of folded proteins.[Citation24] Hydrogen bonds occur when a positively charge hydrogen atom establishes an electrostatic link with electronegative acceptor atoms, such as oxygen, nitrogen, or fluoride.[Citation124]
The traditional notion about hydrogen bonds is that they belong to relatively “weak” electrostatic attractions. However, many studies have shown that hydrogen bonds have partial covalent bond-like properties. Hydrogen bonds also exhibit a cooperative effect when multiple consecutive hydrogen bonds are formed within the hydrogel matrix and the strength (bond energy) of these hydrogen bonds exceeds the sum of the individual strength of these hydrogen bonds, contributing significantly to the mechanical properties of the resulting hydrogels. Therefore, the construction of high-mechanical-strength hydrogels and other materials by hydrogen bonds has received increasing attentions from material scientists.[Citation125–130] For example, Hu et al.[Citation131] designed a new type of “rigid and tough” hydrogel with an excellent elasticity (a high modulus of 28 MPa, a toughness of 9300 J m−3, an extensibility of 800%, and a tensile stress of 2 MPa) formed by the dense clustering of hydrogen bonds (H-clusters) within a loose chemical network. Furthermore, the gel displays a good fatigue-resistance and the complete and extremely fast recovery of the shape and mechanical properties. Moreover, Wang et al. [Citation132] reported the facile fabrication of a novel type of shape Poly(vinyl alcohol) (PVA)–Tannic Acid (TA) memory hydrogel that physically crosslinked by both strong and weak hydrogen bonding (H-bonding). The H-bonding between PVA and TA functions as the “permanent” crosslink and was stronger than the weak H-bonding between the PVA chains, which acted as the “temporary” crosslinking. The reversible breakage and formation of the weaker H-bonds imparted the PVA–TA hydrogels with excellent temperature-responsive shape memory properties. In 2017, Wang et al.[Citation133] reported a novel physical method for fabricating tough hydrogels with excellent mechanical properties and thermoplasticity by utilizing the H-bonds formed between PVA and some small molecular weight (SMW) molecules, such as glycerol, in which both molecules were capable of forming multiple H-bonds. With further research, they dried these PVA–glycerol hydrogels to obtain PVA–glycerol intermolecular complexes (IMCs) with excellent mechanical properties.[Citation134]
Crystallization
Crystallization is not a common approach for preparing physical hydrogels, but is commonly used to form PVA hydrogels and often accompanied by hydrogen bonding. PVA hydrogels are commonly prepared by a repeated freezing-thawing (FT) method and are physically crosslinked via the PVA crystallites formed during the freezing process and the H-bonds formed between the PVA chains,[Citation135–137] making PVA an ideal candidate for constructing tough hydrogels. For instance, Zhao’s group[Citation125] developed a melamine-enhanced PVA physical hydrogel with a therapeutic ultrasound-triggered shape memory ability. The multiple H-bonds between melamine and PVA resulted in a strong hydrogel, and the crystallites formed during the freezing/thawing process endowed the hydrogel with a shape memory ability. A similar study has been performed by Gong et al.[Citation138] Guo et al.[Citation139] designed a dual physically crosslinked hydrogel composed of PVA and poly 6-acrylamidohexanoic acid (PAACA) without any chemical crosslinkers. The H-bonds formed between the PAACA and PVA chains served as the first crosslinking points to construct the three-dimensional network, and the PVA crystalline domains generated during the freezing/thawing process acted as the second physical crosslinkers, which greatly enhanced the mechanical property. Wang et al.[Citation140] reported a type of super-strong and tough hydrogen-bonded PVA/polyacrylic acid (PAA) hydrogel prepared by immersing as-prepared PVA hydrogels in PAA aqueous solutions and subsequently applying a cold-drawing treatment to the hydrogels. In their research, the immersion process introduced PAA chains into the PVA hydrogels, which increased the crosslinking density by hydrogen bonding and, hence, much improved the mechanical properties of the hydrogels with low water contents. Additionally, the cold-drawing process also enabled the formation of more, stronger hydrogen bonds. The above-mentioned combined process impressively generated hydrogels with ultra-high tensile strengths that were even superior to those of biological tissues and most solid-engineered plastics.
Metal-ligand coordination
Coordination chemistry is vital to biological systems because it provides structural integrity to metalloproteins and catalytic centers for many enzymes.[Citation141–143] Coordination bonds also provide structural support to many living tissues.[Citation144,Citation145] One particular example of a coordination interaction is chelation, in which two or more separate binding sites exist on the same ligand with one central atom. In addition, most of the time, chelation occurs between an organic molecule and a central metal atom (of either metal ions or inorganic nanoparticles (NPs)). As the functionality of metal-ligand coordination hydrogels can be endowed from the center metal ions and corresponding ligands, the tenability and kinetic liability of hydrogels can be expected, resulting in the formation of reversible polymeric networks with mechanical strengths that suitable for various biomedical applications.[Citation146–151] A very common example of the application of metal-ligand coordination is alginate-CaCitation2+ bonding (see ). Another classic example is the unique self-healing properties found in mussel adhesive proteins,[Citation152,Citation153] refer to a series of catechol chemistry of bioadhesion, such as 3,4-dihydroxyphenylalanine (DOPA).[Citation145,Citation154,Citation155] Essentially, the alkaline pH condition and oxidant cations in seawater triggered the formation of metal chelates between non-oxidized catechols and multivalent cations, which endows the mussel adhesive proteins with a self-healing ability. Inspired by this metal chelation that occurs in mussels, many biomimetic hydrogels (mainly for self-healing applications) were reported.[Citation141,Citation145,Citation156–163] A recent comprehensive review about the assembly mechanism and application of metal–ligand coordination crosslinked hydrogels was released by Hilborn et al.[Citation141] Zhu and his coworkers[Citation164] assembled a silk fibroin (SF)-based self-healing hydrogel under physiological conditions by coordinating SF microfibers (mSF) with a polysaccharide binder via dynamic metal-ligand coordination (chelated by bisphosphonate ligands of the binder), as well as by generating a second robust dual crosslink via the photopolymerization (see Chemical crosslinking Section) of the acrylamide groups of the binder (Am-HA-BP). Similarly, in He’s research,[Citation165] the Eu (a red luminescent lanthanide metal)-iminodiacetate (IDA) coordination that occurs in a hydrophilic poly(N,N-dimethylacrylamide) matrix was used to prepare an Eu-containing polymer hydrogel demonstrating fast self-healing and tunable fluorochromic properties and responsiveness to five different stimuli (pH, temperature, metal ions, sonication, and force). In the latest research work by Lu et al.,[Citation166] a new class of bioinspired synergistic fluorescence-color switchable polymeric hydrogel actuators based on the supramolecular interaction of dynamic metal-ligand coordination was reported. This robust anisotropic soft actuator was based on smart multicolor fluorescent Eu/Tb-PNIPAMK6APA, which can mimic the synergetic color changing and shape morphing functions of some natural flowers and animals in response to a subtle interplay of various environmental stimuli (e.g., temperature and acidity/alkalinity).
Stereocomplex crystallization
Stereocomplex (SC) crystallization is a special type of crystallization, in which an interlocking polymer stereocomplex forms between two complementary stereoregular (opposite chirality) polymers,[Citation167,Citation168] and demonstrates altered physical properties relative to the parent polymer properties. SC crystallization is usually driven by specific intermolecular interactions (e.g., hydrogen-bonding interactions and van der Waals forces). Polymer chains can form organized, densely packed and strong intermolecular interactions in SCs. Syndiotactic and isotactic polymethyl methacrylate (PMMA) was the first pair of polymers reported by Fox et al.,[Citation169] and until now, poly(lactic acid) (PLA) was studied more. PLA is a representative bio-based, biodegradable, and biocompatible polymer and has been widely used as a polymer matrix to prepare hydrogels for applications as drug delivery vehicles and scaffolds in tissue engineering.[Citation170] Poly(L-lactic acid) (PLLA) and poly(D-lactic acid) (PDLA), two stereoisomers of PLA, can easily crystallize into SCs, forming enantiomeric blends or block copolymers.[Citation168,Citation171–173] Because SC crystallization requires alternative packing of different polymer chains in the same crystalline phase, making the different chains associate together to form physical crosslinking junctions.[Citation172] Therefore, the SC crystallization of polymer segments, which forms physical hydrogels, will be highly effective to enhance the number density and strength of physical crosslinking junctions, as well as improve the mechanical properties of hydrogels.[Citation174] As mentioned previously, compared with homo-crystallized PLA (hc-PLA), SC-PLA has some unique advantages, such as a better ability to crystallize, a lower hydrolytic/enzymatic degradation rate, and enhanced mechanical properties and thermal stabilities. Such characteristics had been utilized to tailor the mechanical properties/stability of hydrogels and to reduce the degradation rate of hydrogels. Many SC-induced hydrogels have been reported in Jing’s review.[Citation171]
Hydrophobic interactions
Hydrophobic interactions exist in both natural and synthetic hydrogels. For example, hydrophobic interactions occur during the heat-induced gelation of some globular proteins, such as soy isolate protein (SPI). When heated is applied to transform a native protein to a denatured protein, hydrophobic groups buried in the SPI core are exposed, and hydrophobic interactions between the proteins lead to aggregation, subsequently, three-dimensional network structures form.[Citation175–177] Moreover, disulfide bonds, repulsive electrostatic interactions, and hydrogen-bond interactions contribute to the heat-induced hydrogel structure.[Citation177]
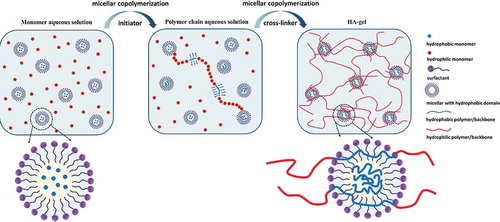
Hydrophobic interactions also exist in synthetic polymers, such as hydrophobic association polymers (HAPs) or hydrophobic association polyelectrolytes (HAPEs). HAPs and HAPEs are synthetic water-soluble polymers/polyelectrolytes containing a small proportion of hydrophobic groups (accounting for 5%–20% of the total amount of polymers), usually in the form of pendant side chains or terminal groups. In aqueous solutions, the hydrophobic groups of HAPs aggregate to minimize their exposure to water, and interactions form between the charged group of HAPEs and ionic surfactants, thereby forming inter- or intra-molecular associations resulting in hydrophobic microdomains.[Citation178,Citation179] Approaches including those related to the molecular weights, distribution sequence of the hydrophobic groups, type, and content of the hydrophobic groups, type and content of the ionic groups, and relative positions of the hydrophobic and ionic groups can be adopted to improve the hydrophobic association ability of HAPEs and HAPs.[Citation180–184] When the concentration of the hydrophobic monomer was above the critical micellar concentration (CMC), the hydrophobic monomers formed stable hydrophobic association domains in the presence of a surfactant. Meanwhile, intermolecular hydrophobic interactions or micro-block copolymerization lead to the formation of a three-dimensional network of polymer chains, resulting in a rapid increase in the apparent viscosity,[Citation178] in which the mixed micelles of the hydrophobic groups and surfactants would act as crosslinking points [Citation179,Citation185](). Hydrophobic association hydrogels (HA-gels) were firstly prepared by Liu et al. [Citation186] by using acrylamide (AAm) as a main component and octyl phenol polyethoxy ether as the hydrophobic segments. Due to their unique reversible, associative structure, excellent mechanical properties, and self-healing properties, [Citation185,Citation187–191] HA-gels have been extensively studied during the past few decades.[Citation191–195] Generally, HA-gels are prepared via micellar copolymerization (see Chemical crosslinking Section), which combines the water-soluble monomers with the hydrophobic monomer-containing micelles [Citation196] . HA-gels display a unique network structure, where the micelles act as physical crosslinking agents to associate with both the inner hydrophobic and external hydrophilic polymer chains.[Citation185,Citation189] When hydrogels are subjected to external forces, the dynamic crosslinking sites (micelles) disperse the stress and prevent the hydrogel from breaking, making HA-gels very suitable for use as self-healing hydrogels. Additionally, the hydrophobic molecular chains entangled within the micelles are unwound or slip to produce large amounts of dissipated energy, which greatly improves the toughness of the hydrogel. Similar research was reported by Okay et al. in 2016.[Citation187] Besides the inner hydrophobic and external hydrophilic polymer types and concentration, surfactants or emulsifiers that stabilize the structure of micelles also deeply impact the structure and properties (e.g., stress and toughness) of HA-gels.[Citation192,Citation197–201]
Conformation transformation
Coil/helix-β‐sheet transformation
Conformation transformations are common in protein-based hydrogels. For instance, silk fibroin (SF) was predominantly composed of helical structures and some β‐sheet content, whereas the SF in water adopted random coil conformations.[Citation202,Citation203] In solution, hydrophobic residues of SF are easily transformed from random coils or α-helices to thermodynamically stable β-sheet structures under some physical factors, such as the pH, vortex shearing, ultrasonication, heating, organic solvents, and electric fields, etc.[Citation203–205] However, most of the SF-based hydrogels exhibit poor mechanical performances, which is a major obstacle for their application. Zhu et al.[Citation206] developed a binary-solvent-induced conformation transition (BSICT) strategy to fabricate high-strength, durable all-silk fibroin hydrogel, in which solvent diffusion-induced conformational transitions from random coils and/or helical structures to β‐sheets were adopted to trigger and regulate SF gelation. Luo et al.[Citation205] obtained hydrogels demonstrating a superior mechanical performance by promoting the formation of small and uniform β-sheet structures via the strong interactions between SF-hydroxypropyl methyl cellulose (HPMC).
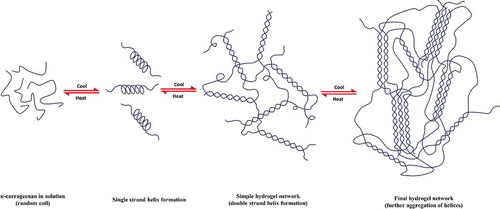
κ-carrageenan can also form hydrogels via a conformation transformation process. In a hot solution above the melting transition temperature, κ-carrageenan is present as a random coil conformation, while upon cooling, it transforms to rigid helical rods (). In the presence of salt (K+, Na+, etc.), the sulfonic group (SOCitation3−) screens the repulsive forces, causing double helices to further aggregate and form stable gels.
Coiled–coil interactions
The coiled-coil is one of the basic folding domains of native proteins and has been widely used in the self-assembly of biological and synthetic materials.[Citation207] Coiled-coils are left-handed super-helical bundles of two or more right-handed α-helices that are ubiquitous in cell matrix proteins and transcription factors. In protein-based hydrogels, coiled-coil folding domains have been used for effective physical crosslinks approaches, in which two or more α-helices are wound around each other to form a superhelix.[Citation208–212] Xu et al.[Citation209] designed three ABA triblock and two AB diblock polypeptides to explore the relationship between the structures of the polypeptides and the process by which they self-assembly into hydrogels and to investigate the parameters influencing the formation and physical properties of hydrogels. The results suggested that the self-assembled hydrogel and resulting physical properties may be influenced by some parameters critical to the hydrogel performance, which mainly contributes to the molecular arrangement of the polymer components.[Citation213] Coiled-coils have also been used to drive the assembly of hybrid hydrogels consisting of water-soluble synthetic polymers and engineered protein domains.[Citation207]
Host-guest interactions
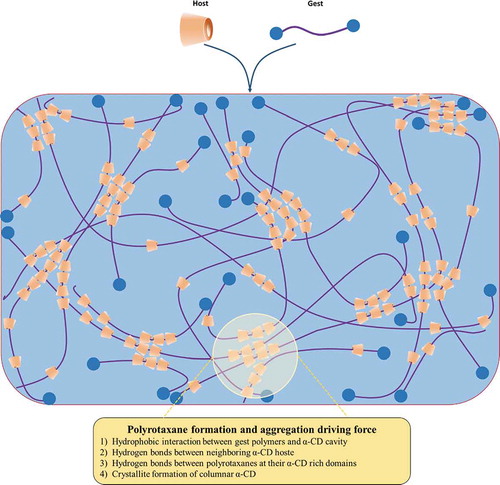
Host-guest interactions are kind of supramolecular interactions that have been widely used in supramolecular systems to create unique architectures, such as self-healing hydrogels.[Citation101] Cyclodextrin (CD) is a class of particularly interesting water-soluble host molecules and is usually used to construct various supramolecular self-assembled systems with appropriate guest molecules, e.g., azobenzene, vanillin, and adamantine, as well as linear polymers, such as poly(ethylene glycol) (PEG), poly(propylene glycol) (PPG), and poly(ε-caprolactone) (PCL).[Citation214–218] CD is a class of cyclic oligosaccharides, in which its most common members include α-, β- and γ-CD, which each comprise 6, 7 and 8 D(+)-glucose units, respectively. Due to its cyclic molecular structure, the glucose units in CD are oriented in a way that causes the outer surface of CD to be hydrophilic while its inner cavity is hydrophobic.[Citation219–221] This circular CD molecule (as a host) allows some long-chain polymers (as guests) to pass through, forming supramolecular polymers called polyrotaxanes, which contain multiple CDs threaded on the polymer chains and are end-capped by bulky molecules (called pseudo-polyrotaxanes if they are not end-capped by bulky molecules) [Citation219,Citation222] ().
Figure 4. Structures of polyrotaxane and pseudo-polyrotaxane formed via CD-based host-guest interactions
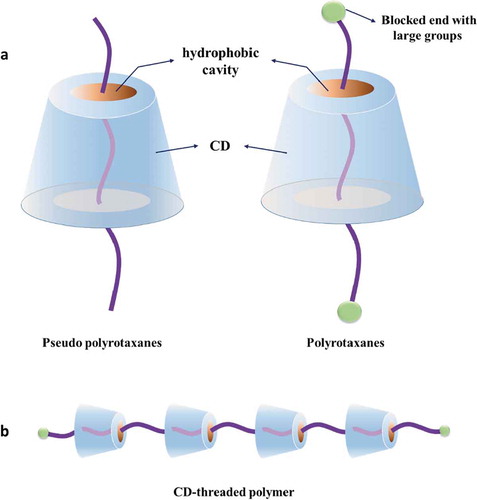
The condition and mechanism of hydrogel formation via CD-based host-guest interactions are shown in .
Molecular specific binding
Molecular hydrogels formed by the self-assembly of small molecules have attracted great research interest in recent years.[Citation223] During the formation process of a molecular hydrogel, a small molecule (a molecular hydrogelator) needs to self-assemble into a 3D matrix of nanofibers, nanorods, or nanospheres that can hold water molecules within the cavities of the 3D matrix.[Citation224] Generally, molecular-specific binding (e.g., antigen–antibody interactions, specific protein–peptide interactions, enzyme-substrate interactions, DNA base–pair interactions) is a very important way of forming self-assembled supramolecules. For example, Yang et al.[Citation224] rationally designed a fusion protein (ULD-TIP-1) with four binding sites and used the protein–peptide interaction to enhance the interactions between the self-assembled nanofibers, thus leading to a 3D fiber network with a high density of crosslinking points and the formation of molecular hydrogels. Fan et al.[Citation225] first synthesized thiolactone-grafted poly(glutamic acid) (PGA-HC) and cysteine-grafted poly(glutamic acid) (PGA-C) precursors. Then, a series of biocompatible and biodegradable hydrogels were formed by utilizing native chemical ligation (NCL) between the thiolactone group of PGA-HC and cysteine group of PGA-C as the crosslinking strategy. Other examples of self-assembled hydrogels are DNA supramolecular hydrogels.[Citation226–228] DNA can be considered to be a block copolymer containing only four kinds of monomers (A, T, C, and G bases). DNA can serve as a reversible crosslinker that modulates the mechanical and rheological properties of a hydrogel, in which DNA can selectively bind to a variety of different molecules, and DNA (aptamers) can bind to hydrogels, extending the range of stimuli to chemical and biological molecules.[Citation229]In recent years, DNA hydrogels have developed very rapidly, and many research reports have emerged.[Citation228,Citation230–237]
The use of the DNA hybridization reaction to prepare crosslinked hydrogel structures also needs to overcome a critical challenge to substantially increase the degree of swelling. The swelling rate of the DNA hydrogel hybridization reaction is generally reported to be only 10%-15%, which is not enough to achieve macroscopic material deformation. To solve this problem, Schulman et al.[Citation228] skillfully designed the DNA hybridization process by adding two polymerizable hairpin DNAs with complementary sequences, in which the DNA crosslinking chain in the original polyacrylamide hydrogel was opened, and DNA hybridization occurred continuously. The reaction extended the length of the DNA crosslinking chain in the hydrogel and increased the degree of swelling of the gel (a 100-fold volumetric hydrogel expansion was achieved by the successive extension of the crosslinks). We strongly recommend a recent review from Yang et al.,[Citation238] in which they highlighted the recent progress on DNA hydrogels from a polymeric perspective, including general design principles, synthesis strategies, and applications.
π–π stacking
π–π stacking is a special kind of space arrangement among aromatic compounds and is a weak interaction that exists in aromatic rings. π–π stacking usually occurs between two molecules that are relatively rich and short of electrons.[Citation28,Citation239,Citation240] π–π stacking is a noncovalent interaction that can also be employed for the design of self-healing hydrogels.[Citation241–243] In Shi’s research, [Citation244] a graphene oxide (GO)/DNA self-healing hydrogel was prepared by heating the mixing solution of GO with the double-stranded DNA at 90°C. The thermal process opened the double-stranded DNA chain to form single-stranded DNA (ssDNA), causing the exposure of the pentose groups on the DNA strand. As a result, the fast binding of the ssDNA chains to the GO sheets happened due to the π–π stacking that occurred between the unwound ssDNA chain and the GO sheet.
Chemical crosslinking
Chemically crosslinked hydrogels (also called true gels) are fabricated via the formation of covalent junctions between two polymer molecules so that the formed hydrogels are generally permanent, non-reversible, and stable. In the most succinct sense, a hydrogel is simply a hydrophilic polymeric network crosslinked in some fashion to produce an elastic structure. Thus, any technique that can be used to create a crosslinked polymer can be used to produce a hydrogel.[Citation2] There two main types of covalent junction approaches are used to prepare chemically crosslinked hydrogels: pathway via monomers and pathway via polymers.
Via monomers
Generally, as a very important technique used by the polymer industry to produce a large proportion of polymers, free-radical polymerization (FRP) is the main pathway to form polymers and to further form hydrogels via monomers. FRP is a form of chain polymerization that proceeds via a sequence of initiation, propagation, chain transfer, and termination reactions.[Citation245] The induced free radicals are mostly produced in pairs by disproportionation reactions of purposely added initiator molecules. There are four common FRP methods (), including bulk polymerization, solution polymerization, suspension polymerization (including inverse-suspension polymerization), and emulsion polymerization (including micellar polymerization). These reactions may take place in any of the three states of matter (i.e., the solid, liquid, and gas states).
Figure 6. Schematic diagram of the four common free-radical polymerization methods. Dimensions are not drawn to scale
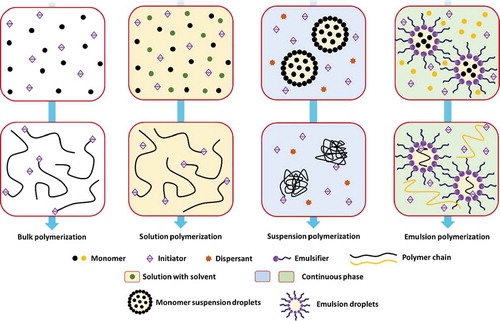
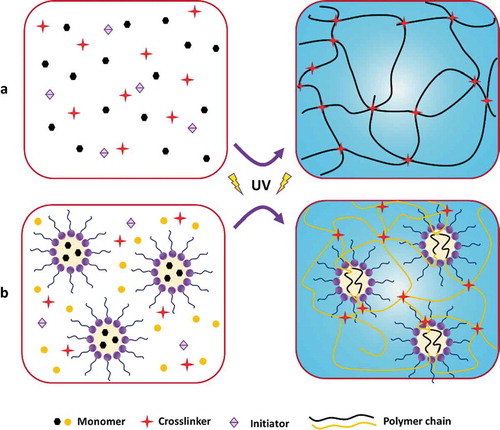
In general, the monomer, initiator (sometimes combined with the use of an activator), and crosslinker are the three integral parts of hydrogels prepared via FRP. The initiator is used to generate sufficient-free radicals, which can also be generated by redox reactions, decomposition reactions, heating, UV-irradiation, high-energy radiation, electrolysis, and plasma initiation. The crosslinker is used to form covalent crosslinks between the polymer chains ().
Figure 7. Schematic diagram of the hydrogels prepared via UV-induced free-radical polymerization. (a) Solution polymerization pathway, and (b) emulsion/micellar polymerization pathway. Dimensions are not drawn to scale
There is no doubt that the research involving conventional and controlled FRP processes is very diverse. For instance, Asatekin’s work[Citation246] demonstrated a new, simple, and reproducible method called interfacially initiated free-radical polymerization (IIFRP) for fabricating membranes with ultrathin (<100 nm), defect-free hydrogel selective layers on porous supports. In the research by Liu et al.,[Citation247] they reported a simple yet versatile method for assembling macroscopic-layered organogel–hydrogel hybrids via UV initiated free-radical polymerization to provide robust properties, such as excellent stretchability, tough interfacial bonds, enduring anti-swelling, and low dehydration.
Via polymers
In the pathway via polymers, monomers should be first polymerized into polymer chains, and then, crosslinking takes place among the polymer chains with the help of crosslinker(s). Hence, it is easy to understand that if a polymer is already made, only the crosslinking process is needed for the hydrogel process. Generally, there are three common pathways for the formation of a hydrogel from a polymer (both natural and synthetic); one is via a crosslinker, while the other is via high-energy radiation crosslinking, and the third pathway is via a chemical reaction[,Citation28,Citation248] such as a Schiff base reaction, Michael addition, and azide-alkyne cycloaddition reactions (click chemistry).
It is difficult to describe the type and amount of crosslinking agents used in synthetic polymers, because the type of polymer is too large, and the crosslinking reaction is complicated. For a synthetic polymer, it is necessary to first form an active site on the polymer chain, and then, a crosslinking reaction occurs between the active sites by using crosslinkers so that a connection point is formed between the polymer chains. In natural polymers, generally, crosslinkers such as glutaraldehyde, epichlorohydrin (ECH), genipin, N-hydroxysuccinimide (NHS), 1-(3-dimethylaminopropyl)-3-ethyl-carbodimide hydrochloride (EDC), and epoxy-based materials are commonly used.[Citation249–258] In particular, genipin is a low-toxicity, natural crosslinking agent derived from the gardenia fruit and can bridge the free amino groups of the lysine or hydroxylysine residues of different polypeptide chains by monomeric or oligomeric crosslinking.[Citation259] Due to its high biocompatibility and low-toxicity, many recent reports regarded genipin as a new bifunctional agent to replace traditional crosslinkers.[Citation260,Citation261]
High-energy radiation, such as gamma radiation or accelerated electrons, offers unique advantages for the synthesis of new materials and modification of existing materials; high-energy radiation techniques are simple, additive-free processes, where reactions such as polymerization, crosslinking, and grafting can be easily controlled.[Citation262,Citation263]
Schiff base reactions that self-crosslink the component polymers bearing amine groups and aldehyde groups to form acylhydrazone bonds (without additional chemical crosslinking reagents) are one kind of intermacromolecular in situ chemical crosslinking systems and have attracted wide attention due to their various advantages, such as biocompatibility, easily controlled reaction rate under mild conditions and suitability for injectable hydrogels.[Citation264–268] Several natural polysaccharides, including alginate, dextran, hyaluronic acid, chondroitin sulfate, carboxymethyl cellulose, and synthetic polypeptides, such as poly(l-glutamic acid) (PLGA), were reported for the preparation of hydrogels via Schiff base reactions.[Citation264,Citation269–276] For the detailed principle and application of Michael additions and click chemistry in hydrogel construction, readers are recommended to read the review from Ghorbani et al.[Citation248]
Enzymatic crosslinking
Enzymatic crosslinking provides the ability to generate strong covalent bonds between polymer chains under mild conditions.[Citation277] Unlike general crosslinking agents, the enzymatic crosslinking reaction is characterized by the fact that the enzyme molecule itself does not become part of the crosslinked molecular structure. Enzymes such as horseradish peroxidase (HRP), [Citation278–280] laccase,[Citation281–284] transglutaminase (TG),[Citation285–289] and tyrosinase[Citation290,Citation291] are commonly used. For example, HRP can catalyze the decomposition of hydrogen peroxide at the expense of aromatic proton donors, that is, to catalyze the coupling of several phenol and aniline derivatives using hydrogen peroxide as an oxidant.[Citation292] The HRP-mediated in situ formation of hydrogels in which researchers have focused has generally used natural polymers, such as hyaluronic acid, dextran, gelatin, poly(aspartic acid), poly(γ-glutamic acid) (γ-PGA), silk protein, and chitosan, as well as synthetic polymers, such as poly(L-glutamic acid) and 4-arm poly(propylene oxide)-poly(ethylene oxide) (PPO-PEO) oligomers.[Citation7,Citation277,Citation293–297]
Multi-crosslinking
Note that the classification of the crosslinking mechanism does not mean that a hydrogel will form base on only one approach. In fact, many hydrogels are induced by combined crosslinking methods, which are more commonly used to generate some complex hydrogels. Andrij Pich and Rienk Eelkema[Citation298] made a detailed comparison of macromolecular hydrogels (MHGs) and supramolecular hydrogels (SHGs) from aspects of gelling mechanisms, structure, function, and responsibility. In conclusions, they said synthetic MHGs and SHGs each have areas where they outperform the other, merging of the two worlds (MHGs and SHGs) seems to be an optimal way to design life‐like adaptive soft hydrogel materials. Xu et al.[Citation299] prepared mechanically superior (high-flexibility and high-toughness) double-crosslinked chitin hydrogels via a simple strategy to induce the formation of chemical and physical crosslinking domains within the chitin hydrogels, which was achieved by sequential chemical crosslinking with epichlorohydrin (ECH) and physical crosslinking via hydrogen bonding, hydrophobic interactions, and the formation of crystalline hydrates in aqueous ethanol solutions. In Zhou’s research, [Citation190] chitosan/PAA polyelectrolyte complex (PEC) hydrogels were first prepared via the in situ polymerization of AAc monomers in concentrated chitosan solutions, and then, he random and evenly distributed electrostatic interactions between the NHCitation3+ of chitosan and COO− of the PAA groups maintained the polymer network of the PEC hydrogels. The PEC hydrogels were then mixed with Ag+. During the Ag+ loading process, part of the electrostatic interaction between the polymer chains was shielded by the free ions, which resulted in the obvious swelling of the hydrogels and the sufficient absorption of Ag+ ions to form more coordination bonds. The dual physical crosslinking (DPC) hydrogels with the desired network topology were finally attained by the reorganization of the electrostatic interactions and coordination bonds. There are other multi-crosslinked hydrogels that you can refer to.[Citation188,Citation300–308]
The structural diversity of hydrogels
Hydrogels can be formed into almost any size and shape. In hydrogels, there are features with lengths spanning from centimeters to sub-nanometers. Generally, hydrogels can be classified into three main categories based on their sizes: macroscopic hydrogels (millimeters to centimeters) in the form of columns, spheres, porous sponges, matrices, films and fibers, microgels, and nanogels. The different forms and sizes (multiscale properties) of hydrogels can lead to large differences in the application and even lead to completely different functional characteristics. For example, according to Li’s review, [Citation5] hydrogels applied to delivery systems would differ in their delivery routes depending upon the different shapes and sizes.
However, the consensus is that, reasonable, fine structures are the basis for imparting hydrogels with excellent mechanical properties and also the basis for hydrogels effectively performing their functions. Owing to the rapid development of hydrogel applications, especially extensively demand for hydrogels with different functions, such as cell cultures, tissue engineering, soft robotics, and ionic devices, it is increasingly more difficult for hydrogels with a single structure to meet these requirements in terms of their mechanical properties and responsiveness to external stimuli. Hence, advanced techniques for fabricating hydrogel structures are being developed to meet the user-specified requirements, prompting researchers to pay increasingly more attention to hydrogels with complex network structures, and indicating that the rational design of structures can achieve the effective regulation of the functional properties of hydrogels. Therefore, the structural diversity of hydrogels is described in this section.
Microgels
Microgels (100 nm-10 μm) are colloidal dispersions (or suspensions) of gel-like particles, as well as macromolecular networks swollen by the solvent.[Citation309–311] Microgels are unique systems, differ from conventional colloids comprising rigid nanoparticles, flexible macromolecules, micelles, or vesicles. However, microgels possess unique properties of both hydrogels and colloidal particles, such as structural integrity, compartmentalization, orthogonal functionalization, softness, deformability, permeability, stimuli-responsiveness, reversible swelling, and adaptivity, and have been found to be promising for a wide range of applications in various fields including controlled drug release, separation technology, bio-, and chemical sensors, etc.[Citation312–326] Microgels can be fabricated mainly via emulsion polymerization with an added surfactant, surfactant-free emulsion polymerization (SFEP), inverse mini-, and microemulsion polymerization, as well as the crosslinking of prepolymer chains, etc.[Citation327,Citation328] Typically, the techniques used to break-up and gel the particles for microgel formation will determine the final microgel properties, including the structure, size, and strength [Citation329] and the presence and amount of crosslinks determine the “colloidal” or “macromolecular” character of the microgel.[Citation330] Considering that microgels have a larger particle size distribution than nanogels and suffer from residual surfactant contamination caused by conventional SFEP, Du et al.,[Citation331] innovatively utilized the reactivity between the unprotected catechol groups of the polymer chains and the radicals of the propagating chains to form a microgel via the SFEP of the acrylamide-type main monomers without using any surfactant stabilizer or crosslinker. Zhang et al.[Citation332] used binary microgel colloidal crystals as a template, in which the large microgels with surface thiol groups were arranged into a close-packed lattice, a few small microgels with surface vinyl groups occupied the tetrahedral or octahedral interstitial sites, and, then, the structure was immobilized via an in situ thiol-ene reaction under UV irradiation.
Considering the fact that microgels are highly porous colloids, the functionalization of the particle surface or interior makes it possible to selectively attach different functions. In addition, the open structure of a swollen microgel allows small molecules to diffuse into the porous colloid, and the diffusion rate can be regulated by the swelling degree of the polymer network.[Citation333,Citation334] Rueping et al. [Citation335] described a new adaptable microgel-based colloidal catalyst system based on temperature-responsive polymer microgels that combine the advantages of homogeneous and heterogeneous catalysts. The new adaptable catalyst system consisted of a porous microgel structure, in which a covalently attached organocatalyst acted as a catalysis center, mimicking the active site of an enzyme. The reviews from Richtering et al.[Citation8] and Agrawal et al.[Citation336] are recommended. Additionally, in Farooqi’s review, [Citation337] the advancement in multifunctional poly(styrene)-poly(N-isopropylacrylamide) based core-shell microgels and their applications were summarized.
Nanogels
Nanogels are nanosized (0-100 nm) hydrogel particles composed of physically or chemically crosslinked polymer networks.[Citation338,Citation339] Nanogels possess features and characteristics of both hydrogels and nanoparticles at the same time.[Citation340] In essence, there is little difference between nanogels and microgels, except the application scenarios caused by the size effects (e.g., nanogels can easily permeate due to their extremely small size and, more specifically, can cross the blood-brain barrier) and some specific functions (e.g., nanogels have non-immunological responses, and invasion by the reticuloendothelial system is prevented) are different.[Citation341,Citation342] Due to the high stability, biodegradability, biocompatibility, large surface area, long circulation time in blood, tunable size, and minimal resources required to manufacture nanogels, nanogels (administered by oral, pulmonary, nasal, ocular, and topical routes) have attracted great attention for nanomedicine applications, for instance, as pharmaceutical drug carriers.[Citation341,Citation343–346] Akiyoshi et al.[Citation347] focused on amphiphilic polysaccharide self-assembled nanogels (self-nanogels) and their application in immunotherapy because one of the most attractive characteristics of self-nanogels is their ability to function as molecular chaperones, which enables them to capture various protein and peptide molecules within their polymer matrix via the formation of hydrophobic interactions.[Citation348] In addition, Bian’s group reported an easy and scalable method for preparing single-chain nanogels (SCNGs) with improved efficiency and application in biomedical area.[Citation349,Citation350]
Core-shell-structured hydrogels
Most of the time, when we mention the core-shell-structured hydrogels, it actually refers to a class of microgels/nanogels with special structures. As illustrated in , these microgels/nanogels couple two chemically crosslinked networks within one particle, [Citation351–353] which results in inhomogeneous but chemically stable single particles. Core-shell microgels are highly stable and can be functionalized and easily recovered since the solid cores are encapsulated by multi-sensitive shells, and thus, these characteristics are preferred over those of homogenous microgels.[Citation354–356] Many studies have examined hydrogels with core-shell structures. For example, Farooqi et al.[Citation357] synthesized polystyrene-poly(N-isopropylmethacrylamide-acrylic acid) core-shell microgels loaded with silver nanoparticles (NPs), which were used as a recyclable catalyst for the rapid reduction of Congo Red dye. The same research group [Citation358] also used a precipitation polymerization method to fabricate nearly mono-dispersed spherical core-shell polymer microgels consisting of an un-responsive polystyrene (PSt) core and a temperature- and pH-responsive poly(N-isopropylmethacrylamide-acrylic acid).
Multi-layered hydrogels
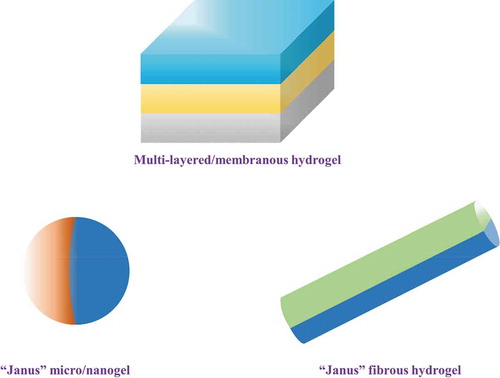
With the increasing requirements for complex functions, homogeneous hydrogels that with uniform bulk properties show more and more limitations, while those hierarchical hydrogels containing multiple layers/membranes show increasing advantages in terms of structural complexity and functional diversity play crucial role in many fields.[Citation84,Citation359–364] In addition to the traditional photo-polymerization technique,[Citation365,Citation366] in recent years, several effective technology paths have been developed for the fabrication of multi-layered hydrogels, such as layer-by-layer (LBL) technique,[Citation367–370] step-wise technique (mainly for onion-like multi-layered hydrogels),[Citation359,Citation361,Citation371–374] sequential electrospinning technique,[Citation375–381] and 3D printing (additive manufacturing) technique.[Citation382–385] Meanwhile, “Janus” structural hydrogels can be regarded as another kind of multi-layered hydrogels (), in which, word “Janus” stems from ancient, two-faced Roman god, to describe the two faces with asymmetry properties nowadays. “Janus-featured” hydrogels with different structures (particle/sphere, membrane, and fiber, etc.) and function for each layer can be particularly important in diverse applications.[Citation247,Citation386–390]
Porous hydrogels
Though hydrogels composed of 3D-crosslinked porous network, which show a significant ability to absorb water. Swelling properties are still one of the key performance indicators of hydrogels for some aspects such as biocompatible scaffolds, superabsorbent materials, high penetration materials, and drug-release vehicles, etc. According to report by Tanaka et al.,[Citation391] the characteristic time (t) of swelling is proportional to the square of a linear dimension (L) of the gel and is also proportional to the diffusion coefficient of the gel network (f), which is defined as t=E2/f. Hydrogels synthesized by traditional methods can obtain a higher swelling rate; however, the swelling kinetics are usually quite slow. Affected by the choice of materials and production process, the swelling and de-swelling time of hydrogels can be from several seconds to several hours, even a few days.[Citation392] Though reducing the size will increase the swelling rate of the hydrogels, it limits the application of the hydrogel in biomedical fields such as drug-controlled release, delivery, and tissue engineering. In order to get rid of the limitation of the size of the hydrogels on the swelling and deswelling rate, introducing a porous structure into a hydrogel is an effective method. Compared with nonporous hydrogels, in porous hydrogels, space among the pores rather than the 3D size of the gel determines the scale criterion of swelling time. The special structure filled with pores inside and on the surface of the porous hydrogels is easy to form a unique capillary channel. Capillary action reduces the water penetration resistance and greatly improves the value of diffusion coefficient (f), as well as the pores, are connected with each other to further increase the swelling/deswelling rate of the hydrogels. Meanwhile, the porosity also increases the high specific surface area of hydrogel networks, helpful for rapid diffusion of molecules into networks.
Porous hydrogels can be divided into three categories according to the pore size range: microporous hydrogels (10-100 nm), macroporous hydrogels (100 nm-10 μm), and supermacroporous hydrogels (tens to hundreds μm). There are several methods for synthesizing porous hydrogels, such as freeze-drying, [Citation393–396] templating, [Citation396–400] solvent-induced phase separation (SIPS), [Citation401–403] gas foaming, [Citation396,Citation404,Citation405] microgel annealing, [Citation406–410] 3D printing, [Citation396,Citation411] electrospinning, [Citation97,Citation396,Citation412] cryogelation, [Citation396,Citation413] and self-crosslinking,[Citation414] etc. It is always very interesting to pay more attention to the porous structure and overall porosity of the hydrogels. In 1998, superporous hydrogels (SPHs) were introduced as a different category of water-absorbent polymer systems was reported, [Citation415] and has made great development since then.[Citation416–421]
Hybrid hydrogels
Hybrid hydrogels are systems created from components of chemically, functionally, and morphologically distinct building blocks, which can include biologically active proteins, peptides, synthetic macromolecules, or nano/microstructures that are interconnected via physical or chemical means.[Citation422–424] That is to say, in theory, except a single structure or component, hydrogels should be hybrid hydrogels. Hence, there are many types of hybrid hydrogels, such as nanocomposite (NC) hydrogels, macromolecular microsphere composite (MMC) hydrogels, interpenetrating network (IPN) hydrogels, double network (DN) hydrogels, and double network-nanocomposite (DN-NC) hydrogels. Since the main function of the five above-mentioned kinds of hybrid hydrogels is to increase the mechanical strength, these hydrogels are also classified as high-strength hydrogels (topological hydrogels and tetra-arm hydrogels are also high-strength hydrogels and will be discussed in the followed Sections).
NC hydrogels
NC hydrogels are series of hybrid hydrogels that incorporate nanostructures into the hydrogel structure. For instance, inorganic nanomaterials include spheres (particles), rods, plates, fibrils, or tube-shapes [Citation425–430] and comprise graphene oxides, [Citation431,Citation432] nanoclays,[Citation433–437] double-layered oxides,[Citation84] and carbon nanodots[Citation438] incorporated into hydrogels with promoted functions, such as improved mechanical properties (e.g., stiffness, toughness, and tissue adhesive properties), bioactivities, and thermal responses. In Zhang’s review, [Citation439] functional nanoparticles/hydrogel composites were comprehensively summarized. Loh et al. [Citation430] comprehensively described the concept, design, and applications of nanoparticle–hydrogel composites, and three different supramolecular hydrogel-nanoparticle designs were summarized (). Generally, the final application of nanoparticle-hydrogel composites determines the approach choice, in which five main approaches are used to obtain a uniform distribution of nanoparticle-hydrogel composites (). In the past decade, more studies have focused on NC hydrogels with different structures, functions, and properties.[Citation440–451] We also recommend a review from De Cola, et al.[Citation27] about nanocomposite injectable hydrogels.
Figure 10. Three different hydrogel-nanoparticle structural designs exist: a) micro- or nano-sized hydrogel particles stabilizing inorganic or polymer nanoparticles, b) nanoparticles non-covalently immobilized in a hydrogel matrix, and c) nanoparticles covalently immobilized in a hydrogel matrix. Reproduced from ref. [Citation430] with permission. Copyright 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
![Figure 10. Three different hydrogel-nanoparticle structural designs exist: a) micro- or nano-sized hydrogel particles stabilizing inorganic or polymer nanoparticles, b) nanoparticles non-covalently immobilized in a hydrogel matrix, and c) nanoparticles covalently immobilized in a hydrogel matrix. Reproduced from ref. [Citation430] with permission. Copyright 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim](/cms/asset/a9e29764-a796-468e-ac29-2d01769f9618/lfri_a_1858313_f0010_oc.jpg)
Figure 11. Five main approaches used to obtain hydrogel-nanoparticle conjugates with uniform distributions: 1) hydrogel formation in a nanoparticle suspension, 2) physically embedding the nanoparticles into a hydrogel matrix after gelation, 3) reactive nanoparticle formation within a preformed gel, 4) crosslinking using nanoparticles to form hydrogels, 5) gel formation using nanoparticles, polymers, and distinct gelator molecules. Reproduced from ref. [Citation430] with permission. Copyright 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
![Figure 11. Five main approaches used to obtain hydrogel-nanoparticle conjugates with uniform distributions: 1) hydrogel formation in a nanoparticle suspension, 2) physically embedding the nanoparticles into a hydrogel matrix after gelation, 3) reactive nanoparticle formation within a preformed gel, 4) crosslinking using nanoparticles to form hydrogels, 5) gel formation using nanoparticles, polymers, and distinct gelator molecules. Reproduced from ref. [Citation430] with permission. Copyright 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim](/cms/asset/e0464394-feab-4f89-868e-df45fc6653d5/lfri_a_1858313_f0011_oc.jpg)
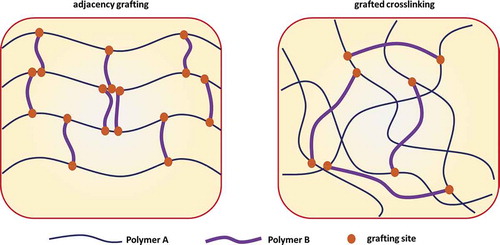
MMC hydrogels
The design concept of MMC hydrogels or microgel composite (MC) hydrogels is very similar to that of NC hydrogels. The only difference is that MMC/MC hydrogels use nanometer or even micron-sized polymer microspheres instead of nanoparticles as crosslinkers, and then, the monomers are grafted onto the surface of the microspheres by polymerization to form a hydrogel (macromolecular microspheres/microgels act as initiators) structure.[Citation424] The structure and possible interactions of the MMC/MC hydrogel are shown in and ,[Citation452] respectively.
Figure 12. Schematic illustrations of an MMC hydrogel structure. The MC hydrogel structure is similar to that of the MMC hydrogel structure as long as the macromolecular microsphere is replaced by a microgel
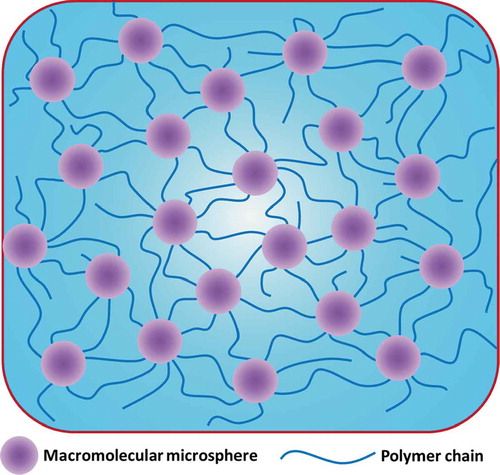
Figure 13. Main possible interactions contributing to the formation and toughening of MMC hydrogels. Reproduced from ref. [Citation452] with permission. Copyright 2013 American Chemical Society
![Figure 13. Main possible interactions contributing to the formation and toughening of MMC hydrogels. Reproduced from ref. [Citation452] with permission. Copyright 2013 American Chemical Society](/cms/asset/5de7dff1-e8f4-494e-b34b-1ed63b007320/lfri_a_1858313_f0013_oc.jpg)
Better mechanical properties and the responsive ability of MMC/MC hydrogels can be obtained by adjusting the type, composition, proportion, size, and content of macromolecular microspheres, and some unique physical properties can be obtained via introducing some polymer groups, endowing the inorganic particles with unique properties.[Citation424,Citation453–456] For example, Huang et al.[Citation457] used a radiation treatment to cause peroxidation on the surface of styrene-acrylic polymer microspheres, which were then used as initiators and crosslinking agents that were uniformly dispersed in an acrylic acid (AA) monomer solution. The thermally initiated monomers were grafted to the surface of the microspheres to obtain a composite hydrogel with a relatively uniform crosslinking density and a relatively regular structure. The research performed by Huang et al. indicated that the grafting density of the microspheres was relatively large, causing the graft chains between the adjacent microspheres to become entangled into the micelles and collapse by strong hydrogen bonding. When an external force was applied, stress was uniformly dispersed throughout all the chains, and the physically entangled graft chain in the micelle has a large stretching range, from the freely collapsed state to the fully extended state, so that the gel has a good elastic deformation ability and effectively dissipates stress. However, although the MMC hydrogel has more good characteristics, research on MMC hydrogels is limited, and the scientific community has paid significantly less attention to MMC hydrogels than to NC hydrogels.
IPN hydrogels
An on-going challenge in the design and application of hydrogels is the conflict between the strength, toughness, and high-water content (>90 wt%).[Citation24] In general, strong materials tend to be brittle, softer materials are usually tougher, and highly hydrated materials are weak. If additional requirements are required other than special functions such as the stimuli responsiveness, this challenge of the mutual exclusivity of the diversified needs will be difficult to overcome.[Citation458,Citation459] However, IPN hydrogels or DN hydrogels may address all of these challenges.
IPNs are aggregated structures in which two or more polymers interpenetrate in the form of a network. In IPN structures, at least one polymer is crosslinked, and the other polymer does not form a covalent bond with the first polymer network.[Citation460–462] The IUPAC definition states that an IPN is “A polymer comprising two or more networks which are at least partially interlaced on a molecular scale but not covalently bonded to each other and cannot be separated unless chemical bonds are broken. A mixture of two or more preformed polymer networks is not an IPN”,[Citation463,Citation464] in which, the clear difference between an IPN and a polymer blend has been described.
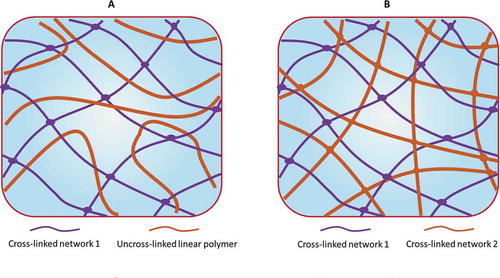
IPN structures can be classified into semi-IPNs (SIPNs) and full-IPNs (FIPNs).[Citation465] SIPNs are composed of one linear polymer entrapped within the network of another crosslinked polymer (), while FIPNs are composed of one crosslinked polymer interpenetrated within anothercrosslinked polymer network (). The IUPAC definition of a SIPN is as follows: “semi-interpenetrating polymer networks are distinguished from interpenetrating polymer networks because the constituent linear or branched polymers can, in principle, be separated from the constituent polymer network(s) without breaking chemical bonds.”[Citation463] Intermolecular entanglement and interpenetration can cause the forced miscibility between components, which enable IPNs to integrate the excellent performance of the different participating components.[Citation466,Citation467] Although not strictly IPNs (according to definition), some polymer structures can be classified as IPN-like (pseudo-IPNs), such as AB-type crosslinked polymers (ABCPs) [Citation468,Citation469] () and thermoplastic IPNs (also called physical crosslinked IPNs) in terms of their structures and preparation methods. Generally, IPNs and semi-IPNs can be prepared by two main synthetic pathways: the in situ synthesis and the sequential synthesis [Citation464] ().
Figure 16. Schematic of the in situ and sequential pathways used to prepare IPNs and semi-IPNs. Reproduced from ref. [Citation464] with permission. Copyright 2011 Elsevier
![Figure 16. Schematic of the in situ and sequential pathways used to prepare IPNs and semi-IPNs. Reproduced from ref. [Citation464] with permission. Copyright 2011 Elsevier](/cms/asset/2c78e793-8773-46ee-9405-f2872c94cf54/lfri_a_1858313_f0016_oc.jpg)
It is easy to define an IPN hydrogel according to the definition of an IPN. Adequate research has shown that the IPN and SIPN structures can improve the mechanical performance and dimensional stability of functional hydrogels, [Citation461,Citation470,Citation471] even the response rate of some stimuli-responsive hydrogels.[Citation472,Citation473]
DN hydrogels
DN hydrogels are special type of IPN hydrogels that possess two different types of network structures, the highly crosslinked polyelectrolyte network acts as the first network, which provides a rigid bracket for the DN, and the poorly crosslinked or non-crosslinked neutral network structure acts as the second network, fills in the rigid network to retain the gel shape and absorb external stress.[Citation474–476] DN hydrogels are attracting increasing attention due to their excellent mechanical strength and other resulting in mechanical properties, [Citation86,Citation476–481] and increasingly more uniquely structured DN hydrogels have been reported. For instance, multiple-network hydrogels, such as triple-network hydrogels, [Citation482,Citation483] DN-NC hydrogels,[Citation484–486] anisotropic liquid crystalline DN hydrogels,[Citation487,Citation488] void-DN hydrogels,[Citation489] and microgel-reinforced DN hydrogels.[Citation89,Citation490,Citation491] Suo’s group formed a type of highly stretchable and tough DN hydrogels that had combined ionically and covalently crosslinked networks.[Citation477] Different from traditional DN hydrogels, the two types of polymer networks were intertwined via covalent crosslinks that formed between the amine groups on the polyacrylamide chain and the carboxyl groups on the alginate chains. Although this DN hydrogel contains ∼90% water, it can be stretched beyond 20 times its initial length and has a fracture energy of ∼9,000 J m−2. In the research of Liu et al.,[Citation492] they formed a kind of hybrid hydrogel composed of a covalently crosslinked polyacrylamide network and a physical shear-thinning dexamethasone phosphate that can separately provide elastic and plastic property. The mechanical strength competition between two networks in a polymeric material can be switched between elasticity and plasticity. When the mechanical strength of the covalent network was greater than the physical network, the gel turned into a typical elastic material. Conversely, the gel displayed a conventional plastic characteristic once the former was weaker than the latter, which can be stretched to form a permanent gel material with prominent anisotropism and high toughness owing to the orientation of the embedded nanofibers.
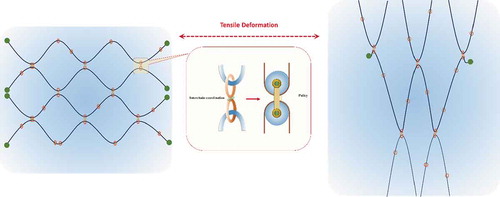
Topological hydrogels
Supramolecules with topological characteristics have attracted great interest.[Citation493] Topological hydrogels are another high-strength hydrogel, and sometimes highly stretchable.[Citation494,Citation495] Topological hydrogels can be fabricated via host-guest interactions (see previous Section) and some physical or chemical crosslinks. Due to the structural features, topological hydrogels are also called slide-ring or slip-link gels. As shown in , in the topological hydrogels, the polymer chains with bulky end groups exhibit neither the covalent crosslinks found in chemical gels nor the attractive interactions found physical gels, but are instead topologically interlocked by figure- eight crosslinks similar to pulleys.[Citation80,Citation495–498] Because of these figure-eight crosslinked networks, the polymer chain in the slide-ring gel can pass through the figure-eight crosslinks acting as pulleys, while the free rings cannot pass and can only slide along an axis of the polymer and tend to keep a random distribution in the network.[Citation495] As a result, the pulley effect changes the length of the network strands between the crosslinks but does not change the number of rings between the crosslinks, relaxing the anisotropic deformation of the polymer chains to generate an isotropic form, resulting in the deformation of the network to form a heterogeneous density distribution of free rings, and endowing the hydrogels with an excellent tensile deformation performance. That is to say, these hydrogels are very stretchable compared with conventional chemical hydrogels.[Citation495,Citation496]
Ke et al.[Citation498] reported the shear-induced formation of a transient hydrogel, in which a synthesized molecular tube acted as the host, PEG acted as the guest (called pseudo-polyrotaxanes), and CuCitation2+ interacted with the pseudo-polyrotaxanes via metal coordination to fabricate the gel network. The final hydrogel was highly stretchable (it can be stretched to up to 30 times its original length) and also demonstrated rapid self-healing upon shaking.
Besides host-guest interactions, a new kind of topological hydrogel with Turing microstructure was reported by Yu et al.[Citation499] Detailed, a dynamic hydrogel composed of cellulose, ionic liquids, and H2O with reversible Turing-pattern microstructures was realized via the construction of a switchable hydrogen-bond topological network. The dynamic hydrogel exhibited diverse tunable, reversible properties including mechanical strength and toughness, viscoelasticity, self-healing, and ionic conductivity. As reported, these dynamic features can be facilely tuned through changing the water content in the gel material. The flexible, transparent, and designable dynamic hydrogel material shows great potential in electronic skin and smart devices.
Tetra-arm hydrogels
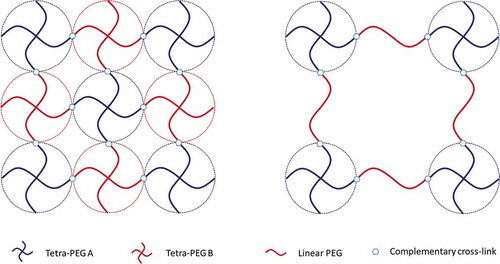
Conventional polymer hydrogels inevitably contain spatial inhomogeneities that reduce their mechanical strength, [Citation500] and thus the fabrication of hydrogels with an excellent uniform network is attracting increasingly more attention. Tetra-arm hydrogels, such as Tera-PEG hydrogels, are tough enough and uniform for applications as structural materials because of their well-defined, nearly ideal network structure that is free from defects or entanglements.[Citation500–503] Tera-PEG hydrogels were first fabricated in 2008[Citation504] and are formed by the cross-end-coupling of two types of tetra-arm PEG or one tetra-arm PEG and one linear-PEG having complementary functional end groups. shows a schematic illustration of tetra-PEG hydrogels.
In Yang’s research, [Citation505] a well-defined ammonolysis-based Tetra-PEG hydrogel sealant with a rapid gelation time, strong tissue adhesion, and high mechanical strength was developed. Shibayama and co-workers [Citation506] fabricated hydrogels using a star tetrafunctional poly(ethylene glycol) with N-hydroxysuccinimidyl ester (tetra-PEG-NHS), and 1,14-diamino-3,6,9,12-tetraoxatetradecane (amino-PEG4-amine) as crosslinker. These hydrogels showed homogeneous and ordered three-dimensional (3D) network without spatial defects. Wang et al. [Citation303] introduced (2-hydroxypropyl)-α-CDs (Hy-α-CDs) into the network of a tetra-PEG gel. Before the gelling process, a poly-pseudorotaxane consisting of a tetra-PEG macromonomer and Hy-α-CD was formed through host-guest interactions (see previous Section). Then, the tetra-PEG hydrogels were formed through click chemistry (by in situ copper (I)-catalyzed azide–alkyne cycloaddition click chemistry).
Emulsion hydrogels
Emulsion hydrogels, also called emulsion gels, are a semi-solid colloidal system with physicochemical and functional properties of both gels and emulsions. Emulsion hydrogels can also be called gel-like emulsions or solid like-emulsions. For example, cheese, yogurt, some dairy desserts, and reformulated meat products are common typical food emulsion gels.[Citation507] Conventional emulsion gels have two main structural forms (), including emulsion-filled gels, in which emulsion droplets are incorporated into a polymeric gel matrix, and emulsion particulate gels comprising a network of aggregated emulsion droplets.[Citation508–511] Furthermore, droplets within the emulsion-filled gels can be classified as either active or inactive fillers.[Citation508,Citation512] Active fillers (so-called bound fillers) are connected to the gel network and contribute to the gel strength, whereas inactive (unbound) fillers have a low chemical affinity towards the gel matrix and do not interact or interact minimally with the gel matrix. [Citation508]The rheological, structural, and microstructural properties of emulsion gels can be affected by controlling the emulsion types,[Citation513–517] matrix materials, and interactions, [Citation518–525] oil content,[Citation526–529] filler and particle size distribution,[Citation512,Citation530,Citation531] gelling methods,[Citation510,Citation528,Citation532] etc.
Figure 19. Schematic representation of emulsion-filled gels and emulsion particulate gels. Reproduced from ref. [Citation508] with permission. Copyright 2019 Elsevier
![Figure 19. Schematic representation of emulsion-filled gels and emulsion particulate gels. Reproduced from ref. [Citation508] with permission. Copyright 2019 Elsevier](/cms/asset/09af048c-bce6-4d70-b6f5-1f98c3cebb85/lfri_a_1858313_f0019_oc.jpg)
Oriented structural hydrogels
Biological soft tissues, such as cartilage, skeletal muscle, the corneas, and blood vessels, exhibit highly anisotropic mechanical properties, thus ensuring that the normal biological functions can adapt to complex physiological environments. In artificial material systems, although the hydrogels and biological soft tissues have the most similar properties, and the three-dimensional hydrophilic polymer network of hydrogels exhibit many excellent characteristics, such as a good water absorption, elasticity, and biocompatibility, in comparison to biological soft tissues, conventional hydrogels are structurally isotropic and homogeneous, which limits their further application. In recent years, oriented/anisotropic structural hydrogels have attracted increasingly more attention.[Citation353,Citation533,Citation534]
The oriented structures are being prepared to create hydrogels with ordered and aligned structures. Oriented hydrogel structures always offer extensive internal reactive surfaces inside the material, as well as oriented (anisotropic) features that can evidently improve the mechanical properties of the hydrogels.[Citation535–537] According to the study performed by Ishida et al.,[Citation538] anisotropic hydrogels can be classified into three main types, including hydrogels with aligned 1D or 2D nanofillers, polymer chain networks, and cavity channels. Generally, several approaches are used to generate oriented (anisotropic) hydrogels,[Citation538–540] including magnetic alignment,[Citation541–545] electric-field alignment,[Citation546–551] strain alignment,[Citation552–560] directional freezing,[Citation561–565] ion alignment,[Citation566–568] electrostatic repulsion,[Citation542,Citation569] and self-assembly.[Citation487,Citation570–574]
Professor Liu reviewed the latest research progress of highly oriented nanocomposite hydrogels in recent years.[Citation539] This review not only summarized the strategies used to prepare oriented structure nanocomposite hydrogels but also compared the advantages and challenges of different strategies. Moreover, the review also elaborated on the anisotropic properties, optical properties, stimuli responsiveness, biological properties, and applications of the oriented hydrogel structures. Because anisotropic hydrogels have been a research focus, there are a large number of related studies (including but not limited to the literature we have listed in this section).[Citation540,Citation575,Citation576]
Application potential of hydrogel in food science
Before starting the discussion of application potential of hydrogel in food science, it is necessary to ask what is food science? and why in food science? Food science is a discipline that closely connected to our human beings, and a discipline that closely connected to “food” and “eat”. Generally, three basic levels are included in food science, one is to obtain healthy and safe food (food nutrition and health, and food safety), the other is to effectively process and preserve food (food engineering and processing, and food safety), and the third is to eat deliciously, comfortably, and conveniently (food engineering and processing). Considering the powerful and versatile functions of hydrogels, there is no doubt that hydrogels are playing, and will play critical roles in modern food science, such as in structuring foods with desired sensorial textures, preserving metastable food structures and increasing shelf-life, designing food packaging materials, nutraceutical delivery and bioavailability, calorie control and risk monitoring for food safety, etc.
There are a lot of literatures about naturally sourced hydrogels prepared from food biopolymers (proteins and polysaccharides),[Citation10,Citation577–580] composed of decellularized extracellular matrix (ECM), Matrigel collagen, chitosan, elastin, fibrin, alginates, hyaluronic acid, pullulan, fibrin, etc., [Citation91,Citation581–592] even DNA,[Citation226,Citation228,Citation231,Citation233,Citation234,Citation593–596] and these hydrogels show excellent application potential in improving quality, nutrition, and bioavailability features of foods. But it does not mean that synthetic source-based hydrogels are useless in food science. Synthetic polymers and their derivatives offer engineers highly versatile materials with physical and chemical properties that can be easily controlled and altered (such as degradability)[Citation55] and show greater potential for forming hydrogels with different structures and functions.
Due to the diversity of the hydrogel (whether natural or synthetic) structures, it brings expecting broader spaces for functional regulation, making the application of hydrogels in the field of food science more tunable. For instances, high-strength hydrogels, such as hybrid hydrogels (NC/MMC/IPN/DN hydrogels), topological hydrogels, and tetra-arm hydrogels show distinct advantages in designing food packaging materials, giving packaging materials considerable strength and toughness, while porous hydrogels may give packaging materials appropriate permeability. Meanwhile, microgels, nanogels, core-shell-structured hydrogels, and emulsion hydrogels are more suitable as platforms of encapsulation and nutraceutical delivery. It is actually difficult to accurately predict the future of hydrogel science, and also difficult to accurately predict the application potential of hydrogel in food science. The outer edge of food science has become more and more broad, and it is no longer as simple as traditional food sciences which mainly focus on edibility. At the same time, the rapid development of materials science has brought more and more new technologies and new materials for the development of food science and hydrogel science, causing cross integration be an inevitable trend. For example, one can deeply imagine what impacts will some special structured hydrogels bring? such as multi-layered hydrogels, self-healing, and oriented structural hydrogels.
Application progress of hydrogels in food science
Both hydrogel science and food science are developing rapidly. In the current review, the application process of hydrogel in food science will be discussed, from perspective of food nutrition and health, food engineering and processing, and food safety.
In food nutrition and health field
Encapsulation and immobilization
Hydrogel, as a kind of soft and wet material that readily processed, can absorb up to several thousand times their dry weight with the three-dimensional porous networks, making itself an idea encapsulation system of water-soluble component. In the past decades, there are numerous reports on the application of hydrogels as encapsulation systems in food field.[Citation597–600] Compared with that in the food field, there are more researches on the use of hydrogels in encapsulation in the medical areas, which are worthy of reference for the scientists of food industry. From a structural point of view, microgels, nanogels, core-shell-structured hydrogels, emulsion hydrogels, and hydrogel nanoparticles are more suitable as platform for encapsulation.
There are similarities but also differences between the research of using hydrogels for immobilization (enzyme, cell, microorganism, etc.) and for encapsulation. On the one hand, generally, the encapsulation only occurs inside the hydrogel network, while the immobilization can occur both inside the hydrogel network and on the surface of hydrogel. On the other hand, the surface and internal structural characteristics of hydrogels have a significant impact on their immobilization ability.[Citation232,Citation601–603] It can be imagined that a highly porous hydrogel with high/super absorption capacity will attract wider attention.
Delivery system
The unique characteristic that can absorb and hold a large amount of water or biological fluids within a three-dimensional network structure makes hydrogels common candidates for applications in delivery system.[Citation604–606] For example, Trojan horse nanoparticles, kind of hydrogel beads that conceal bioactive-loaded nanoparticles, release them under some condition, shows great capacity of nutraceutical delivery.[Citation607–609] Trojan horse nanoparticles were also utilized to encapsulate nanoemulsions or Pickering emulsion in hydrogel beads, where the release depends on the environment.[Citation609,Citation610] In addition, the other properties of hydrogels such as stimuli-responsiveness to heat, pH, and light are particularly useful for controlled release in food nutrition delivery system.[Citation611] In Song’s research,[Citation612] they prepared a pH-responsive semi-IPN hydrogel consisted of peach gum polysaccharide (PGP) and Auricularia polytricha β-glucans (APP) via inverse emulsion cross-linking method. In this semi-IPN hydrogel, PGP chains acted as the crosslinked network via metal-ligand coordination between FeCitation3+ and COO− group in the PGP chains, while APP acted as the second network. On account of stable and dense structure of PGP-APP semi-IPN hydrogels, a significant positive effect (p < 0.05) on the viability of bacteria under gastrointestinal transition model was obtained. Their studies indicated the unique properties of PGP-based hydrogels and potential application as intestinal-targeted delivery systems. Hu et al.[Citation613] developed novel polyelectrolyte complex (PEC) hydrogels by self-assembly of two food-grade polysaccharides salecan and N,N,N-trimethyl chitosan (TMC). Those PEC hydrogels could be suitable carriers for intestinal-targeted nutrient delivery (green tea polyphenols).
It should be noted that there are a large number of studies focus on the application of hydrogels in the development of intelligent systems for drug delivery,[Citation614] such as microgels.[Citation615] In most cases, hydrogels for drug delivery in right place and right time (controlled-release) are designed depending on the swelling or shrinkage by the effect of pH and temperature signals. Although there are differences between the food delivery system and the drug delivery system, the two share a certain degree of similarity. Therefore, it is encouraged to take useful examples from the research cases of application of well-designed structured hydrogels in drug delivery system.
Calorie control
There is an urgent need to develop reduced-fat or reduced-starch products. Hydrogels can also play a good role in reducing calories, by enhancing satiety or by reducing intake.[Citation10] Wu et al.[Citation616] created hydrogel particles from protein and dietary fiber as the soft matter strategies with good texture, which could be healthier replacements for starch granules. Similar study has been reported by McClements et al.[Citation617] Paunov et al.[Citation618] reported the use of a temperature insensitive, food-grade, mixed agar-methylcellulose hydrogel to reduce the caloric density of pancakes that were prepared at temperatures much higher than the boiling point of water. McClements et al.[Citation617,Citation619–622] reported that the application of emulsion hydrogels in reduced-fat food, by replacing animal oils with vegetable oils.[Citation616]
In food engineering and processing field
Food packaging materials
Hydrogel as food packaging material is an important solution to the increasingly serious issue that abundant use and misuse of large molecular weight petroleum-based polymeric materials. Recent research and scientific advances have been promoting biobased biodegradable material for food packaging.[Citation623] The main role of these materials in food packaging is humidity control inside of a packaging.[Citation624] The use of hydrogel can decrease water activity (Aw), slowing down the growth of mold, yeast, and spoilage bacteria on foods such as ready-to-eat meals and hygroscopic products, or to decrease the softening of dry crispy products.[Citation624,Citation625] Regardless of whether the hydrogel plays as traditional or active, or an intelligent packaging material, in addition to traditional concerns such as low-cost, renewability (shelf-life), biodegradability, from the perspective of the hydrogel structure, the core properties to be concerned about are mechanical strength, high absorption or superabsorbent property, and even breathability and transparency. Foe example, in Batista’s review,[Citation624] the potential of hydrogels as absorbent materials applied in food packaging was well summarized. Hence, any attempt to improve performance that good mechanical strength, high absorption, or superabsorbent property, and even good breathability and transparency through effective structural design are worth attentions, such as DN/IPN hydrogels, oriented structural hydrogels, porous hydrogels, tetra-arm hydrogels, and topological hydrogels, etc.
Active packaging interacts directly with the contained food product or with the headspace to reduce or inhibit microbial growth, which leads to the extension of shelf-life and/or the preservation of the food’s sensory characteristics.[Citation624] Hence, hydrogels show great antimicrobial activity when incorporated with different antimicrobial compounds, such as nanoparticles (e.g., silver) or bioactive compounds, have been well studied both in food matrices and in vitro.[Citation626,Citation627] Therefore, hybrid hydrogels such as nanocomposite hydrogels can be selected as potential strategy to overcome these drawbacks (such as limited mechanical and water resistance) from conventional hydrogel, by incorporating nanoparticles into the polymeric structure of the hydrogel.[Citation627–629]
Intelligent packaging communicates quality and safety information to the consumer. Hydrogels also show their potential applications in intelligent packaging system to provide information regarding the freshness of the contained food products or as part of a simple detection method to determine the presence of contaminants.[Citation630,Citation631] In this application area, the structured design of the hydrogels is mainly to serve their functions of smart response.
Food texture perception
Food texture perception is one of the key factors determining the quality of food products. As a soft material, hydrogel has significant texture properties (elasticity, hardness, chewiness, fracture, etc.). Therefore, in addition to being used as a substitute food to reduce calorie intake, hydrogels can also be used to improve the texture or mouthfeel of food. For instance, emulsion hydrogels can impact the texture properties of food.[Citation530,Citation632–636] Interestingly, replacing meat or starch with hydrogels that of excellent texture characteristics, or of low oil content, is also an effective way to reduce calories in food.
Others
In the food industry, hydrogels can also be used for sewage treatment of food processing waste liquids, oil-water separation, masking of unpleasant flavors, and effective prevention of scaling on boilers, pipes, and heat exchanger surfaces. Paunov etal.[Citation637] reported abinary hydrogel system made from two food-grade biopolymers, agar, and methylcellulose (agar-MC), which did not require addition of salt for gelation to occur and has very unusual rheological and thermal properties. These highly thermally stable hydrogels showed melt resistance which might find application in high-temperature food processing and materials templating. In Dickinson’ review,[Citation638] biopolymer-based microgels show potential in rheological control and emulsion stabilization, indicating the ability to preserve metastable food structures and increase food shelf-life.
In food safety field
Adsorption and removal
Due to the fact that hydrogel has high-water retention capacity in its structure, and the 3D hydrophilic polymer network can allow large amounts of free molecules or ions to diffuse throughout the whole structure. In addition, high adsorbent with high porosity, high surface area, and the reuse possibility, causing hydrogel an efficient platform for adsorption or removal operation.[Citation639–642] For example, Pinto et al.[Citation643] developed one promising adsorbent based on chitosan hydrogel scaffold modified with carbon nanotubes, for food dye removal in single and binary aqueous systems. In addition, there are a lot of researches in the field of environmental science on the use of hydrogels to remove heavy metals and other pollutants can also be considered for application in food science.[Citation486,Citation644–651] In these studies, in addition to focusing on the adsorption and removal performance, the mechanical strength of the hydrogel is also important. Hence, hybrid hydrogels are ideal for more attentions.
Risk monitoring
In this field, hydrogel has gained widespread attention acts as (bio)sensor or signal probes to detect hazard or risk factors in food. For example, Wu et al.[Citation652] formed a fluorescent DNA hydrogel aptasensor for sensitive detection of Ochratoxin A (OTA). Su et al.[Citation653] applied Aflatoxin B-1-responsive aptamer-cross-linked hydrogel in a portable pH meter for Aflatoxin B-1 detection. Similar study has been reported by Zhu et al.[Citation654] Meng et al.[Citation655] formed a 2D molecularly imprinted photonic crystal hydrogel sensor for the detection of oxytetracycline in milk. Guo et al.[Citation656] fabricated a ratiometric fluorescent-based portable agarose hydrogel test kit to applied in on-spot assessment of NOCitation2− content within 10 min, which might have broad application prospects in food safety assessment. Similar report can also be found in other research.[Citation654,Citation657–659] In recent years, polymer-based hydrogel solid-phase extraction (SPE) was also used as sampling method in direct onsite fast screening of risk substances, such as mercury (II) (Hg2+) ions,[Citation660] illicit additives on fruit peel,[Citation658] rhodamine B,[Citation661] etc.
Prospective and challenges
In general, hydrogel science covers a wide range of disciplines, and the functions and applications of hydrogels are becoming more and more abundant. Therefore, it is actually difficult to accurately predict the future of hydrogel science. From the structural point of view, the main challenge of the research is that the crosslinking approaches of hydrogel will be more diverse and complex, the rapid developments in hydrogel applications require for more pleuripotent, effective, and targeted regulation of functions, which in turn require more novel, ingenious, and complex rational design of hydrogels.
In addition, the rapid development of materials science has brought more and more new technologies and new materials to the development of food science, causing cross-disciplinary be an inevitable trend. Compared with synthetic hydrogels, although the complexity of hydrogels that commonly used in some foods is increasing, it is still strongly necessary to learn new concepts from synthetic hydrogels in design diverse hydrogel structure, so that design innovative food with improved characteristics and functionalities.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Conflicts of Interest
All the authors confirmed there is no conflicts of interest.
Additional information
Funding
References
- Erol, O.; Pantula, A.; Liu, W.; Gracias, D. H. Transformer Hydrogels: A Review. Adv. Mater. Technol. US. 2019, 4, 1900043. doi: 10.1002/admt.201900043.
- Ahmed, E. M.;. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. doi: 10.1016/j.jare.2013.07.006.
- Kamata, H.; Akagi, Y.; Kayasuga-Kariya, Y.; Chung, U.; Sakai, T. “Nonswellable” Hydrogel without Mechanical Hysteresis. Science. 2014, 343, 873–875. doi: 10.1126/science.1247811.
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A. Review of Trends and Limitations in Hydrogel-Rapid Prototyping for Tissue Engineering. Biomaterials. 2012, 33, 6020–6041. doi: 10.1016/j.biomaterials.2012.04.050.
- Li, J.; Mooney, D. J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. doi: 10.1038/natrevmats.2016.71.
- Shouji, T.; Yamamoto, K.; Kadokawa, J. Chemoenzymatic Synthesis and Self-Assembling Gelation Behavior of Amylose-Grafted Poly(γ-Glutamic Acid). Int. J. Biol. Macromol. 2017, 97, 99–105. doi: 10.1016/j.ijbiomac.2017.01.001.
- Fan, Z.; Zhang, Y.; Zhang, W.; Li, X. In Situ Injectable Poly(γ-Glutamic Acid) Based Biohydrogel Formed by Enzymatic Crosslinking. J. Appl. Polym. Sci. 2015, 132, 42301. doi: 10.1002/app.42301.
- Plamper, F. A.; Richtering, W. Functional Microgels and Microgel Systems. Acc. Chem. Res. 2017, 50, 131–140. doi: 10.1021/acs.accounts.6b00544.
- Elsayed, M. M.;. Hydrogel Preparation Technologies: Relevance Kinetics, Thermodynamics and Scaling up Aspects. J. Polym. Environ. 2019, 27, 871–891. doi: 10.1007/s10924-019-01376-4.
- Cao, Y.; Mezzenga, R. Design Principles of Food Gels. Nat. Food. 2020, 1, 106–118. doi: 10.1038/s43016-019-0009-x.
- Qin, H.; Zhang, T.; Li, N.; Cong, H.; Yu, S. Anisotropic and Self-Healing Hydrogels with Multi-Responsive Actuating Capability. Nat. Commun. 2019, 10, 2202. doi: 10.1038/s41467-019-10243-8.
- Jaspers, M.; Dennison, M.; Mabesoone, M. F. J.; MacKintosh, F. C.; Rowan, A. E.; Kouwer, P. H. J. Ultra-Responsive Soft Matter From Strain-Stiffening Hydrogels. Nat. Commun. 2014, 5, 5808. doi: 10.1038/ncomms6808.
- Park, J.; Pramanick, S.; Park, D.; Yeo, J.; Lee, J.; Lee, H.; Kim, W. J. Therapeutic-Gas-Responsive Hydrogel. Adv. Mater. 2017, 29, 1702859. doi: 10.1002/adma.201702859.
- Yetisen, A. K.; Jiang, N.; Fallahi, A.; Montelongo, Y.; Ruiz-Esparza, G. U.; Tamayol, A.; Zhang, Y. S.; Mahmood, I.; Yang, S.; Kim, K. S.;, et al. Glucose-Sensitive Hydrogel Optical Fibers Functionalized with Phenylboronic Acid. Adv. Mater. 2017, 29, 1606380. doi: 10.1002/adma.201606380.
- Li, C.; Iscen, A.; Palmer, L. C.; Schatz, G. C.; Stupp, S. I. Light-Driven Expansion of Spiropyran Hydrogels. J. Am. Chem. Soc. 2020, 142, 8447–8453. doi: 10.1021/jacs.0c02201.
- Dai, L.; Ma, M.; Xu, J.; Si, C.; Wang, X.; Liu, Z.; Ni, Y. All-Lignin-Based Hydrogel with Fast pH-Stimuli-Responsiveness for Mechanical Switching and Actuation. Chem. Mater. 2020. doi: 10.1021/acs.chemmater.0c01198.
- Wang, H.; Heilshorn, S. C. Adaptable Hydrogel Networks with Reversible Linkages for Tissue Engineering. Adv. Mater. 2015, 27, 3717–3736. doi: 10.1002/adma.201501558.
- Huang, S.; Shuyi, S.; Gan, H.; Linjun, W.; Lin, C.; Danyuan, X.; Zhou, H.; Lin, X.; Qin, Y. Facile Fabrication and Characterization of Highly Stretchable Lignin-Based Hydroxyethyl Cellulose Self-Healing Hydrogel. Carbohyd. Polym. 2019, 223, 115080. doi: 10.1016/j.carbpol.2019.115080.
- Chen, M.; Tian, J.; Liu, Y.; Cao, H.; Li, R.; Wang, J.; Wu, J.; Zhang, Q. Dynamic Covalent Constructed Self-Healing Hydrogel for Sequential Delivery of Antibacterial Agent and Growth Factor in Wound Healing. Chem. Eng. J. 2019, 373, 413–424. doi: 10.1016/j.cej.2019.05.043.
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P. X.; Guo, B. Antibacterial Adhesive Injectable Hydrogels with Rapid Self-Healing, Extensibility and Compressibility as Wound Dressing for Joints Skin Wound Healing. Biomaterials. 2018, 183, 185–199. doi: 10.1016/j.biomaterials.2018.08.044.
- Wei, Z.; Yang, J. H.; Liu, Z. Q.; Xu, F.; Zhou, J. X.; Zrinyi, M.; Osada, Y.; Chen, Y. M. Novel Biocompatible Polysaccharide-Based Self-Healing Hydrogel. Adv. Funct. Mater. 2015, 25, 1352–1359. doi: 10.1002/adfm.201401502.
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P. X. Antibacterial Anti-Oxidant Electroactive Injectable Hydrogel as Self-Healing Wound Dressing with Hemostasis and Adhesiveness for Cutaneous Wound Healing. Biomaterials. 2017, 122, 34–47. doi: 10.1016/j.biomaterials.2017.01.011.
- Su, E.; Yurtsever, M.; Okay, O. A Self-Healing and Highly Stretchable Polyelectrolyte Hydrogel via Cooperative Hydrogen Bonding as A Superabsorbent Polymer. Macromolecules. 2019, 52, 3257–3267. doi: 10.1021/acs.macromol.9b00032.
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C. P.; Kadumudi, F. B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.;, et al. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering?. Adv Sci 2019, 6, 1801664. doi: 10.1002/advs.201801664.
- Li, L.; Yan, B.; Yang, J.; Huang, W.; Chen, L.; Zeng, H. Injectable Self-Healing Hydrogel with Antimicrobial and Antifouling Properties. ACS Appl. Mater. Inter. 2017, 9, 9221–9225. doi: 10.1021/acsami.6b16192.
- Cirillo,; Curcio,; Nicoletta,; Iemma. Injectable Hydrogels for Cancer Therapy over the Last Decade. Pharmaceutics. 2019, 11, 486. doi: 10.3390/pharmaceutics11090486.
- Piantanida, E.; Alonci, G.; Bertucci, A.; De Cola, L. Design of Nanocomposite Injectable Hydrogels for Minimally Invasive Surgery. Acc. Chem. Res. 2019, 52, 2101–2112. doi: 10.1021/acs.accounts.9b00114.
- Tu, Y.; Chen, N.; Li, C.; Liu, H.; Zhu, R.; Chen, S.; Xiao, Q.; Liu, J.; Ramakrishna, S.; He, L. Advances in Injectable Self-Healing Biomedical Hydrogels. Acta Biomater. 2019, 90, 1–20. doi: 10.1016/j.actbio.2019.03.057.
- Zhang, S.; Chen, Y.; Liu, H.; Wang, Z.; Ling, H.; Wang, C.; Ni, J.; Saltik, B. C.; Wang, X.; Meng, X.;, et al.. Room-Temperature-Formed Pedot: Pss Hydrogels Enable Injectable, Soft, and Healable Organic Bioelectronics. Adv. Mater. 2019, e1904752. doi: 10.1002/adma.201904752.
- Li, W.; Liu, X.; Deng, Z.; Chen, Y.; Yu, Q.; Tang, W.; Sun, T. L.; Zhang, Y. S.; Yue, K. Tough Bonding, On-Demand Debonding, and Facile Rebonding between Hydrogels and Diverse Metal Surfaces. Adv. Mater. 2019, 1904732. doi: 10.1002/adma.201904732.
- Yuk, H.; Zhang, T.; Lin, S.; Parada, G. A.; Zhao, X. Tough Bonding of Hydrogels to Diverse Non-Porous Surfaces. Nat. Mater. 2016, 15, 190–196. doi: 10.1038/nmat4463.
- Gan, D.; Huang, Z.; Wang, X.; Jiang, L.; Wang, C.; Zhu, M.; Ren, F.; Fang, L.; Wang, K.; Xie, C.;, et al. Graphene Oxide-Templated Conductive and Redox-Active Nanosheets Incorporated Hydrogels for Adhesive Bioelectronics. Adv. Funct. Mater. 2019, 30, 1907678. doi: 10.1002/adfm.201907678.
- Han, L.; Wang, M.; Prieto-López, L. O.; Deng, X.; Cui, J. Self-Hydrophobization in a Dynamic Hydrogel for Creating Nonspecific Repeatable Underwater Adhesion. Adv. Funct. Mater. 2019, 30, 1907064. doi: 10.1002/adfm.201907064.
- Rose, S.; Prevoteau, A.; Elzière, P.; Hourdet, D.; Marcellan, A.; Leibler, L. Nanoparticle Solutions as Adhesives for Gels and Biological Tissues. Nature. 2014, 505, 382–385. doi: 10.1038/nature12806.
- Cheng, W.; Hu, X.; Wang, D.; Liu, G. Preparation and Characteristics of Corn Straw-Co-Amps-Co-Aa Superabsorbent Hydrogel. Polymers-Basel. 2015, 7, 2431–2445. doi: 10.3390/polym7111522.
- Sidorenko, A.; Krupenkin, T.; Taylor, A.; Fratzl, P.; Aizenberg, J. Reversible Switching of Hydrogel-Actuated Nanostructures Into Complex Micropatterns. Science. 2007, 315, 487–490. doi: 10.1126/science.1135516.
- Chin, S. Y.; Poh, Y. C.; Kohler, A.; Compton, J. T.; Hsu, L. L.; Lau, K. M.; Kim, S.; Lee, B. W.; Lee, F. Y.; Sia, S. K. Additive Manufacturing of Hydrogel-Based Materials for Next-Generation Implantable Medical Devices. Sci. Rob. 2017, 2, eaah6451. doi: 10.1126/scirobotics.aah6451.
- Banerjee, H.; Ren, H. Optimizing Double-Network Hydrogel for Biomedical Soft Robots. Soft Rob. 2017, 4, 191–201. doi: 10.1089/soro.2016.0059.
- Yuk, H.; Lin, S.; Ma, C.; Takaffoli, M.; Fang, N. X.; Zhao, X. Hydraulic Hydrogel Actuators and Robots Optically and Sonically Camouflaged in Water. Nat. Commun. 2017, 8, 14230. doi: 10.1038/ncomms14230.
- Ge, G.; Lu, Y.; Qu, X.; Zhao, W.; Ren, Y.; Wang, W.; Wang, Q.; Huang, W.; Dong, X. Muscle-Inspired Self-Healing Hydrogels for Strain and Temperature Sensor. ACS Nano. 2020, 14, 218–228. doi: 10.1021/acsnano.9b07874.
- Liu, X.; Liu, J.; Lin, S.; Zhao, X. Hydrogel Machines. Mater. Today. 2020. doi: 10.1016/j.mattod.2019.12.026.
- Bakarich, S. E.; Gorkin, R.; Panhuis, M. I. H.; Spinks, G. M. 4D Printing with Mechanically Robust, Thermally Actuating Hydrogels. Macromol. Rapid Comm. 2015, 36, 1211–1217. doi: 10.1002/marc.201500079.
- Lee, H. R.; Woo, J.; Han, S. H.; Lim, S. M.; Lim, S.; Kang, Y. W.; Song, W. J.; Park, J. M.; Chung, T. D.; Joo, Y. C.;, et al. A Stretchable Ionic Diode from Copolyelectrolyte Hydrogels with Methacrylated Polysaccharides. Adv. Funct. Mater. 2019, 29, 1806909. doi: 10.1002/adfm.201806909.
- Sun, J.; Keplinger, C.; Whitesides, G. M.; Suo Z. Ionic Skin. Adv. Mater. 2014, 26, 7608–7614. doi: 10.1002/adma.201403441..
- Kim, C.; Lee, H.; Oh, K. H.; Sun, J. Highly Stretchable, Transparent Ionic Touch Panel. Science. 2016, 353, 682–687. doi: 10.1126/science.aaf8810.
- Larson, C.; Peele, B.; Li, S.; Robinson, S.; Totaro, M.; Beccai, L.; Mazzolai, B.; Shepherd, R. Highly Stretchable Electroluminescent Skin for Optical Signaling and Tactile Sensing. Science. 2016, 351, 1071–1074. doi: 10.1126/science.aac5082.
- Yang, C.; Suo, Z. Hydrogel Ionotronics. Nat. Rev. Mater. 2018, 3, 125–142. doi: 10.1038/s41578-018-0018-7.
- Keplinger, C.; Sun, J. Y.; Foo, C. C.; Rothemund, P.; Whitesides, G. M.; Suo, Z. Stretchable, Transparent, Ionic Conductors. Science. 2013, 341, 984–987. doi: 10.1126/science.1240228.
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel Bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. doi: 10.1039/C8CS00595H.
- Liu, X.; Steiger, C.; Lin, S.; Parada, G. A.; Liu, J.; Chan, H. F.; Yuk, H.; Phan, N. V.; Collins, J.; Tamang, S.;, et al. Ingestible Hydrogel Device. Nat. Commun. 2019, 10, 493. doi: 10.1038/s41467-019-08355-2.
- Ge, G.; Yuan, W.; Zhao, W.; Lu, Y.; Zhang, Y.; Wang, W.; Chen, P.; Huang, W.; Si, W.; Dong, X. Highly Stretchable and Autonomously Healable Epidermal Sensor Based on Multi-Functional Hydrogel Frameworks. J. Mater. Chem. A. 2019, 7, 5949–5956. doi: 10.1039/C9TA00641A.
- Li, J.; Celiz, A. D.; Yang, J.; Yang, Q.; Wamala, I.; Whyte, W.; Seo, B. R.; Vasilyev, N. V.; Vlassak, J. J.; Suo, Z.;, et al. Tough Adhesives for Diverse Wet Surfaces. Science 2017, 357, 378–381. doi: 10.1126/science.aah6362.
- Guo, J. L.; Kim, Y. S.; Xie, V. Y.; Smith, B. T.; Watson, E.; Lam, J.; Pearce, H. A.; Engel, P. S.; Mikos, A. G. Modular, Tissue-Specific, and Biodegradable Hydrogel Cross-Linkers for Tissue Engineering. Sci. Adv. 2019, 5, eaaw7396. doi: 10.1126/sciadv.aaw7396.
- Sharma, B.; Fermanian, S.; Gibson, M.; Unterman, S.; Herzka, D. A.; Cascio, B.; Coburn, J.; Hui, A. Y.; Marcus, N.; Gold, G. E.;, et al. Human Cartilage Repair with a Photoreactive Adhesive-Hydrogel Composite. Sci. Transl. Med. 2013, 5, 167ra6. doi: 10.1126/scitranslmed.3004838.
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of Hydrogel-Based Scaffolds for Tissue Engineering Applications. Biotechnol. Adv. 2017, 35, 530–544. doi: 10.1016/j.biotechadv.2017.05.006.
- Prince, E.; Kumacheva, E. Design and Applications of Man-Made Biomimetic Fibrillar Hydrogels. Nat. Rev. Mater. 2019, 4, 99–115. doi: 10.1038/s41578-018-0077-9.
- Kloxin, A. M.; Kasko, A. M.; Salinas, C. N.; Anseth, K. S. Photodegradable Hydrogels for Dynamic Tuning of Physical and Chemical Properties. Science. 2009, 324, 59. doi: 10.1126/science.1169494.
- Johnson, C. T.; Wroe, J. A.; Agarwal, R.; Martin, K. E.; Guldberg, R. E.; Donlan, R. M.; Westblade, L. F.; Garcia, A. J. Hydrogel Delivery of Lysostaphin Eliminates Orthopedic Implant Infection by Staphylococcus Aureus and Supports Fracture Healing. PANS. 2018, 115, E4960–E4969. doi: 10.1073/pnas.1801013115.
- Zhang, S.; Ermann, J.; Succi, M. D.; Zhou, A.; Hamilton, M. J.; Cao, B.; Korzenik, J. R.; Glickman, J. N.; Vemula, P. K.; Glimcher, L. H.;, et al. An Inflammation-Targeting Hydrogel for Local Drug Delivery in Inflammatory Bowel Disease. Sci. Transl. Med. 2015, 7, 300ra128. doi: 10.1126/scitranslmed.aaa5657.
- Liu, Q.; Zhan, C.; Barhoumi, A.; Wang, W.; Santamaria, C.; McAlvin, J. B.; Kohane, D. S. A Supramolecular Shear-Thinning Anti-Inflammatory Steroid Hydrogel. Adv. Mater. 2016, 28, 6680–6686. doi: 10.1002/adma.201601147.
- Weaver, J. D.; Headen, D. M.; Aquart, J.; Johnson, C. T.; Shea, L. D.; Shirwan, H.; Garcia, A. J. Vasculogenic Hydrogel Enhances Islet Survival, Engraftment, and Function in Leading Extrahepatic Sites. Sci. Adv. 2017, 3, e1700184. doi: 10.1126/sciadv.1700184.
- Ghobril, C.; Grinstaff, M. W. The Chemistry and Engineering of Polymeric Hydrogel Adhesives for Wound Closure: A Tutorial. Chem. Soc. Rev. 2015, 44, 1820–1835. doi: 10.1039/c4cs00332b.
- Brown, T. E.; Anseth, K. S. Spatiotemporal Hydrogel Biomaterials for Regenerative Medicine. Chem. Soc. Rev. 2017, 46, 6532–6552. doi: 10.1039/c7cs00445a.
- Guo, S.; Kang, G.; Phan, D. T.; Hsu, M. N.; Por, Y. C.; Chen, C. H. Polymerization-Induced Phase Separation Formation of Structured Hydrogel Particles via Microfluidics for Scar Therapeutics. Sci. Rep-UK. 2018, 8, 2245. doi: 10.1038/s41598-018-20516-9.
- Li, Y.; Kumacheva, E. Hydrogel Microenvironments for Cancer Spheroid Growth and Drug Screening. Sci. Adv. 2018, 4, eaas8998. doi: 10.1126/sciadv.aas8998.
- Seliktar, D.;. Designing Cell-Compatible Hydrogels for Biomedical Applications. Science. 2012, 336, 1124–1128. doi: 10.1126/science.1214804.
- Huebsch, N.;. Translational Mechanobiology: Designing Synthetic Hydrogel Matrices for Improved in Vitro Models and Cell-Based Therapies. Acta Biomater. 2019, 94, 97–111. doi: 10.1016/j.actbio.2019.05.055.
- Hong, Y.; Zhou, F.; Hua, Y.; Zhang, X.; Ni, C.; Pan, D.; Zhang, Y.; Jiang, D.; Yang, L.; Lin, Q.;, et al. A Strongly Adhesive Hemostatic Hydrogel for the Repair of Arterial and Heart Bleeds. Nat. Commun. 2019, 10, 2060. doi: 10.1038/s41467-019-10004-7.
- Kong, W.; Wang, C.; Jia, C.; Kuang, Y.; Pastel, G.; Chen, C.; Chen, G.; He, S.; Huang, H.; Zhang, J.;, et al. Muscle-Inspired Highly Anisotropic, Strong, Ion-Conductive Hydrogels. Adv. Mater. 2018, 30, 1801934. doi: 10.1002/adma.201801934.
- Lei, Z.; Wu, P. A Supramolecular Biomimetic Skin Combining A Wide Spectrum of Mechanical Properties and Multiple Sensory Capabilities. Nat. Commun. 2018, 9, 1134. doi: 10.1038/s41467-018-03456-w.
- Che, L.; Lei, Z.; Wu, P.; Song, D. A 3D Printable and Bioactive Hydrogel Scaffold to Treat Traumatic Brain Injury. Adv. Funct. Mater. 2019, 29, 1904450. doi: 10.1002/adfm.201904450.
- Wang, Y.; Shang, L.; Chen, G.; Sun, L.; Zhang, X.; Zhao, Y. Bioinspired Structural Color Patch with Anisotropic Surface Adhesion. Sci. Adv. 2020, 6, eaax8258. doi: 10.1126/sciadv.aax8258.
- Beebe, D. J.; Moore, J. S.; Bauer, J. M.; Yu, Q.; Liu, R. H.; Devadoss, C.; Jo, B. H. Functional Hydrogel Structures for Autonomous Flow Control Inside Microfluidic Channels. Nature. 2000, 404, 588–590. doi: 10.1038/35007047.
- Zhang, Y. Z.; Lee, K. H.; Anjum, D. H.; Sougrat, R.; Jiang, Q.; Kim, H.; Alshareef, H. N. Mxenes Stretch Hydrogel Sensor Performance to New Limits. Sci. Adv. 2018, 4, eaat0098. doi: 10.1126/sciadv.aat0098.
- Lei, Z.; Wang, Q.; Sun, S.; Zhu, W.; Wu, P. A Bioinspired Mineral Hydrogel as A Self-Healable, Mechanically Adaptable Ionic Skin for Highly Sensitive Pressure Sensing. Adv. Mater. 2017, 29, 1700321. doi: 10.1002/adma.201700321.
- Darabi, M. A.; Khosrozadeh, A.; Mbeleck, R.; Liu, Y.; Chang, Q.; Jiang, J.; Cai, J.; Wang, Q.; Luo, G.; Xing, M. Skin-Inspired Multifunctional Autonomic-Intrinsic Conductive Self-Healing Hydrogels with Pressure Sensitivity, Stretchability, and 3D Printability. Adv. Mater. 2017, 29, 1700533. doi: 10.1002/adma.201700533.
- Lee, J. S.; Song, J.; Kim, S. O.; Kim, S.; Lee, W.; Jackman, J. A.; Kim, D.; Cho, N. J.; Lee, J. Multifunctional Hydrogel Nano-Probes for Atomic Force Microscopy. Nat. Commun. 2016, 7, 11566. doi: 10.1038/ncomms11566.
- Gupta, S. C.; Baheti, G. L.; Gupta, B. P. Application of Hydrogel System for Neutron Attenuation. Radiat. Phys. Chem. 2000, 59, 103–107. doi: 10.1016/S0969-806X(00)00189-4.
- Ito, K.;. Topological Gels. In Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2015; pp 2528–2534.
- Granick, S.; Rubinstein, M. A. Multitude of Macromolecules. Nat. Mater. 2004, 3, 586–587. doi: 10.1038/nmat1199.
- Katsuno, C.; Konda, A.; Urayama, K.; Takigawa, T.; Kidowaki, M.; Ito, K. Pressure-Responsive Polymer Membranes of Slide-Ring Gels with Movable Cross-Links. Adv. Mater. 2013, 25, 4636–4640. doi: 10.1002/adma.201301252.
- Haraguchi, K.; Takehisa, T. Nanocomposite Hydrogels: A Unique Organic-Inorganic Network Structure with Extraordinary Mechanical, Optical, and Swelling/De-Swelling Properties. Adv. Mater. 2002, 14, 1120–1124. doi: 10.1002/1521-4095(20020816)14:16<120::aid-adma1120>3.0.CO;2-9.
- Xia, L.; Xie, R.; Ju, X.; Wang, W.; Chen, Q.; Chu, L. Nano-Structured Smart Hydrogels with Rapid Response and High Elasticity. Nat. Commun. 2013, 4, 2226.
- Hu, Z.; Chen, G. Novel Nanocomposite Hydrogels Consisting of Layered Double Hydroxide with Ultrahigh Tensibility and Hierarchical Porous Structure at Low Inorganic Content. Adv. Mater. 2014, 26, 5950–5956. doi: 10.1002/adma.201400179.
- Okumura, Y.; Ito, K. The Polyrotaxane Gel: A Topological Gel by Figure-of-Eight Cross-Links. Adv.Mate. 2001, 13, 485–487. doi: 10.1002/1521-4095(200104)13:7<485::aid-adma485>3.0.CO;2-T.
- Tang, Z.; Chen, Q.; Chen, F.; Zhu, L.; Lu, S.; Ren, B.; Zhang, Y.; Yang, J.; Zheng, J. General Principle for Fabricating Natural Globular Protein-Based Double-Network Hydrogels with Integrated Highly Mechanical Properties and Surface Adhesion on Solid Surfaces. Chem. Mater.. 2019, 31, 179–189. doi: 10.1021/acs.chemmater.8b03860.
- Gong, J. P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. doi: 10.1002/adma.200304907..
- Morovati, V.; Dargazany, R. Micro-Mechanical Modeling of the Stress Softening in Double-Network Hydrogels. Int. J. Solids Struct. 2019, 164, 1–11. doi: 10.1016/j.ijsolstr.2019.01.002.
- Hu, J.; Hiwatashi, K.; Kurokawa, T.; Liang, S. M.; Wu, Z. L.; Gong, J. P. Microgel-Reinforced Hydrogel Films with High Mechanical Strength and Their Visible Mesoscale Fracture Structure. Macromolecules. 2011, 44, 7775–7781. doi: 10.1021/ma2016248.
- Yan, X.; Yang, J.; Chen, F.; Zhu, L.; Tang, Z.; Qin, G.; Chen, Q.; Chen, G. Mechanical Properties of Gelatin/Polyacrylamide/Graphene Oxide Nanocomposite Double-Network Hydrogels. Compos. Sci. Technol. 2018, 163, 81–88. doi: 10.1016/j.compscitech.2018.05.011.
- Xiao, S.; Zhao, T.; Wang, J.; Wang, C.; Du, J.; Ying, L.; Lin, J.; Zhang, C.; Hu, W.; Wang, L.;, et al.. Gelatin Methacrylate (Gelma)-based Hydrogels for Cell Transplantation: An Effective Strategy for Tissue Engineering. Stem Cell Rev. Rep. 2019, 15, 664–679. doi:10.1007/s12015-019-09893-4.
- Zhou, X.; Zhao, F.; Guo, Y.; Rosenberger, B.; Yu, G. Architecting Highly Hydratable Polymer Networks to Tune the Water State for Solar Water Purification. Sci. Adv. 2019, 5, eaaw5484. doi: 10.1126/sciadv.aaw5484.
- Hu, N.; Zhang, L.; Yang, C.; Zhao, J.; Yang, Z.; Wei, H.; Liao, H.; Feng, Z.; Fisher, A.; Zhang, Y.;, et al. Three-Dimensional Skeleton Networks of Graphene Wrapped Polyaniline Nanofibers: An Excellent Structure for High-Performance Flexible Solid-State Supercapacitors. Sci. Rep-UK 2016, 6, 19777. doi: 10.1038/srep19777.
- Qu, Y.; Chu, B. Y.; Peng, J. R.; Liao, J. F.; Qi, T. T.; Shi, K.; Zhang, X. N.; Wei, Y. Q.; Qian, Z. Y. A Biodegradable Thermo-Responsive Hybrid Hydrogel: Therapeutic Applications in Preventing the Post-Operative Recurrence of Breast Cancer. NPG Asia Mater. 2015, 7, e207. doi: 10.1038/am.2015.83.
- Wu, Y.; Wang, L.; Qing, Y.; Yan, N.; Tian, C.; Huang, Y. A Green Route to Prepare Fluorescent and Absorbent Nano-Hybrid Hydrogel for Water Detection. Sci. Rep-UK. 2017, 7, 4380. doi: 10.1038/s41598-017-04542-7.
- Alam, M. A.; Takafuji, M.; Ihara, H. Silica Nanoparticle-Crosslinked Thermosensitive Hybrid Hydrogels as Potential Drug-Release Carriers. Polym. J. 2014, 46, 293–300.
- Li, J.; Pan, K.; Tian, H.; Yin, L. Yin Potential of Electrospinning/Electrospraying Technology in the Rational Design of Hydrogel Structures. Macromol. Mater. Eng. 2020, 305(8), 2000285. doi:10.1002/mame.v305.8 8 305 133
- Akhtar, M. F.; Hanif, M.; Ranjha, N. M. Methods of Synthesis of Hydrogels … a Review. Saudi Pharm. J. 2016, 24, 554–559. doi: 10.1016/j.jsps.2015.03.022.
- Zhang, Y. S.; Khademhosseini, A. Advances in Engineering Hydrogels. Science. 2017, 356, eaaf3627. doi: 10.1126/science.aaf3627.
- Dhanjai,; Sinha, A.; Kalambate, P. K.; Mugo, S. M.; Kamau, P.; Chen, J.; Jain, R. Polymer Hydrogel Interfaces in Electrochemical Sensing Strategies: A Review. TrAC Trends Anal. Chem. 2019, 118, 488–501. doi: 10.1016/j.trac.2019.06.014.
- Harada, A.; Takashima, Y.; Nakahata, M. Supramolecular Polymeric Materials via Cyclodextrin–Guest Interactions. Acc. Chem. Res. 2014, 47, 2128–2140. doi: 10.1021/ar500109h.
- Clarke, D. E.; Pashuck, E. T.; Bertazzo, S.; Weaver, J. V. M.; Stevens, M. M. Self-Healing, Self-Assembled Β-sheet Peptide–Poly(γ-Glutamic Acid) Hybrid Hydrogels. J. Am. Chem. Soc. 2017, 139, 7250–7255. doi: 10.1021/jacs.7b00528.
- Jacob, R. S.; Ghosh, D.; Singh, P. K.; Basu, S. K.; Jha, N. N.; Das, S.; Sukul, P. K.; Patil, S.; Sathaye, S.; Kumar, A.;, et al. Self Healing Hydrogels Composed of Amyloid Nano Fibrils for Cell Culture and Stem Cell Differentiation. Biomaterials 2015, 54, 97–105. doi: 10.1016/j.biomaterials.2015.03.002.
- Zhang, G.; Ngai, T.; Deng, Y.; Wang, C. An Injectable Hydrogel with Excellent Self-Healing Property Based on Quadruple Hydrogen Bonding. Macromol. Chem. Phys. 2016, 217, 2172–2181. doi: 10.1002/macp.201600319.
- Miyamae, K.; Nakahata, M.; Takashima, Y.; Harada, A. Self-Healing, Expansion-Contraction, and Shape-Memory Properties of a Preorganized Supramolecular Hydrogel through Host-Guest Interactions. Angew. Chem. Int. Ed. Engl. 2015, 54, 8984–8987. doi: 10.1002/anie.201502957.
- Kakuta, T.; Takashima, Y.; Nakahata, M.; Otsubo, M.; Yamaguchi, H.; Harada, A. Preorganized Hydrogel: Self-Healing Properties of Supramolecular Hydrogels Formed by Polymerization of Host–Guest-Monomers that Contain Cyclodextrins and Hydrophobic Guest Groups. Adv. Mater. 2013, 25, 2849–2853. doi: 10.1002/adma.201205321.
- Gulyuz, U.; Okay, O. Self-Healing Poly(Acrylic Acid) Hydrogels with Shape Memory Behavior of High Mechanical Strength. Macromolecules. 2014, 47, 6889–6899. doi: 10.1021/ma5015116.
- Algi, M. P.; Okay, O. Highly Stretchable Self-Healing Poly(N,N-Dimethylacrylamide) Hydrogels. Eur. Polym. J. 2014, 59, 113–121. doi: 10.1016/j.eurpolymj.2014.07.022.
- Sun, T. L.; Kurokawa, T.; Kuroda, S.; Ihsan, A. B.; Akasaki, T.; Sato, K.; Haque, M. A.; Nakajima, T.; Gong, J. P. Physical Hydrogels Composed of Polyampholytes Demonstrate High Toughness and Viscoelasticity. Nat. Mater. 2013, 12, 932–937. doi: 10.1038/nmat3713.
- Bai, T.; Liu, S.; Sun, F.; Sinclair, A.; Zhang, L.; Shao, Q.; Jiang, S. Zwitterionic Fusion in Hydrogels and Spontaneous and Time-Independent Self-Healing under Physiological Conditions. Biomaterials. 2014, 35, 3926–3933. doi: 10.1016/j.biomaterials.2014.01.077.
- Sun, T. L.; Luo, F.; Kurokawa, T.; Karobi, S. N.; Nakajima, T.; Gong, J. P. Molecular Structure of Self-Healing Polyampholyte Hydrogels Analyzed from Tensile Behaviors. Soft Matter. 2015, 11, 9355–9366. doi: 10.1039/C5SM01423A.
- Shao, C.; Wang, M.; Meng, L.; Chang, H.; Wang, B.; Xu, F.; Yang, J.; Wan, P. Mussel-Inspired Cellulose Nanocomposite Tough Hydrogels with Synergistic Self-Healing, Adhesive, and Strain-Sensitive Properties. Chem. Mater. 2018, 30, 3110–3121. doi: 10.1021/acs.chemmater.8b01172.
- Sun, S.; Mao, L.; Lei, Z.; Yu, S.; Cölfen, H. Hydrogels from Amorphous Calcium Carbonate and Polyacrylic Acid: Bio-Inspired Materials for “Mineral Plastics”. Angew. Chem. Int. Ed. 2016, 55, 11765–11769. doi: 10.1002/anie.201602849.
- Ihsan, A. B.; Sun, T. L.; Kurokawa, T.; Karobi, S. N.; Nakajima, T.; Nonoyama, T.; Roy, C. K.; Luo, F.; Gong, J. P. Self-Healing Behaviors of Tough Polyampholyte Hydrogels. Macromolecules. 2016, 49, 4245–4252. doi: 10.1021/acs.macromol.6b00437.
- Henderson, K. J.; Zhou, T. C.; Otim, K. J.; Shull, K. R. Ionically Cross-Linked Triblock Copolymer Hydrogels with High Strength. Macromolecules. 2010, 43, 6193–6201. doi: 10.1021/ma100963m.
- Tsao, C. T.; Chang, C. H.; Lin, Y. Y.; Wu, M. F.; Wang, J.; Han, J. L.; Hsieh, K. H. Antibacterial Activity and Biocompatibility of a Chitosan-γ-Poly(Glutamic Acid) Polyelectrolyte Complex Hydrogel. Carbohyd. Res. 2010, 345, 1774–1780. doi: 10.1016/j.carres.2010.06.002.
- Liu, Z.; Wang, Y.; Ren, Y.; Jin, G.; Zhang, C.; Chen, W.; Yan, F. Poly(Ionic Liquid) Hydrogel-Based Anti-Freezing Ionic Skin for a Soft Robotic Gripper. Mater. Horiz. 2020, 7, 919–927. doi: 10.1039/C9MH01688K.
- Augst, A. D.; Kong, H. J.; Mooney, D. J. Alginate Hydrogels as Biomaterials. Macromol. Biosci. 2006, 6, 623–633. doi: 10.1002/mabi.200600069.
- Leong, J.; Lam, W.; Ho, K.; Voo, W.; Lee, M. F.; Lim, H.; Lim, S.; Tey, B.; Poncelet, D.; Chan, E. Advances in Fabricating Spherical Alginate Hydrogels with Controlled Particle Designs by Ionotropic Gelation as Encapsulation Systems. Particuology. 2016, 24, 44–60. doi: 10.1016/j.partic.2015.09.004.
- Gulrez, S. K. H.; Al-Assaf, S.; Phillips, G. O. Progress in Molecular and Environmental Bioengineering-From Analysis and Modeling to Technology Applications; Carpi, A., ed.; InTechOpen: London, UK, 2011; pp p 127.
- Nilsen-Nygaard, J.; Hattrem, M. N.; Draget, K. I. Propylene Glycol Alginate (Pga) Gelled Foams: A Systematic Study of Surface Activity and Gelling Properties as A Function of Degree of Esterification. Food Hydrocolloid. 2016, 57, 80–91. doi: 10.1016/j.foodhyd.2016.01.011.
- Hong, S.; Sycks, D.; Chan, H. F.; Lin, S.; Lopez, G. P.; Guilak, F.; Leong, K. W.; Zhao, X. 3D Printing of Highly Stretchable and Tough Hydrogels into Complex, Cellularized Structures. Adv. Mater. 2015, 27, 4035–4040. doi: 10.1002/adma.201501099.
- Chen, Q.; Yan, X.; Zhu, L.; Chen, H.; Jiang, B.; Wei, D.; Huang, L.; Yang, J.; Liu, B.; Zheng, J. Improvement of Mechanical Strength and Fatigue Resistance of Double Network Hydrogels by Ionic Coordination Interactions. Chem. Mater. 2016, 28, 5710–5720. doi: 10.1021/acs.chemmater.6b01920.
- Desiraju, G. R.;. Hydrogen Bridges in Crystal Engineering: Interactions without Borders. Acc. Chem. Res. 2002, 35, 565–573. doi: 10.1021/ar010054t.
- Li, G.; Yan, Q.; Xia, H.; Zhao, Y. Therapeutic-Ultrasound-Triggered Shape Memory of a Melamine-Enhanced Poly(Vinyl Alcohol) Physical Hydrogel. ACS Appl. Mater. Inter. 2015, 7, 12067–12073. doi: 10.1021/acsami.5b02234.
- Li, G.; Zhang, H.; Fortin, D.; Xia, H.; Zhao, Y. Poly(Vinyl Alcohol)-Poly(Ethylene Glycol) Double-Network Hydrogel: A General Approach to Shape Memory and Self-Healing Functionalities. Langmuir. 2015, 31, 11709–11716. doi: 10.1021/acs.langmuir.5b03474.
- Song, P. A.; Xu, Z.; Guo, Q. Bioinspired Strategy to Reinforce PVA with Improved Toughness and Thermal Properties via Hydrogen-Bond Self-Assembly. ACS Macro Lett. 2013, 2, 1100–1104. doi: 10.1021/mz4005265.
- Li, J.; Wang, Z.; Wen, L.; Nie, J.; Yang, S.; Xu, J.; Cheng, S. Z. D. Highly Elastic Fibers Made From Hydrogen-Bonded Polymer Complex. ACS Macro Lett. 2016, 5, 814–818. doi: 10.1021/acsmacrolett.6b00346.
- Tang, L.; Liu, W.; Liu, G. High-Strength Hydrogels with Integrated Functions of H-Bonding and Thermoresponsive Surface-Mediated Reverse Transfection and Cell Detachment. Adv. Mater. 2010, 22, 2652–2656. doi: 10.1002/adma.200904016.
- Dai, X.; Zhang, Y.; Gao, L.; Bai, T.; Wang, W.; Cui, Y.; Liu, W. A. Mechanically Strong, Highly Stable, Thermoplastic, and Self-Healable Supramolecular Polymer Hydrogel. Adv. Mater. 2015, 27, 3566–3571. doi: 10.1002/adma.201500534.
- Hu, X.; Vatankhah-Varnoosfaderani, M.; Zhou, J.; Li, Q.; Sheiko, S. S. Weak Hydrogen Bonding Enables Hard, Strong, Tough, and Elastic Hydrogels. Adv. Mater. 2015, 27, 6899–6905. doi: 10.1002/adma.201503724.
- Chen, Y.; Peng, L.; Liu, T.; Wang, Y.; Shi, S.; Wang, H. Poly(Vinyl Alcohol)–Tannic Acid Hydrogels with Excellent Mechanical Properties and Shape Memory Behaviors. ACS Appl. Mater. Inter. 2016, 8, 27199–27206. doi: 10.1021/acsami.6b08374.
- Shi, S.; Peng, X.; Liu, T.; Chen, Y.; He, C.; Wang, H. Facile Preparation of Hydrogen-Bonded Supramolecular Polyvinyl Alcohol-Glycerol Gels with Excellent Thermoplasticity and Mechanical Properties. Polymer. 2017, 111, 168–176. doi: 10.1016/j.polymer.2017.01.051.
- Liu, T.; Peng, X.; Chen, Y.; Bai, Q.; Shang, C.; Zhang, L.; Wang, H. Hydrogen-Bonded Polymer–Small Molecule Complexes with Tunable Mechanical Properties. Macromol. Rapid Comm. 2018, 39, 1800050. doi: 10.1002/marc.201800050.
- Yokoyama, F.; Masada, I.; Shimamura, K.; Ikawa, T.; Monobe, K. Morphology and Structure of Highly Elastic Poly(Vinyl Alcohol) Hydrogel Prepared by Repeated Freezing-and-Melting. Colloid Polym. Sci. 1986, 264, 595–601. doi: 10.1007/BF01412597.
- Nugent, M. J. D.; Hanley, A.; Tomkins, P. T.; Higginbotham, C. L. Investigation of a Novel Freeze-Thaw Process for the Production of Drug Delivery Hydrogels. J. Mater. Sci.: Mater. Med. 2005, 16, 1149–1158. doi: 10.1007/s10856-005-4722-7.
- Willcox, P. J.; Howie, D. W., Jr.; Schmidt-Rohr, K.; Hoagland, D. A.; Gido, S. P.; Pudjijanto, S.; Kleiner, L. W.; Venkatraman, S. Microstructure of Poly(Vinyl Alcohol) Hydrogels Produced by Freeze/Thaw Cycling. J. Polym. Sci. B Polym. Phys. 1999, 37, 3438–3454. doi: 10.1002/(SICI)1099-0488(19991215)37:24<3438::aid-polb6>3.0.CO;2-9.
- Gong, Z.; Zhang, G.; Zeng, X.; Li, J.; Li, G.; Huang, W.; Sun, R.; Wong, C. High-Strength, Tough, Fatigue Resistant, and Self-Healing Hydrogel Based on Dual Physically Cross-Linked Network. ACS Appl. Mater. Inter. 2016, 8, 24030–24037. doi: 10.1021/acsami.6b05627.
- Guo, P.; Liang, J.; Li, Y.; Lu, X.; Fu, H.; Jing, H.; Guan, S.; Han, D.; Niu, L. High-Strength and Ph-Responsive Self-Healing Polyvinyl Alcohol/Poly 6-Acrylamidohexanoic Acid Hydrogel Based on Dual Physically Cross-Linked Network. Colloids Surf. A. 2019, 571, 64–71. doi: 10.1016/j.colsurfa.2019.03.027.
- Liu, T.; Jiao, C.; Peng, X.; Chen, Y.; Chen, Y.; He, C.; Liu, R.; Wang, H. Super-Strong and Tough Poly(Vinyl Alcohol)/Poly(Acrylic Acid) Hydrogels Reinforced by Hydrogen Bonding. J. Mater. Chem. B. 2018, 6, 8105–8114. doi: 10.1039/C8TB02556H.
- Shi, L.; Ding, P.; Wang, Y.; Zhang, Y.; Ossipov, D.; Hilborn, J. Self-Healing Polymeric Hydrogel Formed by Metal–Ligand Coordination Assembly: Design, Fabrication, and Biomedical Applications. Macromol. Rapid Comm. 2019, 40, 1800837. doi: 10.1002/marc.201800837.
- Haas, K. L.; Franz, K. J. Application of Metal Coordination Chemistry to Explore and Manipulate Cell Biology. Chem. Rev. 2009, 109, 4921–4960. doi: 10.1021/cr900134a.
- Nastri, F.; Chino, M.; Maglio, O.; Bhagi-Damodaran, A.; Lu, Y.; Lombardi, A. Design and Engineering of Artificial Oxygen-Activating Metalloenzymes. Chem. Soc. Rev. 2016, 45, 5020–5054. doi: 10.1039/C5CS00923E.
- Harrington, M. J.; Masic, A.; Holten-Andersen, N.; Waite, J. H.; Fratzl, P. Iron-Clad Fibers: A Metal-Based Biological Strategy for Hard Flexible Coatings. Science. 2010, 328, 216. doi: 10.1126/science.1181044.
- Krogsgaard, M.; Nue, V.; Birkedal, H. Mussel‐Inspired Materials: Self‐Healing through Coordination Chemistry. Chem. Eur. J. 2016, 22, 844–857. doi: 10.1002/chem.201503380.
- Mauro, M.;. Dynamic Metal–Ligand Bonds as Scaffolds for Autonomously Healing Multi‐Responsive Materials. Eur. J. Inorg. Chem. 2018, 2018, 2090–2100. doi: 10.1002/ejic.201800226.
- Mozhdehi, D.; Ayala, S.; Cromwell, O. R.; Guan, Z. Self-Healing Multiphase Polymers Via Dynamic Metal–Ligand Interactions. J. Am. Chem. Soc. 2014, 136, 16128–16131. doi: 10.1021/ja5097094.
- Cook, T. R.; Zheng, Y.; Stang, P. J. Metal–Organic Frameworks and Self-Assembled Supramolecular Coordination Complexes: Comparing and Contrasting the Design, Synthesis, and Functionality of Metal–Organic Materials. Chem. Rev. 2013, 113, 734–777. doi: 10.1021/cr3002824.
- Li, C.; Wang, C.; Keplinger, C.; Zuo, J.; Jin, L.; Sun, Y.; Zheng, P.; Cao, Y.; Lissel, F.; Linder, C.;; et al.. A Highly Stretchable Autonomous Self-Healing Elastomer. Nat. Chem. 2016, 8, 618–624.
- Zhang, J.; Su, C. Metal-Organic Gels: From Discrete Metallogelators to Coordination Polymers. Coordin. Chem. Rev. 2013, 257, 1373–1408. doi: 10.1016/j.ccr.2013.01.005.
- Grindy, S. C.; Learsch, R.; Mozhdehi, D.; Cheng, J.; Barrett, D. G.; Guan, Z.; Messersmith, P. B.; Holten-Andersen, N. Control of Hierarchical Polymer Mechanics with Bioinspired Metal-Coordination Dynamics. Nat. Mater. 2015, 14, 1210–1216. doi: 10.1038/nmat4401.
- Waite, J. H.;. Mussel Power. Nat. Mater. 2008, 7, 8–9. doi: 10.1038/nmat2087.
- Lin, Q.; Gourdon, D.; Sun, C.; Holten-Andersen, N.; Anderson, T. H.; Waite, J. H.; Israelachvili, J. N. Adhesion Mechanisms of the Mussel Foot Proteins Mfp-1 and Mfp-3. Proc. National Academy Sci. 2007, 104, 3782. doi: 10.1073/pnas.0607852104.
- Sedó, J.; Saiz-Poseu, J.; Busqué, F.; Ruiz-Molina, D. Catechol-Based Biomimetic Functional Materials. Adv. Mater. 2013, 25, 653–701. doi: 10.1002/adma.201202343.
- Zhang, W.; Wang, R.; Sun, Z.; Zhu, X.; Zhao, Q.; Zhang, T.; Cholewinski, A.; Yang, F. K.; Zhao, B.; Pinnaratip, R.;, et al. Catechol-Functionalized Hydrogels: Biomimetic Design, Adhesion Mechanism, and Biomedical Applications. Chem. Soc. Rev. 2020, 49, 433–464. doi: 10.1039/C9CS00285E.
- Krogsgaard, M.; Behrens, M. A.; Pedersen, J. S.; Birkedal, H. Self-Healing Mussel-Inspired Multi-Ph-Responsive Hydrogels. Biomacromolecules. 2013, 14, 297–301. doi: 10.1021/bm301844u.
- Li, L.; Smitthipong, W.; Zeng, H. Mussel-Inspired Hydrogels for Biomedical and Environmental Applications. Polym. Chem. UK. 2015, 6, 353–358. doi: 10.1039/C4PY01415D.
- Hou, S.; Ma, P. X. Stimuli-Responsive Supramolecular Hydrogels with High Extensibility and Fast Self-Healing via Precoordinated Mussel-Inspired Chemistry. Chem. Mater. 2015, 27, 7627–7635. doi: 10.1021/acs.chemmater.5b02839.
- Liu, Y.; Meng, H.; Qian, Z.; Fan, N.; Choi, W.; Zhao, F.; Lee, B. P. A Moldable Nanocomposite Hydrogel Composed of A Mussel-Inspired Polymer and A Nanosilicate as A Fit-to-Shape Tissue Sealant. Angew. Chem. Int. Ed. 2017, 56, 4224–4228. doi: 10.1002/anie.201700628.
- Han, L.; Lu, X.; Wang, M.; Gan, D.; Deng, W.; Wang, K.; Fang, L.; Liu, K.; Chan, C. W.; Tang, Y.;, et al. A Mussel-Inspired Conductive, Self-Adhesive, and Self-Healable Tough Hydrogel as Cell Stimulators and Implantable Bioelectronics. Small 2017, 13, 1601916. doi: 10.1002/smll.201601916.
- Holten-Andersen, N.; Harrington, M. J.; Birkedal, H.; Lee, B. P.; Messersmith, P. B.; Lee, K. Y. C.; Waite, J. H. Ph-Induced Metal-Ligand Cross-Links Inspired by Mussel Yield Self-Healing Polymer Networks with Near-Covalent Elastic Moduli. Proc. National Academy Sci. 2011, 108, 2651. doi: 10.1073/pnas.1015862108.
- Zhang, Z.; Gao, Z.; Wang, Y.; Guo, L.; Yin, C.; Zhang, X.; Hao, J.; Zhang, G.; Chen, L. Eco-Friendly, Self-Healing Hydrogels for Adhesive and Elastic Strain Sensors, Circuit Repairing, and Flexible Electronic Devices. Macromolecules. 2019, 52, 2531–2541. doi: 10.1021/acs.macromol.8b02466.
- Jing, X.; Mi, H.; Lin, Y.; Enriquez, E.; Peng, X.; Turng, L. Highly Stretchable and Biocompatible Strain Sensors Based on Mussel-Inspired Super-Adhesive Self-Healing Hydrogels for Human Motion Monitoring. ACS Appl. Mater. Inter. 2018, 10, 20897–20909. doi: 10.1021/acsami.8b06475.
- Shi, L.; Wang, F.; Zhu, W.; Xu, Z.; Fuchs, S.; Hilborn, J.; Zhu, L.; Ma, Q.; Wang, Y.; Weng, X.;, et al. Self-Healing Silk Fibroin-Based Hydrogel for Bone Regeneration: Dynamic Metal-Ligand Self-Assembly Approach. Adv. Funct. Mater. 2017, 27, 1700591. doi: 10.1002/adfm.201700591.
- Weng, G.; Thanneeru, S.; He, J. Dynamic Coordination of Eu–Iminodiacetate to Control Fluorochromic Response of Polymer Hydrogels to Multistimuli. Adv. Mater. 2018, 30, 1706526. doi: 10.1002/adma.201706526.
- Wei, S.; Lu, W.; Le, X.; Ma, C.; Lin, H.; Wu, B.; Zhang, J.; Theato, P.; Chen, T. Bioinspired Synergistic Fluorescence-Color Switchable Polymeric Hydrogel Actuator. Angew. Chem. Int. Ed. 2019. doi: 10.1002/anie.201908437.
- Slager, J.; Domb, A. J. Biopolymer Stereocomplexes. Adv. Drug Deliver. Rev. 2003, 55, 549–583. doi: 10.1016/S0169-409X(03)00042-5.
- Tsuji, H.;. Poly(Lactic Acid) Stereocomplexes: A Decade of Progress. Adv. Drug Deliver. Rev. 2016, 107, 97–135. doi: 10.1016/j.addr.2016.04.017.
- Fox, T. G.; Garrett, B. S.; Goode, W. E.; Gratch, S.; Kincaid, J. F.; Spell, A.; Stroupe, J. D. Crystalline Polymers of Methyl Methacrylate. J. Am. Chem. Soc. 1958, 80, 1768–1769. doi: 10.1021/ja01540a068.
- Basu, A.; Kunduru, K. R.; Doppalapudi, S.; Domb, A. J.; Khan, W. Poly(Lactic Acid) Based Hydrogels. Adv. Drug Deliver. Rev. 2016, 107, 192–205. doi: 10.1016/j.addr.2016.07.004.
- Jing, Y.; Quan, C.; Liu, B.; Jiang, Q.; Zhang, C. A Mini Review on the Functional Biomaterials Based on Poly(Lactic Acid) Stereocomplex. Polym. Rev. 2016, 56, 262–286. doi: 10.1080/15583724.2015.1111380.
- Wei, X.; Bao, R.; Cao, Z.; Yang, W.; Xie, B.; Yang, M. Stereocomplex Crystallite Network in Asymmetric Plla/ Pdla Blends: Formation, Structure, and Confining Effect On the Crystallization Rate of Homocrystallites. Macromolecules. 2014, 47, 1439–1448. doi: 10.1021/ma402653a.
- Lim, D. W.; Park, T. G. Stereocomplex Formation between Enantiomeric Pla–Peg–Pla Triblock Copolymers: Characterization and Use as Protein-Delivery Microparticulate Carriers. J. Appl. Polym. Sci. 2000, 75, 1615–1623. doi: 10.1002/(SICI)1097-4628(20000328)75:13<615::aid-app7>3.0.CO;2-L.
- Cao, H.; Chang, X.; Mao, H.; Zhou, J.; Wu, Z. L.; Shan, G.; Bao, Y.; Pan, P. Stereocomplexed Physical Hydrogels with High Strength and Tunable Crystallizability. Soft Matter. 2017, 13, 8502–8510. doi: 10.1039/c7sm01491k.
- Savadkoohi, S.; Farahnaky, A. Dynamic Rheological and Thermal Study of the Heat-Induced Gelation of Tomato-Seed Proteins. J. Food Eng. 2012, 113, 479–485. doi: 10.1016/j.jfoodeng.2012.06.010.
- Cordobes, F.; Partal, P.; Guerrero, A. Rheology and Microstructure of Heat-Induced Egg Yolk Gels. Rheol. Acta. 2004, 43, 184–195. doi: 10.1007/s00397-003-0338-3.
- Gosal, W. S.; Ross-Murphy, S. B. Globular Protein Gelation. Curr. Opin. Colloid Interface Sci. 2000, 5, 188–194. doi: 10.1016/S1359-0294(00)00057-1.
- Ma, J.; Cui, P.; Zhao, L.; Huang, R. Synthesis and Solution Behavior of Hydrophobic Association Water-Soluble Polymers Containing Arylalkyl Group. Eur. Polym. J. 2002, 38, 1627–1633. doi: 10.1016/S0014-3057(02)00034-4.
- Liu, C.; Liu, X.; Yu, J.; Gao, G.; Liu, F. Network Structure and Mechanical Properties of Hydrophobic Association Hydrogels: Surfactant Effect I. J. Appl. Polym. Sci. 2015, 132, 41222. doi: 10.1002/app.41222.
- Han, Y.; Tan, J.; Wang, D.; Xu, K.; An, H. Novel Approach to Promote the Hydrophobic Association: Introduction of Short Alkyl Chains into Hydrophobically Associating Polyelectrolytes. J. Appl. Polym. Sci. 2019, 136, 47581. doi: 10.1002/app.47581.
- Gruber, A.; Isik, D.; Fontanezi, B. B.; Boettcher, C.; Schaefer-Korting, M.; Klinger, D. A Versatile Synthetic Platform for Amphiphilic Nanogels with Tunable Hydrophobicity. Polym. Chem. UK 2018, 9, 5572–5584. doi: 10.1039/c8py01123k..
- Li, B.; Thompson, M. E. Phase Transition in Amphiphilic Poly(N-Isopropylacrylamide): Controlled Gelation. Phys. Chem. Chem. Phys. 2018, 20, 13623–13631. doi: 10.1039/C8CP01609G..
- Manouras, T.; Koufakis, E.; Anastasiadis, S. H.; Vamvakaki, M. A Facile Route towards Pdmaema Homopolymer Amphiphiles. Soft Matter. 2017, 13, 3777–3782. doi: 10.1039/c7sm00365j.
- Xiong, C.; Peng, K.; Tang, X.; Ye, Z.; Shi, Y.; Yang, H. Co2-Responsive Self-Healable Hydrogels Based on Hydrophobically-Modified Polymers Bridged by Wormlike Micelles. RSC Adv. 2017, 7, 34669–34675. doi: 10.1039/C7RA06418G.
- Liu, Y.; Li, Z.; Niu, N.; Zou, J.; Liu, F. A Simple Coordination Strategy for Preparing A Complex Hydrophobic Association Hydrogel. J. Appl. Polym. Sci. 2018, 135, 46400. doi: 10.1002/app.46400.
- Jiang, G.; Liu, C.; Liu, X.; Zhang, G.; Yang, M.; Liu, F. Construction and Properties of Hydrophobic Association Hydrogels with High Mechanical Strength and Reforming Capability. Macromol. Mater. Eng. 2009, 294, 815–820. doi: 10.1002/mame.200900160.
- Can, V.; Kochovski, Z.; Reiter, V.; Severin, N.; Siebenbürger, M.; Kent, B.; Just, J.; Rabe, J. P.; Ballauff, M.; Okay, O. Nanostructural Evolution and Self-Healing Mechanism of Micellar Hydrogels. Macromolecules. 2016, 49, 2281–2287. doi: 10.1021/acs.macromol.6b00156.
- Jeon, I.; Cui, J.; Illeperuma, W. R.; Aizenberg, J.; Vlassak, J. J. Extremely Stretchable and Fast Self-Healing Hydrogels. Adv. Mater. 2016, 28, 4678–4683. doi: 10.1002/adma.201600480.
- Yang, F.; Ren, B.; Cai, Y.; Tang, J.; Li, D.; Wang, T.; Feng, Z.; Chang, Y.; Xu, L.; Zheng, J. Mechanically Tough and Recoverable Hydrogels via Dual Physical Crosslinkings. J. Polym. Sci. B Polym. Phys. 2018, 56, 1294–1305. doi: 10.1002/polb.24729.
- Cao, J.; Li, J.; Chen, Y.; Zhang, L.; Zhou, J. Dual Physical Crosslinking Strategy to Construct Moldable Hydrogels with Ultrahigh Strength and Toughness. Adv. Funct. Mater. 2018, 28, 1800739. doi: 10.1002/adfm.201800739.
- Jiang, H.; Duan, L.; Ren, X.; Gao, G. Hydrophobic Association Hydrogels with Excellent Mechanical and Self-Healing Properties. Eur. Polym. J. 2019, 112, 660–669. doi: 10.1016/j.eurpolymj.2018.10.031.
- Li, S.; Gao, Y.; Jiang, H.; Duan, L.; Tough, G. G. Sticky and Remoldable Hydrophobic Association Hydrogel Regulated by Polysaccharide and Sodium Dodecyl Sulfate as Emulsifiers. Carbohyd. Polym. 2018, 201, 591–598. doi: 10.1016/j.carbpol.2018.08.100.
- Tuncaboylu, D. C.; Sari, M.; Oppermann, W.; Okay, O. Tough and Self-Healing Hydrogels Formed via Hydrophobic Interactions. Macromolecules. 2011, 44, 4997–5005. doi: 10.1021/ma200579v.
- Hao, J.; Weiss, R. A. Viscoelastic and Mechanical Behavior of Hydrophobically Modified Hydrogels. Macromolecules. 2011, 44, 9390–9398. doi: 10.1021/ma202130u.
- Mihajlovic, M.; Staropoli, M.; Appavou, M.; Wyss, H. M.; Pyckhout-Hintzen, W.; Sijbesma, R. P. Tough Supramolecular Hydrogel Based on Strong Hydrophobic Interactions in a Multiblock Segmented Copolymer. Macromolecules. 2017, 50, 3333–3346. doi: 10.1021/acs.macromol.7b00319.
- Abdurrahmanoglu, S.; Can, V.; Okay, O. Design of High-Toughness Polyacrylamide Hydrogels by Hydrophobic Modification. Polymer. 2009, 50, 5449–5455. doi: 10.1016/j.polymer.2009.09.042.
- Jiang, G.; Liu, F.; Liu, X.; Liu, C.; Chen, Q.; Zhang, G.; Yang, M. Network Structure and Compositional Effects on Tensile Mechanical Properties of Hydrophobic Association Hydrogels with High Mechanical Strength. Polymer. 2010, 51, 1507–1515. doi: 10.1016/j.polymer.2010.01.061.
- Tuncaboylu, D. C.; Sahin, M.; Argun, A.; Oppermann, W.; Okay, O. Dynamics and Large Strain Behavior of Self-Healing Hydrogels with and without Surfactants, Macromolecules. 2012, 45, 1991–2000. doi:10.1021/ma202672y
- Akay, G.; Hassan-Raeisi, A.; Tuncaboylu, D. C.; Orakdogen, N.; Abdurrahmanoglu, S.; Oppermann, W.; Okay, O. Self-Healing Hydrogels Formed in Catanionic Surfactant Solutions. Soft Matter. 2013, 9, 2254–2261. doi: 10.1039/c2sm27515e.
- Gulyuz, U.; Okay, O. Self-Healing Poly(Acrylic Acid) Hydrogels: Effect of Surfactant. Macromol. Symp. 2015, 358, 232–238. doi: 10.1002/masy.201500063.
- Xu, J.; Ren, X.; Gao, G. Salt-Inactive Hydrophobic Association Hydrogels with Fatigue Resistant and Self-Healing Properties. Polymer. 2018, 150, 194–203. doi: 10.1016/j.polymer.2018.07.045.
- Trabbic, K. A.; Yager, P. Comparative Structural Characterization of Naturally- and Synthetically-Spun Fibers of Bombyx Mori Fibroin. Macromolecules. 1998, 31, 462–471. doi: 10.1021/ma9708860.
- Su, D.; Yao, M.; Liu, J.; Zhong, Y.; Chen, X.; Shao, Z. Enhancing Mechanical Properties of Silk Fibroin Hydrogel through Restricting the Growth of Beta-Sheet Domains. ACS Appl. Mater. Inter. 2017, 9, 17489–17498. doi: 10.1021/acsami.7b04623.
- Lin, Y.; Xia, X.; Shang, K.; Elia, R.; Huang, W.; Cebe, P.; Leisk, G.; Omenetto, F.; Kaplan, D. L. Tuning Chemical and Physical Cross-Links in Silk Electrogels for Morphological Analysis and Mechanical Reinforcement. Biomacromolecules. 2013, 14, 2629–2635. doi: 10.1021/bm4004892.
- Luo, K.; Yang, Y.; Shao, Z. Physically Crosslinked Biocompatible Silk-Fibroin-Based Hydrogels with High Mechanical Performance. Adv. Funct. Mater. 2016, 26, 872–880. doi: 10.1002/adfm.201503450.
- Zhu, Z.; Ling, S.; Yeo, J.; Zhao, S.; Tozzi, L.; Buehler, M. J.; Omenetto, F.; Li, C.; Kaplan, D. L. High-Strength, Durable All-Silk Fibroin Hydrogels with Versatile Processability toward Multifunctional Applications. Adv. Funct. Mater. 2018, 28, 1704757. doi: 10.1002/adfm.201704757.
- Yang, J.; Xu, C.; Wang, C.; Kopeček, J. Refolding Hydrogels Self-Assembled from N-(2-Hydroxypropyl)Methacrylamide Graft Copolymers by Antiparallel Coiled-Coil Formation. Biomacromolecules. 2006, 7, 1187–1195. doi: 10.1021/bm051002k.
- Wang, C.; Stewart, R. J.; KopeČek, J. Hybrid Hydrogels Assembled from Synthetic Polymers and Coiled-Coil Protein Domains. Nature. 1999, 397, 417–420. doi: 10.1038/17092.
- Xu, C.; Kopeček, J. Genetically Engineered Block Copolymers: Influence of the Length and Structure of the Coiled-Coil Blocks on Hydrogel Self-Assembly. Pharm. Res.-Dordr. 2008, 25, 674–682. doi: 10.1007/s11095-007-9343-z.
- Wang, C.; Kopeček, J.; Stewart, R. J. Hybrid Hydrogels Cross-Linked by Genetically Engineered Coiled-Coil Block Proteins. Biomacromolecules. 2001, 2, 912–920. doi: 10.1021/bm0155322.
- Lupas, A.;. Coiled Coils: New Structures and New Functions. Trends Biochem. Sci. 1996, 21, 375–382. doi: 10.1016/S0968-0004(96)10052-9.
- Yang, J.; Xu, C.; Kopečková, P.; Kopeček, J. Hybrid Hydrogels Self-Assembled From Hpma Copolymers Containing Peptide Grafts. Macromol. Biosci. 2006, 6, 201–209. doi: 10.1002/mabi.200500208..
- Kopeček, J.;. Swell Gels. Nature. 2002, 417, 389–391. doi: 10.1038/417388a.
- van de Manakker, F.; van der Pot, M.; Vermonden, T.; van Nostrum, C. F.; Hennink, W. E. Self-Assembling Hydrogels Based on β-Cyclodextrin/Cholesterol Inclusion Complexes. Macromolecules. 2008, 41, 1766–1773. doi: 10.1021/ma702607r.
- Wang, Y.; Zhou, L.; Sun, G.; Xue, J.; Jia, Z.; Zhu, X.; Yan, D. Construction of Different Supramolecular Polymer Systems by Combining the Host–Guest and Hydrogen-Bonding Interactions. J. Polym. Sci. B Polym. Phys. 2008, 46, 1114–1120. doi: 10.1002/polb.21444.
- Choi, S.; Kwon, T.; Coskun, A.; Choi, J. W. Highly Elastic Binders Integrating Polyrotaxanes for Silicon Microparticle Anodes in Lithium Ion Batteries. Science. 2017, 357, 279. doi: 10.1126/science.aal4373.
- Kato, K.; Inoue, K.; Kidowaki, M.; Ito, K. Organic-Inorganic Hybrid Slide-Ring Gels: Polyrotaxanes Consisting of Poly(Dimethylsiloxane) and γ-Cyclodextrin and Subsequent Topological Cross-Linking. Macromolecules. 2009, 42, 7129–7136. doi: 10.1021/ma9011895.
- Gotoh, H.; Liu, C.; Imran, A. B.; Hara, M.; Seki, T.; Mayumi, K.; Ito, K.; Takeoka, Y. Optically Transparent, High-Toughness Elastomer Using a Polyrotaxane Cross-Linker as a Molecular Pulley. Sci. Adv. 2018, 4, eaat7629. doi: 10.1126/sciadv.aat7629.
- Yao, X.; Huang, P.; Nie, Z. Cyclodextrin-Based Polymer Materials: From Controlled Synthesis to Applications. Prog. Polym. Sci. 2019, 93, 1–35. doi: 10.1016/j.progpolymsci.2019.03.004.
- Hu, Q.; Tang, G.; Chu, P. K. Cyclodextrin-Based Host–Guest Supramolecular Nanoparticles for Delivery: From Design to Applications. Acc. Chem. Res. 2014, 47, 2017–2025. doi: 10.1021/ar500055s.
- Davis, M. E.; Brewster, M. E. Cyclodextrin-Based Pharmaceutics: Past, Present and Future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. doi: 10.1038/nrd1576.
- Liu, K. L.; Zhang, Z.; Li, J. Supramolecular Hydrogels Based on Cyclodextrin-Polymer Polypseudorotaxanes: Materials Design and Hydrogel Properties. Soft Matter. 2011, 7, 1129–11297. doi: 10.1039/c1sm06340e.
- Collier, J. H.; Rudra, J. S.; Gasiorowski, J. Z.; Jung, J. P. Multi-Component Extracellular Matrices Based On Peptide Self-Assembly. Chem. Soc. Rev. 2010, 39, 3413–3424. doi: 10.1039/B914337H.
- Zhang, X.; Chu, X.; Wang, L.; Wang, H.; Liang, G.; Zhang, J.; Long, J.; Yang, Z. Rational Design of a Tetrameric Protein to Enhance Interactions between Self-Assembled Fibers Gives Molecular Hydrogels. Angew. Chem. Int. Ed. 2012, 51, 4388–4392. doi: 10.1002/anie.201108612.
- Fan, Z.; Cheng, P.; Liu, M.; Li, D.; Liu, G.; Zhao, Y.; Ding, Z.; Chen, F.; Wang, B.; Tan, X.;, et al. Poly(Glutamic Acid) Hydrogels Crosslinked Via Native Chemical Ligation. New J. Chem. 2017, 41, 8656–8662. doi: 10.1039/C7NJ00439G.
- Kahn, J. S.; Hu, Y.; Willner, I. Stimuli-Responsive Dna-Based Hydrogels: From Basic Principles to Applications. Acc. Chem. Res. 2017, 50, 680–690. doi: 10.1021/acs.accounts.6b00542.
- Wang, J.; Chao, J.; Liu, H.; Su, S.; Wang, L.; Huang, W.; Willner, I.; Fan, C. Clamped Hybridization Chain Reactions for the Self-Assembly of Patterned DNA Hydrogels. Angew. Chem. Int. Ed. 2017, 56, 2171–2175. doi: 10.1002/anie.201610125.
- Cangialosi, A.; Yoon, C.; Liu, J.; Huang, Q.; Guo, J.; Nguyen, T. D.; Gracias, D. H.; Schulman, R. DNA Sequence–Directed Shape Change of Photopatterned Hydrogels via High-Degree Swelling. Science. 2017, 357, 1126–1130. doi: 10.1126/science.aan3925.
- Liu, J.;. Oligonucleotide-Functionalized Hydrogels as Stimuli Responsive Materials and Biosensors. Soft Matter. 2011, 7, 6757–6767. doi: 10.1039/C1SM05284E.
- Xing, Y.; Cheng, E.; Yang, Y.; Chen, P.; Zhang, T.; Sun, Y.; Yang, Z.; Liu, D. Self-Assembled DNA Hydrogels with Designable Thermal and Enzymatic Responsiveness. Adv. Mater. 2011, 23, 1117–1121. doi: 10.1002/adma.201003343.
- Li, F.; Lyu, D.; Liu, S.; Guo, W. DNA Hydrogels and Microgels for Biosensing and Biomedical Applications. Adv. Mater. 2019, 1806538. doi: 10.1002/adma.201806538.
- Song, J.; He, W.; Shen, H.; Zhou, Z.; Li, M.; Su, P.; Yang, Y. Self-Assembly of a Magnetic Dna Hydrogel as a New Biomaterial for Enzyme Encapsulation with Enhanced Activity and Stability. Chem. Commun. 2019, 55, 2449–2452. doi: 10.1039/C8CC09717H.
- Shao, Y.; Jia, H.; Cao, T.; Liu, D. Supramolecular Hydrogels Based On DNA Self-Assembly. Acc. Chem. Res. 2017, 50, 659–668. doi: 10.1021/acs.accounts.6b00524.
- Wang, D.; Hu, Y.; Liu, P.; Luo, D. Bioresponsive DNA Hydrogels: Beyond the Conventional Stimuli Responsiveness. Acc. Chem. Res. 2017, 50, 733–739. doi: 10.1021/acs.accounts.6b00581.
- Cao, T.; Jia, H.; Dong, Y.; Gui, S.; Liu, D. In Situ Formation of Covalent Second Network in a DNA Supramolecular Hydrogel and Its Application for 3D Cell Imaging. ACS Appl. Mater. Inter. 2020, 12, 4185–4192. doi: 10.1021/acsami.9b11534.
- English, M. A.; Soenksen, L. R.; Gayet, R. V.; de Puig, H.; Angenent-Mari, N. M.; Mao, A. S.; Nguyen, P. Q.; Collins, J. J. Programmable Crispr-Responsive Smart Materials. Science. 2019, 365, 780–785. doi: 10.1126/science.aaw5122.
- Tang, J.; Yao, C.; Gu, Z.; Jung, S.; Luo, D.; Yang, D. Super-Soft and Super-Elastic Dna Robot with Magnetically Driven Navigational Locomotion for Cell Delivery in Confined Space. Angew. Chem. Int. Ed. 2020, 59, 2490–2495. doi: 10.1002/anie.201913549.
- Li, F.; Tang, J.; Geng, J.; Luo, D.; Yang, D. Polymeric DNA Hydrogel: Design, Synthesis and Applications. Prog. Polym. Sci. 2019, 98, 101163. doi: 10.1016/j.progpolymsci.2019.101163.
- Kawase, T.; Kurata, H. Ball-, Bowl-, And Belt-Shaped Conjugated Systems and their Complexing Abilities: Exploration of the Concave−Convex π-π Interaction. Chem. Rev. 2006, 106, 5250–5273. doi: 10.1021/cr0509657.
- Cai, W.; Xu, D.; Qian, L.; Wei, J.; Xiao, C.; Qian, L.; Lu, Z.; Cui, S. Force-Induced Transition of π-π Stacking in a Single Polystyrene Chain. J. Am. Chem. Soc. 2019, 141, 9500–9503. doi: 10.1021/jacs.9b03490.
- Jayawarna, V.; Ali, M.; Jowitt, T. A.; Miller, A. F.; Saiani, A.; Gough, J. E.; Ulijn, R. V. Nanostructured Hydrogels for Three‐Dimensional Cell Culture through Self‐Assembly of Fluorenylmethoxycarbonyl–Dipeptides. Adv. Mater. 2006, 18, 611–614. doi: 10.1002/adma.200501522.
- Smith, A. M.; Williams, R. J.; Tang, C.; Coppo, P.; Collins, R. F.; Turner, M. L.; Saiani, A.; Ulijn, R. V. Fmoc-Diphenylalanine Self Assembles to a Hydrogel via a Novel Architecture Based on Π–Π Interlocked Β-sheets. Adv. Mater. 2008, 20, 37–41. doi: 10.1002/adma.200701221.
- Li, F.; Zhu, Y.; You, B.; Zhao, D.; Ruan, Q.; Zeng, Y.; Ding, C. Smart Hydrogels Co-Switched by Hydrogen Bonds and Π–Π Stacking for Continuously Regulated Controlled-Release System. Adv. Funct. Mater. 2010, 20, 669–676. doi: 10.1002/adfm.200901245.
- Xu, Y.; Wu, Q.; Sun, Y.; Bai, H.; Shi, G. Three-Dimensional Self-Assembly of Graphene Oxide and Dna into Multifunctional Hydrogels. ACS Nano. 2010, 4, 7358–7362. doi: 10.1021/nn1027104.
- Noble, B. B.; Coote, M. L. First Principles Modelling of Free-Radical Polymerisation Kinetics. Int. Rev. Phys. Chem. 2013, 32, 467–513. doi: 10.1080/0144235X.2013.797277.
- Sadeghi, I.; Yi, H.; Asatekin, A. A. Method for Manufacturing Membranes with Ultrathin Hydrogel Selective Layers for Protein Purification: Interfacially Initiated Free Radical Polymerization (Iifrp). Chem. Mater. 2018, 30, 1265–1276. doi: 10.1021/acs.chemmater.7b04598.
- Zhao, T.; Wang, G.; Hao, D.; Chen, L.; Liu, K.; Liu, M. Macroscopic Layered Organogel–Hydrogel Hybrids with Controllable Wetting and Swelling Performance. Adv. Funct. Mater. 2018, 28, 1800793. doi: 10.1002/adfm.201800793.
- Nezhad-Mokhtari, P.; Ghorbani, M.; Roshangar, L.; Soleimani Rad, J. A. Review on the Construction of Hydrogel Scaffolds by Various Chemically Techniques for Tissue Engineering. Eur. Polym. J. 2019, 117, 64–76. doi: 10.1016/j.eurpolymj.2019.05.004.
- Pérez-Madrigal, M. M.; Edo, M. G.; Díaz, A.; Puiggalí, J.; Alemán, C. Poly-γ-Glutamic Acid Hydrogels as Electrolyte for Poly(3,4-Ethylenedioxythiophene)-Based Supercapacitors. J. Phys. Chem. C. 2017, 121, 3182–3193. doi: 10.1021/acs.jpcc.6b10693.
- Lone, S.; Yoon, D. H.; Lee, H.; Cheong, I. W. Gelatin–Chitosan Hydrogel Particles for Efficient Removal of Hg(II) from Wastewater. Environ. Sci. 2019, 5, 83–90. doi: 10.1039/C8EW00678D.
- Fu, F.; Chen, Z.; Zhao, Z.; Wang, H.; Shang, L.; Gu, Z.; Zhao, Y. Bio-Inspired Self-Healing Structural Color Hydrogel. P. Natl. Acad. Sci. USA. 2017, 114, 5900–5905. doi: 10.1073/pnas.1703616114.
- Yu, S.; Zhang, X.; Tan, G.; Tian, L.; Liu, D.; Liu, Y.; Yang, X.; Pan, W. A. Novel pH-Induced Thermosensitive Hydrogel Composed of Carboxymethyl Chitosan and Poloxamer Cross-Linked by Glutaraldehyde for Ophthalmic Drug Delivery. Carbohyd. Polym. 2017, 155, 208–217. doi: 10.1016/j.carbpol.2016.08.073..
- Muharam, S.; Yuningsih, L. M.; Sumitra, M. R. Characterization of Superabsorbent Hydrogel Based on Epichlorohydrin Crosslink and Carboxymethyl Functionalization of Cassava Starch. Am. Inst. Phys. Conf. Ser. 2017, 1862, 030083.
- Huang, Y.; Liu, M.; Wang, L.; Gao, C.; Xi, S. A Novel Triple-Responsive Poly(3-Acrylamidephenylboronic Acid-Co-2-(Dimethylamino) Ethyl Methacrylate)/(β-Cyclodextrin-Epichlorohydrin)Hydrogels: Synthesis and Controlled Drug Delivery. React. Funct. Polym. 2011, 71, 666–673. doi: 10.1016/j.reactfunctpolym.2011.03.007.
- Mak, Y. W.; Leung, W. W. Crosslinking of Genipin and Autoclaving in Chitosan-Based Nanofibrous Scaffolds: Structural and Physiochemical Properties. J. Mater. Sci. 2019, 54, 10941–10962. doi: 10.1007/s10853-019-03649-8.
- Výborný, K.; Vallová, J.; Kočí, Z.; Kekulová, K.; Jiráková, K.; Jendelová, P.; Hodan, J.; Kubinová. Genipin and Edc Crosslinking of Extracellular Matrix Hydrogel Derived from Human Umbilical Cord for Neural Tissue Repair. Sci. Rep-UK. 2019, 9, 1–15. doi: 10.1038/s41598-019-47059-x.
- Sung, H.; Chang, W.; Ma, C.; Lee, M. Crosslinking of Biological Tissues Using Genipin and/Or Carbodiimide. J. Biomed. Mater. Res. A. 2003, 64A, 427–438. doi: 10.1002/jbm.a.10346.
- Yu, Y.; Feng, R.; Li, J.; Wang, Y.; Song, Y.; Tan, G.; Liu, D.; Liu, W.; Yang, X.; Pan, H.;, et al. A Hybrid Genipin-Crosslinked Dual-Sensitive Hydrogel/Nanostructured Lipid Carrier Ocular Drug Delivery Platform. Asian J. Pharm. Sci. 2019, 14, 423–434. doi: 10.1016/j.ajps.2018.08.002.
- Liu, Y.; Cai, Z.; Sheng, L.; Ma, M.; Xu, Q.; Jin, Y. Structure-Property of Crosslinked Chitosan/Silica Composite Films Modified by Genipin and Glutaraldehyde under Alkaline Conditions. Carbohyd. Polym. 2019, 215, 348–357. doi: 10.1016/j.carbpol.2019.04.001.
- Bi, L.; Cao, Z.; Hu, Y.; Song, Y.; Yu, L.; Yang, B.; Mu, J.; Huang, Z.; Han, Y. Effects of Different Cross-Linking Conditions on the Properties of Genipin-Cross-Linked Chitosan/Collagen Scaffolds for Cartilage Tissue Engineering. J. Mater. Sci.: Mater. Med. 2011, 22, 51–62. doi: 10.1007/s10856-010-4177-3.
- Klein, M. P.; Hackenhaar, C. R.; Lorenzoni, A. S. G.; Rodrigues, R. C.; Costa, T. M. H.; Ninow, J. L.; Hertz, P. F. Chitosan Crosslinked with Genipin as Support Matrix for Application in Food Process: Support Characterization and Β-d-galactosidase Immobilization. Carbohyd. Polym. 2016, 137, 184–190. doi: 10.1016/j.carbpol.2015.10.069.
- Arndt, K.; Schmidt, T.; Reichelt, R. Thermo-Sensitive Poly(Methyl Vinyl Ether) Micro-Gel Formed by High Energy Radiation. Polymer. 2001, 42, 6785–6791. doi: 10.1016/S0032-3861(01)00164-1.
- Kim, M. H.; Kim, B. S.; Lee, J.; Cho, D.; Kwon, O. H.; Park, W. H. Silk Fibroin/Hydroxyapatite Composite Hydrogel Induced by Gamma-Ray Irradiation for Bone Tissue Engineering. Biomater. Res. 2017, 21, 12. doi: 10.1186/s40824-017-0098-2.
- Yan, S.; Wang, T.; Li, X.; Jian, Y.; Zhang, K.; Li, G.; Yin, J. Fabrication of Injectable Hydrogels Based on Poly(L-Glutamic Acid) and Chitosan. RSC Adv. 2017, 7, 17005–17019. doi: 10.1039/C7RA01864A.
- Joy, J.; Pereira, J.; Aid-Launais, R.; Pavon-Djavid, G.; Ray, A. R.; Letourneur, D.; Meddahi-Pellé, A.; Gupta, B. Gelatin-Oxidized Carboxymethyl Cellulose Blend Based Tubular Electrospun Scaffold for Vascular Tissue Engineering. Int. J. Biol. Macromol. 2018, 107, 1922–1935. doi: 10.1016/j.ijbiomac.2017.10.071.
- Ju, H.; Zhu, F.; Xing, H.; Wu, Z. L.; Huang, F. Ultrastiff Hydrogels Prepared by Schiff’s Base Reaction of Bis(P‐Formylphenyl) Sebacate and Pillar[5]Arene Appended with Multiple Hydrazides. Macromol. Rapid Comm. 2017, 38, 1700232. doi: 10.1002/marc.201700232.
- Molina, B. G.; Cianga, L.; Bendrea, A.; Cianga, I.; Del Valle, L. J.; Estrany, F.; Alemán, C.; Armelin, E. Amphiphilic Polypyrrole-Poly(Schiff Base) Copolymers with Poly(Ethylene Glycol) Side Chains: Synthesis, Properties and Applications. Polym. Chem. UK. 2018, 9, 4218–4232. doi: 10.1039/C8PY00762D.
- Cañas, A. I.; Delgado, J. P.; Gartner, C. Biocompatible Scaffolds Composed of Chemically Crosslinked Chitosan and Gelatin for Tissue Engineering. J. Appl. Polym. Sci. 2016, 133, 43814. doi: 10.1002/app.43814.
- Shi, J.; Guobao, W.; Chen, H.; Zhong, W.; Qiu, X.; Xing, M. M. Q. Schiff Based Injectable Hydrogel for in Situ Ph-Triggered Delivery of Doxorubicin for Breast Tumor Treatment. Polym. Chem. UK 2014, 5, 6180–6189. doi: 10.1039/C4PY00631C.
- Zhao, X.; Li, P.; Guo, B.; Ma, P. X. Antibacterial and Conductive Injectable Hydrogels Based on Quaternized Chitosan-Graft-Polyaniline/Oxidized Dextran for Tissue Engineering. Acta Biomater. 2015, 26, 236–248. doi: 10.1016/j.actbio.2015.08.006.
- Li, L.; Wang, N.; Jin, X.; Deng, R.; Nie, S.; Sun, L.; Wu, Q.; Wei, Y.; Gong, C. Biodegradable and Injectable in Situ Cross-Linking Chitosan-Hyaluronic Acid Based Hydrogels for Postoperative Adhesion Prevention. Biomaterials. 2014, 35, 3903–3917. doi: 10.1016/j.biomaterials.2014.01.050.
- Sheng, X.; Li, X.; Li, M.; Zhang, R.; Deng, S.; Yang, W.; Chang, G.; Ye, X. An Injectable Oxidized Carboxymethyl Cellulose/Polyacryloyl Hydrazide Hydrogel Via Schiff Base Reaction. Aust. J. Chem. 2018, 71, 74. doi: 10.1071/CH17214.
- Shen, Y.; Li, X.; Huang, Y.; Chang, G.; Cao, K.; Yang, J.; Zhang, R.; Sheng, X.; Ye, X. Ph and Redox Dual Stimuli-Responsive Injectable Hydrogels Based on Carboxymethyl Cellulose Derivatives. Macromol. Res. 2016, 24, 602–608. doi: 10.1007/s13233-016-4077-6.
- Fan, M.; Ma, Y.; Tan, H.; Jia, Y.; Zou, S.; Guo, S.; Zhao, M.; Huang, H.; Ling, Z.; Chen, Y.;, et al. Covalent and Injectable Chitosan-Chondroitin Sulfate Hydrogels Embedded with Chitosan Microspheres for Drug Delivery and Tissue Engineering. Mater. Sci. Eng C 2017, 71, 67–74. doi: 10.1016/j.msec.2016.09.068.
- Yan, S.; Zhang, X.; Zhang, K.; Di, H.; Feng, L.; Li, G.; Fang, J.; Cui, L.; Chen, X.; Yin, J. Injectable in Situ Forming Poly(L-Glutamic Acid) Hydrogels for Cartilage Tissue Engineering. J. Mater. Chem. B. 2016, 4, 947–961. doi: 10.1039/C5TB01488C.
- Yan, S.; Wang, T.; Feng, L.; Zhu, J.; Zhang, K.; Chen, X.; Cui, L.; Yin, J. Injectable in Situ Self-Cross-Linking Hydrogels Based on Poly(L-Glutamic Acid) and Alginate for Cartilage Tissue Engineering. Biomacromolecules. 2014, 15, 4495–4508. doi: 10.1021/bm501313t.
- Sun Han Chang, R.; Lee, J. C.; Pedron, S.; Harley, B. A. C.; Rogers, S. A. Rheological Analysis of the Gelation Kinetics of an Enzyme Cross-Linked Peg Hydrogel. Biomacromolecules. 2019, 20, 2198–2206. doi: 10.1021/acs.biomac.9b00116.
- Lee, F.; Bae, K. H.; Kurisawa, M. Injectable Hydrogel Systems Crosslinked by Horseradish Peroxidase. Biomed. Mater. 2015, 11, 014101. doi: 10.1088/1748-6041/11/1/014101..
- Sakai, S.; Taya, M. On-Cell Surface Cross-Linking of Polymer Molecules by Horseradish Peroxidase Anchored to Cell Membrane for Individual Cell Encapsulation in Hydrogel Sheath. ACS Macro Lett. 2014, 3, 972–975. doi: 10.1021/mz5004322.
- Lei, K.; Sun, Y.; Sun, C.; Zhu, D.; Zheng, Z.; Wang, X. Fabrication of a Controlled in Situ Forming Polypeptide Hydrogel with a Good Biological Compatibility and Shapeable Property. ACS Appl. Mater. Inter. 2019, 2, 1751–1761. doi: 10.1021/acsabm.9b00157.
- Zhang, Y.; Zhang, Y.; Wang, Q.; Fan, X. Preparation and Properties of a Chitosan–Hyaluronic Acid-Polypyrrole Conductive Hydrogel Catalyzed by Laccase. J. Polym. Environ. 2017, 25, 526–532. doi: 10.1007/s10924-016-0831-2.
- Huber, D.; Tegl, G.; Baumann, M.; Sommer, E.; Gorji, E. G.; Borth, N.; Schleining, G.; Nyanhongo, G. S.; Guebitz, G. M. Chitosan Hydrogel Formation Using Laccase Activated Phenolics as Cross-Linkers. Carbohyd. Polym. 2017, 157, 814–822. doi: 10.1016/j.carbpol.2016.10.012.
- Chen, H.; Gan, J.; Ji, A.; Song, S.; Yin, L. Development of Double Network Gels Based On Soy Protein Isolate and Sugar Beet Pectin Induced by Thermal Treatment and Laccase Catalysis. Food Chem. 2019, 292, 188–196. doi: 10.1016/j.foodchem.2019.04.059.
- Deng, C.; Liu, Y.; Li, J.; Yadav, M. P.; Yin, L. Diverse Rheological Properties, Mechanical Characteristics and Microstructures of Corn Fiber Gum/Soy Protein Isolate Hydrogels Prepared by Laccase and Heat Treatment. Food Hydrocolloid. 2018, 76, 113–122. doi: 10.1016/j.foodhyd.2017.01.012.
- Zhao, L.; Li, X.; Zhao, J.; Ma, S.; Ma, X.; Fan, D.; Zhu, C.; Liu, Y. A Novel Smart Injectable Hydrogel Prepared by Microbial Transglutaminase and Human-Like Collagen: Its Characterization and Biocompatibility. Mater. Sci. Eng C. 2016, 68, 317–326. doi: 10.1016/j.msec.2016.05.108.
- Hu, H.; Zhu, X.; Hu, T.; Cheung, I. W. Y.; Pan, S.; Li-Chan, E. C. Y. Effect of Ultrasound Pre-Treatment On Formation of Transglutaminase-Catalysed Soy Protein Hydrogel as a Riboflavin Vehicle for Functional Foods. J. Funct. Foods. 2015, 19, 182–193. doi: 10.1016/j.jff.2015.09.023.
- Chen, P.; Yang, K.; Wu, C.; Yu, J.; Lin, F.; Sun, J. Fabrication of Large Perfusable Macroporous Cell-Laden Hydrogel Scaffolds Using Microbial Transglutaminase. Acta Biomater. 2014, 10, 912–920. doi: 10.1016/j.actbio.2013.11.009.
- Guo, J.; Jin, Y.; Yang, X.; Yu, S.; Yin, S.; Qi, J. Computed Microtomography and Mechanical Property Analysis of Soy Protein Porous Hydrogel Prepared by Homogenizing and Microbial Transglutaminase Cross-Linking. Food Hydrocolloid. 2013, 31, 220–226. doi: 10.1016/j.foodhyd.2012.10.023.
- Tsai, C.; Hong, Y.; Lee, R. J.; Cheng, N.; Yu, J. Enhancement of Human Adipose-Derived Stem Cell Spheroid Differentiation in an in Situ Enzyme-Crosslinked Gelatin Hydrogel. J. Mater. Chem. B. 2019, 7, 1064–1075. doi: 10.1039/C8TB02835D.
- Kim, S. H.; Lee, S. H.; Lee, J. E.; Park, S. J.; Kim, K.; Kim, I. S.; Lee, Y. S.; Hwang, N. S.; Kim, B. G. Tissue Adhesive, Rapid Forming, and Sprayable Ecm Hydrogel via Recombinant Tyrosinase Crosslinking. Biomaterials. 2018, 178, 401–412. doi: 10.1016/j.biomaterials.2018.04.057.
- Choi, Y. R.; Kim, E. H.; Lim, S.; Choi, Y. S. Efficient Preparation of a Permanent Chitosan/Gelatin Hydrogel Using an Acid-Tolerant Tyrosinase. Biochem. Eng. J. 2018, 129, 50–56. doi: 10.1016/j.bej.2017.10.016.
- Kobayashi, S.; Uyama, H.; Kimura, S. Enzymatic Polymerization. Chem. Rev. 2001, 101, 3793–3818. doi: 10.1021/cr990121l.
- Wang, R.; Zhou, B.; Liu, W.; Feng, X.; Li, S.; Yu, D.; Chang, J.; Chi, B.; Xu, H. Fast in Situ Generated Ɛ-polylysine-poly (Ethylene Glycol) Hydrogels as Tissue Adhesives and Hemostatic Materials Using an Enzyme-Catalyzed Method. J. Biomater. Appl. 2015, 29, 1167–1179. doi: 10.1177/0885328214553960.
- Singh, S.; Topuz, F.; Hahn, K.; Albrecht, K.; Groll, J. Embedding of Active Proteins and Living Cells in Redox-Sensitive Hydrogels and Nanogels through Enzymatic Cross-Linking. Angew. Chem. Int. Ed. Engl. 2013, 52, 3000–3003. doi: 10.1002/anie.201206266.
- Tabatabai, A. P.; Partlow, B. P.; Raia, N. R.; Kaplan, D. L.; Blair, D. L. Silk Molecular Weight Influences the Kinetics of Enzymatically Cross-Linked Silk Hydrogel Formation. Langmuir. 2018, 34, 15383–15387. doi: 10.1021/acs.langmuir.8b02950.
- Partlow, B. P.; Hanna, C. W.; Rnjak Kovacina, J.; Moreau, J. E.; Applegate, M. B.; Burke, K. A.; Marelli, B.; Mitropoulos, A. N.; Omenetto, F. G.; Kaplan, D. L. Highly Tunable Elastomeric Silk Biomaterials. Adv. Funct. Mater. 2014, 24, 4615–4624. doi: 10.1002/adfm.201400526.
- Ren, C. D.; Kurisawa, M.; Chung, J. E.; Ying, J. Y. Liposomal Delivery of Horseradish Peroxidase for Thermally Triggered Injectable Hyaluronic Acid–Tyramine Hydrogel Scaffolds. J. Mater. Chem. B. 2015, 3, 4663–4670. doi: 10.1039/C4TB01832J.
- Eelkema, R.; Pich, A. Pros and Cons: Supramolecular or Macromolecular: What Is Best for Functional Hydrogels with Advanced Properties?. Adv. Mater. 2020, 32, 1906012. doi: 10.1002/adma.201906012.
- Xu, D.; Huang, J.; Zhao, D.; Ding, B.; Zhang, L.; Cai, J. High-Flexibility, High-Toughness Double-Cross-Linked Chitin Hydrogels by Sequential Chemical and Physical Cross-Linkings. Adv. Mater. 2016, 28, 5844–5849. doi: 10.1002/adma.201600448.
- Zhang, X.; Wang, J.; Jin, H.; Wang, S.; Song, W. Bioinspired Supramolecular Lubricating Hydrogel Induced by Shear Force. J. Am. Chem. Soc. 2018, 140, 3186–3189. doi: 10.1021/jacs.7b12886.
- Chen, F.; Chen, Q.; Zhu, L.; Tang, Z.; Li, Q.; Qin, G.; Yang, J.; Zhang, Y.; Ren, B.; Zheng, J. General Strategy to Fabricate Strong and Tough Low-Molecular-Weight Gelator-Based Supramolecular Hydrogels with Double Network Structure. Chem. Mater. 2018, 30, 1743–1754. doi: 10.1021/acs.chemmater.8b00063.
- Lei, Z.; Wu, P. A Highly Transparent and Ultra-Stretchable Conductor with Stable Conductivity during Large Deformation. Nat. Commun. 2019, 10, 3429. doi: 10.1038/s41467-019-11364-w.
- Li, Z.; Zheng, Z.; Su, S.; Yu, L.; Wang, X. Preparation of a High-Strength Hydrogel with Slidable and Tunable Potential Functionalization Sites. Macromolecules. 2016, 49, 373–386. doi: 10.1021/acs.macromol.5b02359.
- Li, Z.; Zheng, Z.; Su, S.; Yu, L.; Wang, X. Hydroxypropyl-β-Cd Vs. Its α-Homologue for a 3D Modified Polyrotaxane Network Formation and Properties: The Relationship between Modified Cd and Polymer Revealed through Comparison. Soft Matter. 2016, 12, 7089–7101. doi: 10.1039/C6SM01368F.
- Jeong, D.; Joo, S.; Shinde, V. V.; Jung, S. Triple-Crosslinked β-Cyclodextrin Oligomer Self-Healing Hydrogel Showing High Mechanical Strength, Enhanced Stability and Ph Responsiveness. Carbohyd. Polym. 2018, 198, 563–574. doi: 10.1016/j.carbpol.2018.06.117.
- Du, R.; Xu, Z.; Zhu, C.; Jiang, Y.; Yan, H.; Wu, H. C.; Vardoulis, O.; Cai, Y.; Zhu, X.; Bao, Z.;, et al. A Highly Stretchable and Self-Healing Supramolecular Elastomer Based on Sliding Crosslinks and Hydrogen Bonds. Adv. Funct. Mater. 2020, 30, 1907139. doi: 10.1002/adfm.201907139.
- Li, S.; Chen, N.; Li, X.; Li, Y.; Xie, Z.; Ma, Z.; Zhao, J.; Hou, X.; Yuan, X. Bioinspired Double‐Dynamic‐Bond Crosslinked Bioadhesive Enables Post‐Wound Closure Care. Adv. Funct. Mater. 2020, 30, 2000130. doi: 10.1002/adfm.202000130.
- Han, J.; Cui, Y.; Han, X.; Liang, C.; Liu, W.; Luo, D.; Yang, D. Super-Soft Dna/ Dopamine-Grafted-Dextran Hydrogel as Dynamic Wire for Electric Circuits Switched by a Microbial Metabolism Process. Adv Sci. 2020, n/a, 2000684. doi: 10.1002/advs.202000684.
- Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L. M. Multifunctionality in Metal@Microgel Colloidal Nanocomposites. J. Mater. Chem. A. 2013, 1, 20–26. doi: 10.1039/C2TA00112H.
- Karg, M.;. Multifunctional Inorganic/ Organic Hybrid Microgels. Colloid Polym. Sci. 2012, 290, 673–688. doi: 10.1007/s00396-012-2644-8.
- Lu, Y.; Ballauff, M. Thermosensitive Core–Shell Microgels: From Colloidal Model Systems to Nanoreactors. Prog. Polym. Sci. 2011, 36, 767–792. doi: 10.1016/j.progpolymsci.2010.12.003.
- Brijitta, J.; Schurtenberger, P. Responsive Hydrogel Colloids: Structure, Interactions, Phase Behavior, and Equilibrium and Nonequilibrium Transitions of Microgel Dispersions. Curr. Opin. Colloid Interface Sci. 2019, 40, 87–103. doi: 10.1016/j.cocis.2019.02.005.
- Destribats, M.; Wolfs, M.; Pinaud, F.; Lapeyre, V.; Sellier, E.; Schmitt, V.; Ravaine, V. Pickering Emulsions Stabilized by Soft Microgels: Influence of the Emulsification Process on Particle Interfacial Organization and Emulsion Properties. Langmuir. 2013, 29, 12367–12374. doi: 10.1021/la402921b.
- Shin, D. S.; Tokuda, E. Y.; Leight, J. L.; Miksch, C. E.; Brown, T. E.; Anseth, K. S. Synthesis of Microgel Sensors for Spatial and Temporal Monitoring of Protease Activity. ACS Biomater. Sci. Eng. 2018, 4, 378–387. doi: 10.1021/acsbiomaterials.7b00017.
- Aliberti, A.; Ricciardi, A.; Giaquinto, M.; Micco, A.; Bobeico, E.; La Ferrara, V.; Ruvo, M.; Cutolo, A.; Cusano, A. Microgel Assisted Lab-On-Fiber Optrode. Sci. Rep-UK. 2017, 7, 14459. doi: 10.1038/s41598-017-14852-5.
- Li, B.; Kappl, M.; Han, L.; Cui, J.; Zhou, F.; Del Campo, A. Goosebumps-Inspired Microgel Patterns with Switchable Adhesion and Friction. Small. 2019, 1902376. doi: 10.1002/smll.201902376.
- Majerská, M.; Jakubec, M.; Klimša, V.; Rimpelová, S.; Král, V.; Štěpánek, F. Microgel Bioreactors for Cancer Cell Targeting by Ph-Dependent Generation of Radicals. Mol. Pharm. 2019, 16, 3275–3283. doi: 10.1021/acs.molpharmaceut.9b00531.
- Zhou, X.; Chen, F.; Lu, H.; Kong, L.; Zhang, S.; Zhang, W.; Nie, J.; Du, B.; Wang, X. Ionic Microgel Loaded with Gold Nanoparticles for the Synergistic Dual-Drug Delivery of Doxorubicin and Diclofenac Sodium. Ind. Eng. Chem. Res. 2019, 58, 10922–10930. doi: 10.1021/acs.iecr.9b01904.
- Kwok, M.; Sun, G.; Ngai, T. Microgel Particles at Interfaces: Phenomena, Principles, and Opportunities in Food Sciences. Langmuir. 2019, 35, 4205–4217. doi: 10.1021/acs.langmuir.8b04009.
- Chen, Z.; Lv, X.; Zhao, M.; Zhang, P.; Ren, X.; Mei, X. Encapsulation of Green Tea Polyphenol by Ph Responsive, Antibacterial, Alginate Microgels Used for Minimally Invasive Treatment of Bone Infection. Colloids Surf. B. 2018, 170, 648–655. doi: 10.1016/j.colsurfb.2018.06.065.
- Li, F.; Truong, V. X.; Fisch, P.; Levinson, C.; Glattauer, V.; Zenobi-Wong, M.; Thissen, H.; Forsythe, J. S.; Frith, J. E. Cartilage Tissue Formation through Assembly of Microgels Containing Mesenchymal Stem Cells. Acta Biomater. 2018, 77, 48–62. doi: 10.1016/j.actbio.2018.07.015.
- Yao, M.; Li, B.; Ye, H.; Huang, W.; Luo, Q.; Xiao, H.; McClements, D. J.; Li, L. Enhanced Viability of Probiotics (Pediococcus Pentosaceus Li05) by Encapsulation in Microgels Doped with Inorganic Nanoparticles. Food Hydrocolloid. 2018, 83, 246–252. doi: 10.1016/j.foodhyd.2018.05.024.
- Rabiee, H.; Jin, B.; Yun, S.; Dai, S. Gas-Responsive Cationic Microgels for Forward Osmosis Desalination. Chem. Eng. J. 2018, 347, 424–431. doi: 10.1016/j.cej.2018.04.148.
- Nyström, L.; Strömstedt, A. A.; Schmidtchen, A.; Malmsten, M. Peptide-Loaded Microgels as Antimicrobial and Anti-Inflammatory Surface Coatings. Biomacromolecules. 2018, 19, 3456–3466. doi: 10.1021/acs.biomac.8b00776.
- Gregoritza, M.; Abstiens, K.; Graf, M.; Goepferich, A. M. Fabrication of Antibody-Loaded Microgels Using Microfluidics and Thiol-Ene Photoclick Chemistry. Eur. J. Pharm. Biopharm. 2018, 127, 194–203. doi: 10.1016/j.ejpb.2018.02.024.
- Duffy, C.; O’Sullivan, M.; Jacquier, J. Preparation of Novel Chitosan Iron Microgel Beads for Fortification Applications. Food Hydrocolloid. 2018, 84, 608–615. doi: 10.1016/j.foodhyd.2018.06.045.
- Zhou, X.; Zhou, Y.; Nie, J.; Ji, Z.; Xu, J.; Zhang, X.; Du, B. Thermosensitive Ionic Microgels via Surfactant-Free Emulsion Copolymerization and in Situ Quaternization Cross-Linking. ACS Appl. Mater. Inter. 2014, 6, 4498–4513. doi: 10.1021/am500291n.
- Hou, Y.; Xie, W.; Achazi, K.; Cuellar-Camacho, J. L.; Melzig, M. F.; Chen, W.; Haag, R. Injectable Degradable PVA Microgels Prepared by Microfluidic Technology for Controlled Osteogenic Differentiation of Mesenchymal Stem Cells. Acta Biomater. 2018, 77, 28–37. doi: 10.1016/j.actbio.2018.07.003.
- Saavedra Isusi, G. I.; Karbstein, H. P.; van der Schaaf, U. S. Microgel Particle Formation: Influence of Mechanical Properties of Pectin-Based Gels on Microgel Particle Size Distribution. Food Hydrocolloid. 2019, 94, 105–113. doi: 10.1016/j.foodhyd.2019.02.053.
- Mourran, A.; Wu, Y.; Gumerov, R. A.; Rudov, A. A.; Potemkin, I. I.; Pich, A.; Möller, M. When Colloidal Particles Become Polymer Coils. Langmuir. 2016, 32, 723–730. doi: 10.1021/acs.langmuir.5b03931.
- Xue, J.; Zhang, Z.; Nie, J.; Du, B. Formation of Microgels by Utilizing the Reactivity of Catechols with Radicals. Macromolecules. 2017, 50, 5285–5292. doi: 10.1021/acs.macromol.7b01304.
- Yao, L.; Li, Q.; Guan, Y.; Zhu, X. X.; Zhang, Y. Tetrahedral, Octahedral, and Triangular Dipyramidal Microgel Clusters with Thermosensitivity Fabricated from Binary Colloidal Crystals Template and Thiol–Ene Reaction. ACS Macro Lett. 2018, 7, 80–84. doi: 10.1021/acsmacrolett.7b00935.
- Nayak, S.; Lyon, L. A. Soft Nanotechnology with Soft Nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 7686–7708. doi: 10.1002/anie.200501321.
- Lehmann, S.; Seiffert, S.; Richtering, W. Diffusion of Guest Molecules within Sensitive Core–Shell Microgel Carriers. J. Colloid Interfaces Sci. 2014, 431, 204–208. doi: 10.1016/j.jcis.2014.06.014.
- Borrmann, R.; Palchyk, V.; Pich, A.; Rueping, M. Reversible Switching and Recycling of Adaptable Organic Microgel Catalysts (Microgelzymes) for Asymmetric Organocatalytic Desymmetrization. ACS Catal. 2018, 8, 7991–7996. doi: 10.1021/acscatal.8b01408.
- Agrawal, G.; Agrawal, R. Stimuli-Responsive Microgels and Microgel-Based Systems: Advances in the Exploitation of Microgel Colloidal Properties and Their Interfacial Activity. Polymers-Basel. 2018, 10, 418. doi: 10.3390/polym10040418.
- Naseem, K.; Begum, R.; Wu, W.; Irfan, A.; Farooqi, Z. H. Advancement in Multi-Functional Poly(Styrene)-Poly(N-Isopropylacrylamide) Based Core–Shell Microgels and Their Applications. Polym. Rev. 2018, 58, 288–325. doi: 10.1080/15583724.2017.1423326.
- Ramos, J.; Forcada, J.; Hidalgo-Alvarez, R. Cationic Polymer Nanoparticles and Nanogels: From Synthesis to Biotechnological Applications. Chem. Rev. 2014, 114, 367–428. doi: 10.1021/cr3002643.
- Ye, Y.; Yu, J.; Gu, Z. Versatile Protein Nanogels Prepared by in Situ Polymerization. Macromol. Chem. Phys. 2016, 217, 333–343. doi: 10.1002/macp.201500296.
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel Nanoparticles in Drug Delivery. Adv. Drug Deliver. Rev. 2008, 60, 1638–1649. doi: 10.1016/j.addr.2008.08.002.
- Kendre, P. N.; Satav, T. S. Current Trends and Concepts in the Design and Development of Nanogel Carrier Systems. Polym. Bull. 2019, 76, 1595–1617. doi: 10.1007/s00289-018-2430-y.
- Nita, L. E.; Chiriac, A. P.; Diaconu, A.; Tudorachi, N.; Mititelu-Tartau, L. Multifunctional Nanogels with Dual Temperature and Ph Responsiveness. Int. J. Pharm. 2016, 515, 165–175. doi: 10.1016/j.ijpharm.2016.10.017.
- Molina, M.; Asadian-Birjand, M.; Balach, J.; Bergueiro, J.; Miceli, E.; Calderón, M. Stimuli-Responsive Nanogel Composites and Their Application in Nanomedicine. Chem. Soc. Rev. 2015, 44, 6161–6186. doi: 10.1039/c5cs00199d.
- Kabanov, A. V.; Vinogradov, S. V. Nanogels as Pharmaceutical Carriers: Finite Networks of Infinite Capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. doi: 10.1002/anie.200900441.
- Asadian-Birjand, M.; Sousa-Herves, A.; Steinhilber, D.; Cuggino, J. C.; Calderon, M. Functional Nanogels for Biomedical Applications. Curr. Med. Chem. 2012, 19, 5029.
- Gao, Y.; Xie, J.; Chen, H.; Gu, S.; Zhao, R.; Shao, J.; Jia, L. Nanotechnology-Based Intelligent Drug Design for Cancer Metastasis Treatment. Biotechnol. Adv. 2014, 32, 761–777. doi: 10.1016/j.biotechadv.2013.10.013.
- Tahara, Y.; Akiyoshi, K. Current Advances in Self-Assembled Nanogel Delivery Systems for Immunotherapy. Adv. Drug Deliver. Rev. 2015, 95, 65–76. doi: 10.1016/j.addr.2015.10.004.
- Sasaki, Y.; Akiyoshi, K. Nanogel Engineering for New Nanobiomaterials: From Chaperoning Engineering to Biomedical Applications. Chem. Rec. 2010, 10, 366–376. doi: 10.1002/tcr.201000008.
- Chen, X.; Li, R.; Wong, S. H. D.; Wei, K.; Cui, M.; Chen, H.; Jiang, Y.; Yang, B.; Zhao, P.; Xu, J.;, et al. Conformational Manipulation of Scale-Up Prepared Single-Chain Polymeric Nanogels for Multiscale Regulation of Cells. Nat. Commun. 2019, 10, 2705. doi: 10.1038/s41467-019-10640-z.
- Chen, X.; Lai, N. C.; Wei, K.; Li, R.; Cui, M.; Yang, B.; Wong, S. H. D.; Deng, Y.; Li, J.; Shuai, X.;, et al.. Biomimetic Presentation of Cryptic Ligands via Single-Chain Nanogels for Synergistic Regulation of Stem Cells. ACS Nano. 2020. doi: 10.1021/acsnano.9b08564..
- Richtering, W.; Pich, A. The Special Behaviours of Responsive Core-Shell Nanogels. Soft Matter. 2012, 8, 11423–11430. doi: 10.1039/c2sm26424b.
- Wang, L.; Wu, Y.; Guo, B.; Ma, P. X. Nanofiber Yarn/Hydrogel Core–Shell Scaffolds Mimicking Native Skeletal Muscle Tissue for Guiding 3D Myoblast Alignment, Elongation, and Differentiation. ACS Nano. 2015, 9, 9167–9179. doi: 10.1021/acsnano.5b03644.
- Wang, L.; Wu, Y.; Hu, T.; Ma, P. X.; Guo, B. Aligned Conductive Core-Shell Biomimetic Scaffolds Based on Nanofiber Yarns/Hydrogel for Enhanced 3D Neurite Outgrowth Alignment and Elongation. Acta Biomater. 2019, 96, 175–187. doi: 10.1016/j.actbio.2019.06.035.
- Hellweg, T.; Dewhurst, C. D.; Eimer, W.; Kratz, K. Pnipam-Co-Polystyrene Core-Shell Microgels: Structure, Swelling Behavior, and Crystallization. Langmuir. 2004, 20, 4330–4335. doi: 10.1021/la0354786.
- Liu, J.; Wang, Q.; Sun, Y.; Lin, H.; Liang, J.; Fan, Y.; Zhang, X. A Core-Shell Structured Collagen Hydrogel Microsphere with Removable Superparamagnetic Alginate Coating for Cell Coculture and Rapid Separation. Mater. Lett. 2019, 249, 49–52. doi: 10.1016/j.matlet.2019.04.059.
- Yao, D.; Zhang, Z.; Zheng, Y.; Wang, Y.; Wang, D. Enhanced Mechanical Property of Polyacrylic Acid Composite Hydrogel by the Synergistic Role of Core-Shell Structured Sio2@Pdmaema Nano-Objects. Compos. Sci. Technol. 2019, 181, 107711. doi: 10.1016/j.compscitech.2019.107711.
- Naseem, K.; Farooqi, Z. H.; Begum, R.; Wu, W.; Irfan, A.; Al-Sehemi, A. G. Silver Nanoparticles Engineered Polystyrene-Poly(N-Isopropylmethacrylamide-Acrylic Acid) Core Shell Hybrid Polymer Microgels for Catalytic Reduction of Congo Red. Macromol. Chem. Phys. 2018, 219, 1800211. doi: 10.1002/macp.201800211.
- Naseem, K.; Begum, R.; Wu, W.; Usman, M.; Irfan, A.; Al-Sehemi, A. G.; Farooqi, Z. H. Adsorptive Removal of Heavy Metal Ions Using Polystyrene-Poly(N-Isopropylmethacrylamide-Acrylic Acid) Core/Shell Gel Particles: Adsorption Isotherms and Kinetic Study. J. Mol. Liq. 2019, 277, 522–531. doi: 10.1016/j.molliq.2018.12.054.
- Ladet, S.; David, L.; Domard, A. Multi-Membrane Hydrogels. Nature. 2008, 452, 76–79.
- O’Leary, L. E. R.; Fallas, J. A.; Bakota, E. L.; Kang, M. K.; Hartgerink, J. D. Multi-Hierarchical Self-Assembly of a Collagen Mimetic Peptide from Triple Helix to Nanofibre and Hydrogel. Nat. Chem. 2011, 3, 821–828. doi: 10.1038/nchem.1123.
- Liu, G.; Ding, Z.; Yuan, Q.; Xie, H.; Gu, Z. Multi-Layered Hydrogels for Biomedical Applications. Front. Chem. 2018, 6, 439.
- Martinez-Sanz, M.; Mikkelsen, D.; Flanagan, B.; Gidley, M. J.; Gilbert, E. P. Multi-Scale Model for the Hierarchical Architecture of Native Cellulose Hydrogels. Carbohydr. Polym. 2016, 147, 542–555. doi: 10.1016/j.carbpol.2016.03.098.
- Xue, B.; Wang, W.; Qin, J.; Nijampatnam, B.; Murugesan, S.; Kozlovskaya, V.; Zhang, R.; Velu, S. E.; Kharlampieva, E. Highly Efficient Delivery of Potent Anticancer Iminoquinone Derivative by Multilayer Hydrogel Cubes. Acta Biomater. 2017, 58, 386–398. doi: 10.1016/j.actbio.2017.06.004.
- Fischer, M.; Vahdatzadeh, M.; Konradi, R.; Friedrichs, J.; Maitz, M. F.; Freudenberg, U.; Werner, C. Multilayer Hydrogel Coatings to Combine Hemocompatibility and Antimicrobial Activity. Biomaterials. 2015, 56, 198–205. doi: 10.1016/j.biomaterials.2015.03.056.
- Steinmetz, N. J.; Aisenbrey, E. A.; Westbrook, K. K.; Qi, H. J.; Bryant, S. J. Mechanical Loading Regulates Human Msc Differentiation in a Multi-Layer Hydrogel for Osteochondral Tissue Engineering. Acta Biomater. 2015, 21, 142–153. doi: 10.1016/j.actbio.2015.04.015.
- Hume, S. L.; Hoyt, S. M.; Walker, J. S.; Sridhar, B. V.; Ashley, J. F.; Bowman, C. N.; Bryant, S. J. Alignment of Multi-Layered Muscle Cells within Three-Dimensional Hydrogel Macrochannels. Acta Biomater. 2012, 8, 2193–2202. doi: 10.1016/j.actbio.2012.02.001.
- Fujie, T.; Matsutani, N.; Kinoshita, M.; Okamura, Y.; Saito, A.; Takeoka, S. Adhesive, Flexible, and Robust Polysaccharide Nanosheets Integrated for Tissue-Defect Repair. Adv. Funct. Mater. 2009, 19, 2560–2568. doi: 10.1002/adfm.200900103.
- Chen, X.; Wu, W.; Guo, Z.; Xin, J.; Li, J. Controlled Insulin Release From Glucose-Sensitive Self-Assembled Multilayer Films Based On 21-Arm Star Polymer. Biomaterials. 2010, 32, 1759–1766. doi: 10.1016/j.biomaterials.2010.11.002.
- Wang, H.; Zha, G.; Du, H.; Gao, L.; Li, X.; Shen, Z.; Zhu, W. Facile Fabrication of Ultrathin Antibacterial Hydrogel Films via Layer-by-Layer “Click” Chemistry. Polym. Chem. UK 2014, 5, 6489–6494. doi: 10.1039/C4PY00900B.
- Lu, Y.; Wu, Y.; Liang, J.; Libera, M. R.; Sukhishvili, S. A. Self-Defensive Antibacterial Layer-by-Layer Hydrogel Coatings with Ph-Triggered Hydrophobicity. Biomaterials. 2015, 45, 64–71. doi: 10.1016/j.biomaterials.2014.12.048.
- Ladet, S. G.; Tahiri, K.; Montembault, A. S.; Domard, A. J.; Corvol, M. T. M. Multi-Membrane Chitosan Hydrogels as Chondrocytic Cell Bioreactors. Biomaterials. 2011, 32, 5354–5364. doi: 10.1016/j.biomaterials.2011.04.012.
- Nie, J.; Lu, W.; Ma, J.; Yang, L.; Wang, Z.; Qin, A.; Hu, Q. Orientation in Multi-Layer Chitosan Hydrogel: Morphology, Mechanism, and Design Principle. Sci. Rep-UK. 2015, 5, 7635. doi: 10.1038/srep07635.
- He, M.; Zhao, Y.; Duan, J.; Wang, Z.; Chen, Y.; Zhang, L. Fast Contact of Solid–Liquid Interface Created High Strength Multi-Layered Cellulose Hydrogels with Controllable Size. ACS Appl. Mater. Inter. 2014, 6, 1872–1878. doi: 10.1021/am404855q.
- Zarket, B. C.; Raghavan, S. R. Onion-Like Multilayered Polymer Capsules Synthesized by a Bioinspired Inside-Out Technique. Nat. Commun. 2017, 8, 193. doi: 10.1038/s41467-017-00077-7.
- Davidson, M. D.; Ban, E.; Schoonen, A. C. M.; Lee, M.; D’Este, M.; Shenoy, V. B.; Burdick, J. A. Mechanochemical Adhesion and Plasticity in Multifiber Hydrogel Networks. Adv. Mater. 2020, 32, 1905719. doi: 10.1002/adma.201905719..
- Laha, A.; Sharma, C. S.; Majumdar, S. Sustained Drug Release From Multi-Layered Sequentially Crosslinked Electrospun Gelatin Nanofiber Mesh. Mater. Sci. Eng C. 2017, 76, 782–786. doi: 10.1016/j.msec.2017.03.110.
- Yang, G.; Lin, H.; Rothrauff, B. B.; Yu, S.; Tuan, R. S. Multilayered Polycaprolactone/Gelatin Fiber-Hydrogel Composite for Tendon Tissue Engineering. Acta Biomater. 2016, 35, 68–76. doi: 10.1016/j.actbio.2016.03.004.
- Okuda, T.; Tominaga, K.; Kidoaki, S. Time-Programmed Dual Release Formulation by Multilayered Drug-Loaded Nanofiber Meshes. J. Control. Release. 2010, 143, 258–264. doi: 10.1016/j.jconrel.2009.12.029.
- Liu, L.; Ghaemi, A.; Gekle, S.; Agarwal, S. One‐Component Dual Actuation: Poly(Nipam) Can Actuate to Stable 3D Forms with Reversible Size Change. Adv. Mater. 2016, 28, 9792–9796. doi: 10.1002/adma.201603677.
- Jiang, S.; Liu, F.; Lerch, A.; Ionov, L.; Agarwal, S. Unusual and Superfast Temperature‐Triggered Actuators. Adv. Mater. 2015, 27, 4865–4870. doi: 10.1002/adma.201502133.
- Liu, L.; Jiang, S.; Sun, Y.; Agarwal, S. Giving Direction to Motion and Surface with Ultra‐Fast Speed Using Oriented Hydrogel Fibers. Adv. Funct. Mater. 2016, 26, 1021–1027. doi: 10.1002/adfm.201503612.
- Mora-Boza, A.; Wlodarczyk-Biegun, M. K.; Del, C. A.; Vazquez-Lasa, B.; Roman, J. S. Glycerylphytate as an Ionic Crosslinker for 3D Printing of Multi-Layered Scaffolds with Improved Shape Fidelity and Biological Features. Biomater. Sci. 2020, 8, 506–516. doi: 10.1039/c9bm01271k.
- Liu, J.; Li, L.; Suo, H.; Yan, M.; Yin, J.; Fu, J. 3D Printing of Biomimetic Multi-Layered Gelma/Nha Scaffold for Osteochondral Defect Repair. Mater. Des. 2019, 171, 107708. doi: 10.1016/j.matdes.2019.107708.
- Yousefi, A.; Hoque, M. E.; Prasad, R. G. S. V.; Uth, N. Current Strategies in Multiphasic Scaffold Design for Osteochondral Tissue Engineering: A Review. J. Biomed. Mater. Res. A. 2015, 103, 2460–2481. doi: 10.1002/jbm.a.35356.
- Odent, J.; Vanderstappen, S.; Toncheva, A.; Pichon, E.; Wallin, T. J.; Wang, K.; Shepherd, R. F.; Dubois, P.; Raquez, J. Hierarchical Chemomechanical Encoding of Multi-Responsive Hydrogel Actuators via 3D Printing. J. Mater. Chem. A. 2019, 7, 15395–15403. doi: 10.1039/C9TA03547H.
- Wu, J.; Zhang, D.; He, X.; Wang, Y.; Xiao, S.; Chen, F.; Fan, P.; Zhong, M.; Tan, J.; Yang, J. “Janus-featured” Hydrogel with Antifouling and Bacteria-Releasing Properties. Ind. Eng. Chem. Res. 2019, 58, 17792–17801. doi: 10.1021/acs.iecr.9b02984.
- Zhang, F.; Fan, J.; Zhang, P.; Liu, M.; Meng, J.; Jiang, L.; Wang, S. A Monolithic Hydro/ Organo Macro Copolymer Actuator Synthesized Via Interfacial Copolymerization. NPG Asia Mater. 2017, 9, e380–e380. doi: 10.1038/am.2017.61.
- Motealleh, A.; Seda Kehr, N. Janus Nanocomposite Hydrogels for Chirality-Dependent Cell Adhesion and Migration. ACS Appl. Mater. Inter. 2017, 9, 33674–33682. doi: 10.1021/acsami.7b10871.
- Peng, X.; Liu, T.; Shang, C.; Jiao, C.; Wang, H. Mechanically Strong Janus Poly(N-Isopropylacrylamide)/Graphene Oxide Hydrogels as Thermo-Responsive Soft Robots. Chin. J. Polym. Sci. 2017, 35, 1268–1275. doi: 10.1007/s10118-017-1970-1.
- Ghosh, S.; Schurtenberger, P. Microfluidic Production of Snowman-Shaped Janus Hydrogel Particles. Colloids Surf. A. 2019, 573, 205–210. doi: 10.1016/j.colsurfa.2019.04.034.
- Tanaka, T.; Fillmore, D. J. Kinetics of Swelling of Gels. J. Chem. Phys. 1979, 70, 1214–1218. doi: 10.1063/1.437602.
- Omidian, H.; Rocca, J. G.; Park, K. Advances in Superporous Hydrogels. J. Control. Release. 2005, 102, 3–12. doi: 10.1016/j.jconrel.2004.09.028.
- Bencherif, S. A.; Sands, R. W.; Bhatta, D.; Arany, P.; Verbeke, C. S.; Edwards, D. A.; Mooney, D. J. Injectable Preformed Scaffolds with Shape-Memory Properties. Proc. Natl. Acad. Sci. USA 2012, 109, 19590–19595. doi: 10.1073/pnas.1211516109.
- Koshy, S. T.; Ferrante, T. C.; Lewin, S. A.; Mooney, D. J. Injectable, Porous, and Cell-Responsive Gelatin Cryogels. Biomaterials. 2014, 35, 2477–2487. doi: 10.1016/j.biomaterials.2013.11.044.
- Koshy, S. T.; Zhang, D.; Grolman, J. M.; Stafford, A. G.; Mooney, D. J. Injectable Nanocomposite Cryogels for Versatile Protein Drug Delivery. Acta Biomater. 2018, 65, 36–43. doi: 10.1016/j.actbio.2017.11.024.
- De France, K. J.; Xu, F.; Hoare, T. Structured Macroporous Hydrogels: Progress, Challenges, and Opportunities. Adv. Healthc. Mater. 2018, 7, 1700927. doi: 10.1002/adhm.201700927.
- Ma, X.; Zhao, Z.; Wang, H.; Liu, Y.; Xu, Y.; Zhang, J.; Chen, B.; Li, L.; Zhao, Y. P-Glycoprotein Antibody Decorated Porous Hydrogel Particles for Capture and Release of Drug-Resistant Tumor Cells. Adv. Healthc. Mater. 2019, 8, 1900136. doi: 10.1002/adhm.201900136.
- Yan, K.; Xu, F.; Li, S.; Li, Y.; Chen, Y.; Wang, D. Ice-Templating of Chitosan/Agarose Porous Composite Hydrogel with Adjustable Water-Sensitive Shape Memory Property and Multi-Staged Degradation Performance. Colloids Surf. B. 2020, 190, 110907. doi: 10.1016/j.colsurfb.2020.110907.
- Pei, Y.; Guo, D.; An, Q.; Xiao, Z.; Zhai, S.; Zhai, B. Hydrogels with Diffusion-Facilitated Porous Network for Improved Adsorption Performance. Korean J. Chem. Eng. 2018, 35, 2384–2393. doi: 10.1007/s11814-018-0181-y.
- Liu, S.; Jin, M.; Chen, Y.; Gao, H.; Shi, X.; Cheng, W.; Ren, L.; Wang, Y. High Internal Phase Emulsions Stabilised by Supramolecular Cellulose Nanocrystals and Their Application as Cell-Adhesive Macroporous Hydrogel Monoliths. J. Mater. Chem. B. 2017, 5, 2671–2678. doi: 10.1039/C7TB00145B.
- Bailey, B. M.; Hui, V.; Fei, R.; Grunlan, M. A. Tuning Peg-Da Hydrogel Properties Via Solvent-Induced Phase Separation (Sips). J. Mater. Chem. 2011, 21, 18776–18782. doi: 10.1039/C1JM13943F.
- Bailey, B. M.; Fei, R.; Munoz-Pinto, D.; Hahn, M. S.; Grunlan, M. A. Pdmsstar–Peg Hydrogels Prepared via Solvent-Induced Phase Separation (Sips) and Their Potential Utility as Tissue Engineering Scaffolds. Acta Biomater. 2012, 8, 4324–4333. doi: 10.1016/j.actbio.2012.07.034.
- Lee, Y. P.; Liu, H. Y.; Lin, P. C.; Lee, Y. H.; Yu, L. R.; Hsieh, C. C.; Shih, P. J.; Shih, W. P.; Wang, I. J.; Yen, J. Y.;, et al. Facile Fabrication of Superporous and Biocompatible Hydrogel Scaffolds for Artificial Corneal Periphery. Colloids Surf. B. 2019, 175, 26–35. doi: 10.1016/j.colsurfb.2018.11.013.
- Ji, C.; Annabi, N.; Hosseinkhani, M.; Sivaloganathan, S.; Dehghani, F. Fabrication of Poly-Dl-Lactide/Polyethylene Glycol Scaffolds Using the Gas Foaming Technique. Acta Biomater. 2012, 8, 570–578. doi: 10.1016/j.actbio.2011.09.028.
- Dehghani, F.; Annabi, N. Engineering Porous Scaffolds Using Gas-Based Techniques. Curr. Opin. Biotechnol. 2011, 22, 661–666. doi: 10.1016/j.copbio.2011.04.005.
- Cui, Z. K.; Kim, S.; Baljon, J. J.; Wu, B. M.; Aghaloo, T.; Lee, M. Microporous Methacrylated Glycol Chitosan-Montmorillonite Nanocomposite Hydrogel for Bone Tissue Engineering. Nat. Commun. 2019, 10, 3523. doi: 10.1038/s41467-019-11511-3.
- Griffin, D. R.; Weaver, W. M.; Scumpia, P. O.; Di Carlo, D.; Segura, T. Accelerated Wound Healing by Injectable Microporous Gel Scaffolds Assembled from Annealed Building Blocks. Nat. Mater. 2015, 14, 737–744. doi: 10.1038/nmat4294.
- Truong, N. F.; Kurt, E.; Tahmizyan, N.; Lesher-Pérez, S. C.; Chen, M.; Darling, N. J.; Xi, W.; Segura, T. Microporous Annealed Particle Hydrogel Stiffness, Void Space Size, and Adhesion Properties Impact Cell Proliferation, Cell Spreading, and Gene Transfer. Acta Biomater. 2019, 94, 160–172. doi: 10.1016/j.actbio.2019.02.054.
- Isaac, A.; Jivan, F.; Xin, S.; Hardin, J.; Luan, X.; Pandya, M.; Diekwisch, T. G. H.; Alge, D. L. Microporous Bio-Orthogonally Annealed Particle Hydrogels for Tissue Engineering and Regenerative Medicine. ACS Biomater. Sci. Eng. 2019, 5, 6395–6404. doi: 10.1021/acsbiomaterials.9b01205.
- Hou, S.; Lake, R.; Park, S.; Edwards, S.; Jones, C.; Jeong, K. J. Injectable Macroporous Hydrogel Formed by Enzymatic Cross-Linking of Gelatin Microgels. ACS App. Bio Mater. 2018, 1, 1430–1439. doi: 10.1021/acsabm.8b00380.
- Highley, C. B.; Rodell, C. B.; Burdick, J. A. Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Adv. Mater. 2015, 27, 5075–5079. doi: 10.1002/adma.201501234.
- Nedjari, S.; Schlatter, G.; Hébraud, A. Thick Electrospun Honeycomb Scaffolds with Controlled Pore Size. Mater. Lett. 2015, 142, 180–183. doi: 10.1016/j.matlet.2014.11.118.
- Tam, R. Y.; Fisher, S. A.; Baker, A. E. G.; Shoichet, M. S. Transparent Porous Polysaccharide Cryogels Provide Biochemically Defined, Biomimetic Matrices for Tunable 3D Cell Culture. Chem. Mater. 2016, 28, 3762–3770. doi: 10.1021/acs.chemmater.6b00627.
- Chen, Y.; Huang, L.; Dai, X.; Tian, Q.; Yu, M.; Agheb, M.; Chan, H. N.; Poon, E.; Guo, Z.; Boheler, K. R.;, et al. Facile Formation of a Microporous Chitosan Hydrogel Based On Self-Crosslinking. J. Mater. Chem. B. 2017, 5, 9291–9299. doi: 10.1039/C7TB02736B.
- Chen, J.; Park, H.; Park, K. Synthesis of Superporous Hydrogels: Hydrogels with Fast Swelling and Superabsorbent Properties. J. Biomed. Mater. Res. 1999, 44, 53–62.
- El-Said, I. A.; Aboelwafa, A. A.; Khalil, R. M.; ElGazayerly, O. N. Baclofen Novel Gastroretentive Extended Release Gellan Gum Superporous Hydrogel Hybrid System: In Vitro and in Vivo Evaluation. Drug. Deliv. 2016, 23, 101–112. doi: 10.3109/10717544.2014.905654.
- Souza, J. F.; Costa, G. P.; Luque, R.; Alves, D.; Fajardo, A. R. Polysaccharide-Based Superporous Hydrogel Embedded with Copper Nanoparticles: A Green and Versatile Catalyst for the Synthesis of 1,2,3-Triazoles. Catal. Sci. Technol. 2019, 9, 136–145. doi: 10.1039/C8CY01796D.
- Yin, L.; Ding, J.; Zhang, J.; He, C.; Tang, C.; Yin, C. Polymer Integrity Related Absorption Mechanism of Superporous Hydrogel Containing Interpenetrating Polymer Networks for Oral Delivery of Insulin. Biomaterials. 2010, 31, 3347–3356. doi: 10.1016/j.biomaterials.2010.01.045.
- Gils, P. S.; Ray, D.; Sahoo, P. K. Characteristics of Xanthan Gum-Based Biodegradable Superporous Hydrogel. Int. J. Biol. Macromol. 2009, 45, 364–371. doi: 10.1016/j.ijbiomac.2009.07.007.
- Shi, X.; Wang, W.; Wang, A. pH-Responsive Sodium Alginate-Based Superporous Hydrogel Generated by an Anionic Surfactant Micelle Templating. Carbohyd. Polym. 2013, 94, 449–455. doi: 10.1016/j.carbpol.2013.01.019.
- Ashraf, M. U.; Hussain, I.; Hussain, S. Z.; Hussain, M. A.; Muhammad, G.; Haseeb, M. T.; Bashir, S. A Superporous and Superabsorbent Glucuronoxylan Hydrogel from Quince (Cydonia Oblanga): Stimuli Responsive Swelling, On-Off Switching and Drug Release. Int. J. Biol. Macromol. 2017, 95, 138–144. doi: 10.1016/j.ijbiomac.2016.11.057.
- Kopeček, J.; Yang, J. Smart Self-Assembled Hybrid Hydrogel Biomaterials. Angew. Chem. Int. Ed. 2012, 51, 7396–7417. doi: 10.1002/anie.201201040.
- Palmese, L. L.; Thapa, R. K.; Sullivan, M. O.; Kiick, K. L. Hybrid Hydrogels for Biomedical Applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157. doi: 10.1016/j.coche.2019.02.010.
- Xu, K.; Liang, X.; Li, P.; Deng, Y.; Pei, X.; Tan, Y.; Zhai, K.; Wang, P. Tough, Stretchable Chemically Cross-Linked Hydrogel Using Core–Shell Polymer Microspheres as Cross-Linking Junctions. Polymer. 2017, 118, 58–67. doi: 10.1016/j.polymer.2017.04.055.
- Li, Q.; Barrett, D. G.; Messersmith, P. B.; Holten-Andersen, N. Controlling Hydrogel Mechanics Via Bio-Inspired Polymer–Nanoparticle Bond Dynamics. ACS Nano. 2016, 10, 1317–1324. doi: 10.1021/acsnano.5b06692.
- Schexnailder, P.; Schmidt, G. Nanocomposite Polymer Hydrogels. Colloid Polym. Sci. 2009, 287, 1–11. doi: 10.1007/s00396-008-1949-0.
- Li, H.; Jiang, H.; Haraguchi, K. U. Thermoresponsive Nanocomposite Hydrogels Composed of Ternary Polymer-Clay-Silica Networks. Macromolecules. 2018, 51, 529–539. doi: 10.1021/acs.macromol.7b02305.
- Jiang, D.; Liu, Z.; Han, J.; Wu, X. A Tough Nanocomposite Hydrogel for Antifouling Application with Quaternized Hyperbranched Pei Nanoparticles Crosslinking. RSC Adv. 2016, 6, 60530–60536. doi: 10.1039/C6RA07335B.
- Tian, S.; Jiang, D.; Pu, J.; Sun, X.; Li, Z.; Wu, B.; Zheng, W.; Liu, W.; Liu, Z. A New Hybrid Silicone-Based Antifouling Coating with Nanocomposite Hydrogel for Durable Antifouling Properties. Chem. Eng. J. 2019, 370, 1–9. doi: 10.1016/j.cej.2019.03.185.
- Thoniyot, P.; Tan, M. J.; Karim, A. A.; Young, D. J.; Loh, X. J. Nanoparticle-Hydrogel Composites: Concept, Design, and Applications of These Promising, Multi-Functional Materials. Adv Sci. 2015, 2, 1400010. doi: 10.1002/advs.201400010.
- Liu, R.; Liang, S.; Tang, X.; Yan, D.; Li, X.; Yu, Z. Tough and Highly Stretchable Graphene Oxide/Polyacrylamide Nanocomposite Hydrogels. J. Mater. Chem. 2012, 22, 14160–14167. doi: 10.1039/c2jm32541a.
- Arshad, F.; Selvaraj, M.; Zain, J.; Banat, F.; Haija, M. A. Polyethylenimine Modified Graphene Oxide Hydrogel Composite as an Efficient Adsorbent for Heavy Metal Ions. Sep. Purif. Technol. 2019, 209, 870–880. doi: 10.1016/j.seppur.2018.06.035.
- Sheikhi, A.; Afewerki, S.; Oklu, R.; Gaharwar, A. K.; Khademhosseini, A. Effect of Ionic Strength on Shear-Thinning Nanoclay–Polymer Composite Hydrogels. Biomater Sci. UK 2018, 6, 2073–2083. doi: 10.1039/C8BM00469B.
- Lee, M.; Aida, T.; Lee, E.; Mynar, J. L.; Okuro, K.; Yoshida, M.; Wang, Q.; Kinbara, K. High-Water-Content Mouldable Hydrogels by Mixing Clay and a Dendritic Molecular Binder. Nature. 2010, 463, 339–343. doi: 10.1038/nature08693.
- Gao, G.; Du, G.; Sun, Y.; Fu, J. Self-Healable, Tough, and Ultrastretchable Nanocomposite Hydrogels Based on Reversible Polyacrylamide/Montmorillonite Adsorption. ACS Appl. Mater. Inter. 2015, 7, 5029–5037. doi: 10.1021/acsami.5b00704.
- Yao, C.; Liu, Z.; Yang, C.; Wang, W.; Ju, X.; Xie, R.; Chu, L. Poly(N-Isopropylacrylamide)-Clay Nanocomposite Hydrogels with Responsive Bending Property as Temperature-Controlled Manipulators. Adv. Funct. Mater. 2015, 25, 2980–2991. doi: 10.1002/adfm.201500420.
- Feng, K.; Hung, G.; Yang, X.; Liu, M. High-Strength and Physical Cross-Linked Nanocomposite Hydrogel with Clay Nanotubes for Strain Sensor and Dye Adsorption Application. Compos. Sci. Technol. 2019, 181, 107701. doi: 10.1016/j.compscitech.2019.107701.
- Hu, M.; Yang, Y.; Gu, X.; Hu, Y.; Du, Z.; Wang, C. Novel Nanocomposite Hydrogels Consisting of C‐Dots with Excellent Mechanical Properties. Macromol. Mater. Eng. 2015, 300, 1043–1048. doi: 10.1002/mame.201500141.
- Cheng, C.; Zhang, C.; Wang, D. Using Hydrogel to Diversify the Adaptability and Applicability of Functional Nanoparticles: From Nanotech-Flavored Jellies to Artificial Enzymes. Langmuir. 2019, 35, 8612–8628. doi: 10.1021/acs.langmuir.9b00254.
- Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite Hydrogels: 3D Polymer–Nanoparticle Synergies for On-Demand Drug Delivery. ACS Nano. 2015, 9, 4686–4697. doi: 10.1021/acsnano.5b01433.
- Sun, X.; Sun, H.; Li, H.; Peng, H. Developing Polymer Composite Materials: Carbon Nanotubes Or Graphene?. Adv. Mater. 2013, 25, 5153–5176. doi: 10.1002/adma.201301926.
- Han, L.; Lu, X.; Liu, K.; Wang, K.; Fang, L.; Weng, L.; Zhang, H.; Tang, Y.; Ren, F.; Zhao, C.;, et al. Mussel-Inspired Adhesive and Tough Hydrogel Based on Nanoclay Confined Dopamine Polymerization. ACS Nano 2017, 11, 2561–2574. doi: 10.1021/acsnano.6b05318.
- Shin, S. R.; Bae, H.; Cha, J. M.; Mun, J. Y.; Chen, Y.; Tekin, H.; Shin, H.; Farshchi, S.; Dokmeci, M. R.; Tang, S.;, et al. Carbon Nanotube Reinforced Hybrid Microgels as Scaffold Materials for Cell Encapsulation. ACS Nano 2012, 6, 362–372. doi: 10.1021/nn203711s.
- Basuki, J. S.; Qie, F.; Mulet, X.; Suryadinata, R.; Vashi, A. V.; Peng, Y. Y.; Li, L.; Hao, X.; Tan, T.; Hughes, T. C. Photo-Modulated Therapeutic Protein Release from a Hydrogel Depot Using Visible Light. Angewandte Chemie. 2017, 56, 966–971. doi: 10.1002/anie.201610618.
- Jalani, G.; Naccache, R.; Rosenzweig, D. H.; Haglund, L.; Vetrone, F.; Cerruti, M. Photocleavable Hydrogel-Coated Upconverting Nanoparticles: A Multifunctional Theranostic Platform for Nir Imaging and On-Demand Macromolecular Delivery. J. Am. Chem. Soc. 2016, 138, 1078–1083. doi: 10.1021/jacs.5b12357.
- Wang, P.; Sun, J.; Lou, Z.; Fan, F.; Hu, K.; Sun, Y.; Gu, N. Assembly‐Induced Thermogenesis of Gold Nanoparticles in the Presence of Alternating Magnetic Field for Controllable Drug Release of Hydrogel. Adv. Mater. 2016, 28, 10801–10808. doi: 10.1002/adma.201603632.
- Zhao, F.; Bae, J.; Zhou, X.; Guo, Y.; Yu, G. Nanostructured Functional Hydrogels as an Emerging Platform for Advanced Energy Technologies. Adv. Mater. 2018, 30, 1801796. doi: 10.1002/adma.201801796.
- Li, L.; Wang, Y.; Pan, L.; Shi, Y.; Cheng, W.; Shi, Y.; Yu, G. A Nanostructured Conductive Hydrogels-Based Biosensor Platform for Human Metabolite Detection. Nano Lett. 2015, 15, 1146–1151. doi: 10.1021/nl504217p.
- Qin, H.; Zhang, T.; Li, H.; Cong, H.; Antonietti, M.; Yu, S. Dynamic Au-Thiolate Interaction Induced Rapid Self-Healing Nanocomposite Hydrogels with Remarkable Mechanical Behaviors. Chem-Us. 2017, 3, 691–705. doi: 10.1016/j.chempr.2017.07.017.
- Zhang, K.; Yuan, W.; Wei, K.; Yang, B.; Chen, X.; Li, Z.; Zhang, Z.; Bian, L. Highly Dynamic Nanocomposite Hydrogels Self-Assembled by Metal Ion-Ligand Coordination. Small. 2019, 15, 1900242. doi: 10.1002/smll.201900242.
- Zhang, K.; Jia, Z.; Yang, B.; Feng, Q.; Xu, X.; Yuan, W.; Li, X.; Chen, X.; Duan, L.; Wang, D.;, et al. Adaptable Hydrogels Mediate Cofactor-Assisted Activation of Biomarker-Responsive Drug Delivery via Positive Feedback for Enhanced Tissue Regeneration. Adv Sci 2018, 5, 1800875. doi: 10.1002/advs.201800875.
- Jiang, F.; Huang, T.; He, C.; Brown, H. R.; Wang, H. Interactions Affecting the Mechanical Properties of Macromolecular Microsphere Composite Hydrogels. J. Phys. Chem. B. 2013, 117, 13679–13687. doi: 10.1021/jp4069587.
- Zhao, J.; Jiao, K.; Yang, J.; He, C.; Wang, H. Mechanically Strong and Thermosensitive Macromolecular Microsphere Composite Poly(N-Isopropylacrylamide) Hydrogels. Polymer. 2013, 54, 1596–1602. doi: 10.1016/j.polymer.2013.01.025.
- Sun, Y.; Liu, S.; Du, G.; Gao, G.; Fu, J. Multi-Responsive and Tough Hydrogels Based on Triblock Copolymer Micelles as Multi-Functional Macro-Crosslinkers. Chem. Commun. 2015, 51, 8512–8515. doi: 10.1039/c4cc10094h.
- Li, P.; Xu, K.; Tan, Y.; Lu, C.; Li, Y.; Wang, H.; Liang, X.; Wang, P. The Astonishing Progress in Performance of Hydrogel Triggered by the Structure Evolution of Cross-Linking Junctions. RSC Adv. 2014, 4, 37812–37815. doi: 10.1039/C4RA07541B.
- Li, P.; Xu, K.; Tan, Y.; Lu, C.; Li, Y.; Wang, P. A Novel Fabrication Method of Temperature-Responsive Poly(Acrylamide) Composite Hydrogel with High Mechanical Strength. Polymer. 2013, 54, 5830–5838. doi: 10.1016/j.polymer.2013.08.019.
- Huang, T.; Xu, H. G.; Jiao, K. X.; Zhu, L. P.; Brown, H. R.; Wang, H. L.; Novel, A. Hydrogel with High Mechanical Strength: A Macromolecular Microsphere Composite Hydrogel. Adv. Mater. 2007, 19, 1622–1626. doi: 10.1002/adma.200602533.
- Ritchie, R. O.;. The Conflicts between Strength and Toughness. Nat. Mater. 2011, 10, 817–822. doi: 10.1038/nmat3115.
- Zhu, H.; Zhu, S.; Jia, Z.; Parvinian, S.; Li, Y.; Vaaland, O.; Hu, L.; Li, T. Anomalous Scaling Law of Strength and Toughness of Cellulose Nanopaper. Proc. National Academy Sci. 2015, 112, 8971. doi: 10.1073/pnas.1502870112.
- Dragan, E. S.;. Design and Applications of Interpenetrating Polymer Network Hydrogels. A Review. Chem. Eng. J. 2014, 243, 572–590. doi: 10.1016/j.cej.2014.01.065.
- Berrebi, M.; Fabre-Francke, I.; Lavédrine, B.; Fichet, O. Development of Organic Glass Using Interpenetrating Polymer Networks with Enhanced Resistance Towards Scratches and Solvents. Eur. Polym. J. 2015, 63, 132–140. doi: 10.1016/j.eurpolymj.2014.12.010.
- Zhang, Y.; Liu, J.; Huang, L.; Wang, Z.; Wang, L. Design and Performance of a Sericin-Alginate Interpenetrating Network Hydrogel for Cell and Drug Delivery. Sci. Rep-UK. 2015, 5, 12374. doi: 10.1038/srep12374.
- Jenkins, A. D.; Kratochvíl, P.; Stepto, R. F. T.; Suter, U. W. Glossary of Basic Terms in Polymer Science (Iupac Recommendations 1996). Pure Appl. Chem. 1996, 68, 2305. doi: 10.1351/pac199668122287.
- Chikh, L.; Delhorbe, V.; Fichet, O. (Semi-)interpenetrating Polymer Networks as Fuel Cell Membranes. J. Membr. Sci. 2011, 368, 1–17. doi: 10.1016/j.memsci.2010.11.020.
- Yue, Y.; Han, J.; Han, G.; French, A. D.; Qi, Y.; Wu, Q. Cellulose Nanofibers Reinforced Sodium Alginate-Polyvinyl Alcohol Hydrogels: Core-Shell Structure Formation and Property Characterization. Carbohyd. Polym. 2016, 147, 155–164. doi: 10.1016/j.carbpol.2016.04.005.
- Varganici, C.; Rosu, L.; Rosu, D.; Simionescu, B. C. Miscibility Studies of Some Semi-Interpenetrating Polymer Networks Based on an Aromatic Polyurethane and Epoxy Resin. Compos. B Eng. 2013, 50, 273–278. doi: 10.1016/j.compositesb.2013.02.005.
- Qu, C.; Li, X.; Yang, Z.; Zhang, X.; Wang, T.; Wang, Q.; Chen, S. Damping, Thermal, and Mechanical Performances of a Novel Semi-Interpenetrating Polymer Networks Based on Polyimide/Epoxy. J. Appl. Polym. Sci. 2019, 136, 48032. doi: 10.1002/app.48032.
- Venkataramani, S.; Kannan, T.; Radhakrishnan, G. Novel Ab Crosslinked Polymer Networks Based on 1-Vinylimidazole- Terminated Polyurethane and Poly(Methyl Methacrylate). Polym. Int. 2006, 55, 1209–1214. doi: 10.1002/pi.2042.
- Venkataramani, S.; Kannan, T.; Das, P. J.; Radhakrishnan, G. Novel Ab Crosslinked Polymer Networks from Telechelic 4-Vinylbenzyl Carbamate Terminated Polyurethanes and Different Vinyl Monomers. Polym. Adv. Technol. 2009, 20, 892–898. doi: 10.1002/pat.1326.
- Yang, J.; Wang, N.; Chiu, H. Preparation and Characterization of Poly(Vinyl Alcohol)/Sodium Alginate Blended Membrane for Alkaline Solid Polymer Electrolytes Membrane. J. Membr. Sci. 2014, 457, 139–148. doi: 10.1016/j.memsci.2014.01.034.
- Park, S.; Edwards, S.; Hou, S.; Boudreau, R.; Yee, R.; Jeong, K. J. A Multi-Interpenetrating Network (Ipn) Hydrogel with Gelatin and Silk Fibroin. Biomater. Sci. UK 2019, 7, 1276–1280. doi: 10.1039/C8BM01532E.
- Dadfar, S. M. R.; Pourmahdian, S.; Tehranchi, M. M.; Dadfar, S. M. Novel Dual-Responsive Semi-Interpenetrating Polymer Network Hydrogels for Controlled Release of Anticancer Drugs. J. Biomed. Mater. Res. A. 2019, 107, 2327–2339. doi: 10.1002/jbm.a.36741.
- Ding, C.; Zhang, M.; Ma, M.; Zheng, J.; Yang, Q.; Feng, R. Thermal and Ph Dual-Responsive Hydrogels Based on Semi-Interpenetrating Network of Poly(N-Isopropylacrylamide) and Collagen Nanofibrils. Polym. Int. 2019, 68, 1468–1477. doi: 10.1002/pi.5852.
- Gu, Z.; Huang, K.; Luo, Y.; Zhang, L.; Kuang, T.; Chen, Z.; Liao, G. Double Network Hydrogel for Tissue Engineering, Wiley Interdisciplinary Reviews:. Nanomed. Nanobiotechnol. 2018, 10, e1520. doi: 10.1002/wnan.1520.
- Golafshan, N.; Gharibi, H.; Kharaziha, M.; Fathi, M. A Facile One-Step Strategy for Development of a Double Network Fibrous Scaffold for Nerve Tissue Engineering. Biofabrication. 2017, 9, 025008. doi: 10.1088/1758-5090/aa68ed.
- Gong, J. P.;. Why are Double Network Hydrogels so Tough?. Soft Matter. 2010, 6, 2583–2590. doi: 10.1039/b924290b.
- Sun, J.; Zhao, X.; Illeperuma, W. R. K.; Chaudhuri, O.; Oh, K. H.; Mooney, D. J.; Vlassak, J. J.; Suo, Z. Highly Stretchable and Tough Hydrogels. Nature. 2012, 489, 133–136.
- Zhao, Y.; Xia, B. H.; Wang, L.; Wang, R. J.; Meng, D. N.; Liu, Y.; Zhou, J. W.; Lian, H. Q.; Zu, L.; Cui, X. G.;, et al. Tough and Tear‐Resistant Double‐Network Hydrogels Based on a Facile Strategy: Micellar Polymerization Followed by Solution Polymerization. Macromol. Mater. Eng. 2018, 303, 1700527. doi: 10.1002/mame.201700527.
- Yang, Y.; Wang, X.; Yang, F.; Shen, H.; Wu, D. A Universal Soaking Strategy to Convert Composite Hydrogels into Extremely Tough and Rapidly Recoverable Double-Network Hydrogels. Adv. Mater. 2016, 28, 7178–7184. doi: 10.1002/adma.201601742.
- Nonoyama, T.; Wada, S.; Kiyama, R.; Kitamura, N.; Mredha, M. T.; Zhang, X.; Kurokawa, T.; Nakajima, T.; Takagi, Y.; Yasuda, K.;, et al. Double-Network Hydrogels Strongly Bondable to Bones by Spontaneous Osteogenesis Penetration. Adv. Mater. 2016, 28, 6740–6745. doi: 10.1002/adma.201601030.
- Li, Y.; Yang, L.; Zeng, Y.; Wu, Y.; Wei, Y.; Tao, L. Self-Healing Hydrogel with a Double Dynamic Network Comprising Imine and Borate Ester Linkages. Chem. Mater. 2019, 31, 315576–315583. doi: 10.1021/acs.chemmater.9b01301.
- Feng, S.; Li, Q.; Wang, S.; Wang, B.; Hou, Y.; Zhang, T. Tunable Dual Temperature-Pressure Sensing and Parameter Self-Separating Based on Ionic Hydrogel via Multisynergistic Network Design. ACS Appl. Mater. Interfaces. 2019, 11, 21049–21057. doi: 10.1021/acsami.9b05214.
- Zhang, H.; Qadeer, A.; Mynarcik, D.; Chen, W. Delivery of Rosiglitazone from an Injectable Triple Interpenetrating Network Hydrogel Composed of Naturally Derived Materials. Biomaterials. 2011, 32, 890–898. doi: 10.1016/j.biomaterials.2010.09.053.
- Zhang, H.; Wang, X.; Huang, H.; Yang, B.; Wang, C.; Sun, H. Nanocomposite Interpenetrating Hydrogels with High Toughness and Good Self-Recovery. Colloid Polym. Sci. 2019, 297, 821–830. doi: 10.1007/s00396-019-04512-7.
- Li, Z.; Shen, J.; Ma, H.; Lu, X.; Shi, M.; Li, N.; Ye, M. Preparation and Characterization of pH- and Temperature-Responsive Nanocomposite Double Network Hydrogels. Mater. Sci. Eng C 2013, 33, 1951–1957. doi: 10.1016/j.msec.2013.01.004.
- Zhuang, Y.; Yu, F.; Chen, H.; Zheng, J.; Ma, J.; Chen, J. Alginate/ Graphene Double-Network Nanocomposite Hydrogel Beads with Low-Swelling, Enhanced Mechanical Properties, and Enhanced Adsorption Capacity. J. Mater. Chem. A. 2016, 4, 10885–10892. doi: 10.1039/C6TA02738E.
- Yang, W.; Furukawa, H.; Gong, J. P. Highly Extensible Double-Network Gels with Self-Assembling Anisotropic Structure. Adv. Mater. 2008, 20, 4499–4503. doi: 10.1002/adma.200801396.
- Mredha, M. T. I.; Kitamura, N.; Nonoyama, T.; Wada, S.; Goto, K.; Zhang, X.; Nakajima, T.; Kurokawa, T.; Takagi, Y.; Yasuda, K.;, et al. Anisotropic Tough Double Network Hydrogel from Fish Collagen and Its Spontaneous in Vivo Bonding to Bone. Biomaterials 2017, 132, 85–95. doi: 10.1016/j.biomaterials.2017.04.005.
- Nakajima, T.; Furukawa, H.; Tanaka, Y.; Kurokawa, T.; Gong, J. P. Effect of Void Structure on the Toughness of Double Network Hydrogels. J. Polym. Sci. B Polym. Phys. 2011, 49, 1246–1254. doi: 10.1002/polb.22293.
- Zhao, Y.; Chen, S.; Hu, J.; Yu, J.; Feng, G.; Yang, B.; Li, C.; Zhao, N.; Zhu, C.; Xu, J. Microgel-Enhanced Double Network Hydrogel Electrode with High Conductivity and Stability for Intrinsically Stretchable and Flexible All-Gel-State Supercapacitor. ACS Appl. Mater. Inter. 2018, 10, 19323–19330. doi: 10.1021/acsami.8b05224.
- Hu, J.; Kurokawa, T.; Nakajima, T.; Wu, Z. L.; Liang, S. M.; Gong, J. P. Fracture Process of Microgel-Reinforced Hydrogels under Uniaxial Tension. Macromolecules. 2014, 47, 3587–3594. doi: 10.1021/ma5008545.
- Ming, Z.; Pang, Y.; Liu, J. Switching Between Elasticity and Plasticity by Network Strength Competition. Adv. Mater. 2020, 32, 1906870. doi: 10.1002/adma.201906870.
- Chen, S.; He, H.; Tang, G.; Wu, B.; Ma, M.; Shi, Y.; Wang, X. Topological Structure Influences On the Gel Formation Process and Mechanical Properties of L-Lysine Based Supramolecular Gels. RSC Adv. 2015, 5, 101437–101443. doi: 10.1039/C5RA17991B.
- Bin Imran, A.; Esaki, K.; Gotoh, H.; Seki, T.; Ito, K.; Sakai, Y.; Takeoka, Y. Extremely Stretchable Thermosensitive Hydrogels by Introducing Slide-Ring Polyrotaxane Cross-Linkers and Ionic Groups into the Polymer Network. Nat. Commun. 2014, 5, 5124. doi: 10.1038/ncomms6124.
- Noda, Y.; Hayashi, Y.; Ito, K. From Topological Gels to Slide-Ring Materials. J. Appl. Polym. Sci. 2014, 131, 40509. doi: 10.1002/app.40509.
- Ito, K.;. Slide-Ring Materials Using Topological Supramolecular Architecture. Curr. Opin. Solid State Mater. Sci. 2010, 14, 28–34. doi: 10.1016/j.cossms.2009.08.005.
- Steck, J.; Yang, J.; Suo, Z. Covalent Topological Adhesion. ACS Macro Lett. 2019, 8, 754–758. doi: 10.1021/acsmacrolett.9b00325.
- Ke, H.; Yang, L. P.; Xie, M.; Chen, Z.; Yao, H.; Jiang, W. Shear-Induced Assembly of a Transient yet Highly Stretchable Hydrogel Based on Pseudopolyrotaxanes. Nat. Chem. 2019, 11, 470–477. doi: 10.1038/s41557-019-0235-8.
- Zhao, D.; Zhu, Y.; Cheng, W.; Xu, G.; Wang, Q.; Liu, S.; Li, J.; Chen, C.; Yu, H.; Hu, L. A Dynamic Gel with Reversible and Tunable Topological Networks and Performances. Matter. 2020, 2, 390–403. doi: 10.1016/j.matt.2019.10.020.
- Shibayama, M.;. Exploration of Ideal Polymer Networks. Macromol. Symp. 2017, 372, 7–13. doi: 10.1002/masy.201600122.
- Ishikawa, A.; Sakai, T.; Fujii, K. An Ionic Liquid Gel with Ultralow Concentrations of Tetra-Arm Polymers: Gelation Kinetics and Mechanical and Ion-Conducting Properties. Polymer. 2019, 166, 38–43. doi: 10.1016/j.polymer.2019.01.044.
- Matsunaga, T.; Asai, H.; Akagi, Y.; Sakai, T.; Chung, U.; Shibayama, M. Sans Studies On Tetra-Peg Gel Under Uniaxial Deformation. Macromolecules. 2011, 44, 1203–1210. doi: 10.1021/ma102658e.
- Shibayama, M.; Li, X.; Sakai, T. Precision Polymer Network Science with Tetra-Peg Gels-a Decade History and Future. Colloid Polym. Sci. 2019, 297, 1–12. doi: 10.1007/s00396-018-4423-7.
- Sakai, T.; Matsunaga, T.; Yamamoto, Y.; Ito, C.; Yoshida, R.; Suzuki, S.; Sasaki, N.; Shibayama, M.; Chung, U. Design and Fabrication of a High-Strength Hydrogel with Ideally Homogeneous Network Structure from Tetrahedron-Like Macromonomers. Macromolecules. 2008, 41, 5379–5384. doi: 10.1021/ma800476x.
- Bu, Y.; Zhang, L.; Sun, G.; Sun, F.; Liu, J.; Yang, F.; Tang, P.; Wu, D. Tetra-Peg Based Hydrogel Sealants for in Vivo Visceral Hemostasis. Adv. Mater. 2019, 31, 1901580. doi: 10.1002/adma.201901580.
- Li, X.; Nakagawa, S.; Tsuji, Y.; Watanabe, N.; Shibayama, M. Polymer Gel with a Flexible and Highly Ordered Three-Dimensional Network Synthesized via Bond Percolation. Sci. Adv. 2019, 5, eaax8647. doi: 10.1126/sciadv.aax8647.
- Lu, Y.; Mao, L.; Hou, Z.; Miao, S.; Gao, Y. Development of Emulsion Gels for the Delivery of Functional Food Ingredients: From Structure to Functionality. Food Eng. Rev. 2019, 11, 245–258. doi: 10.1007/s12393-019-09194-z.
- Farjami, T.; Madadlou, A. An Overview on Preparation of Emulsion-Filled Gels and Emulsion Particulate Gels. Trends Food Sci. Tech. 2019, 86, 85–94. doi: 10.1016/j.tifs.2019.02.043.
- Torres, O.; Murray, B.; Sarkar, A. Emulsion Microgel Particles: Novel Encapsulation Strategy for Lipophilic Molecules. Trends Food Sci. Tech. 2016, 55, 98–108. doi: 10.1016/j.tifs.2016.07.006.
- Zhu, Y.; Chen, X.; McClements, D. J.; Zou, L.; Liu, W. pH-, Ion- and Temperature-Dependent Emulsion Gels: Fabricated by Addition of Whey Protein to Gliadin-Nanoparticle Coated Lipid Droplets. Food Hydrocolloid. 2018, 77, 870–878. doi: 10.1016/j.foodhyd.2017.11.032.
- Su, J.; Guo, Q.; Chen, Y.; Dong, W.; Mao, L.; Gao, Y.; Yuan, F. Characterization and Formation Mechanism of Lutein Pickering Emulsion Gels Stabilized by β-Lactoglobulin-Gum Arabic Composite Colloidal Nanoparticles. Food Hydrocolloid. 2020, 98, 105276. doi: 10.1016/j.foodhyd.2019.105276.
- Koç, H.; Drake, M.; Vinyard, C. J.; Essick, G.; van de Velde, F.; Foegeding, E. A. Emulsion Filled Polysaccharide Gels: Filler Particle Effects on Material Properties, Oral Processing, and Sensory Texture. Food Hydrocolloid. 2019, 94, 311–325. doi: 10.1016/j.foodhyd.2019.03.018.
- Jiang, Y.; Liu, L.; Wang, B.; Yang, X.; Chen, Z.; Zhong, Y.; Zhang, L.; Mao, Z.; Xu, H.; Sui, X. Polysaccharide-Based Edible Emulsion Gel Stabilized by Regenerated Cellulose. Food Hydrocolloid. 2019, 91, 232–237. doi: 10.1016/j.foodhyd.2019.01.028.
- Soltani, S.; Madadlou, A. Gelation Characteristics of the Sugar Beet Pectin Solution Charged with Fish Oil-Loaded Zein Nanoparticles. Food Hydrocolloid. 2015, 43, 664–669. doi: 10.1016/j.foodhyd.2014.07.030.
- Wang, M.; Wang, M.; Zhang, S.; Chen, J. Pickering Gel Emulsion Stabilized by Enzyme Immobilized Polymeric Nanoparticles: A Robust and Recyclable Biocatalyst System for Biphasic Catalysis. React. Chem. Eng. 2019, 4, 1459–1465. doi: 10.1039/C9RE00158A.
- Xu, Y.; Liu, T.; Tang, C. Novel Pickering High Internal Phase Emulsion Gels Stabilized Solely by Soy β-Conglycinin. Food Hydrocolloid. 2019, 88, 21–30. doi: 10.1016/j.foodhyd.2018.09.031.
- Liu, W.; Gao, H.; McClements, D. J.; Zhou, L.; Wu, J.; Zou, L. Stability, Rheology, and β-Carotene Bioaccessibility of High Internal Phase Emulsion Gels. Food Hydrocolloid. 2019, 88, 210–217. doi: 10.1016/j.foodhyd.2018.10.012.
- Partanen, R.; Forssell, P.; Mackie, A.; Blomberg, E. Interfacial Cross-Linking of β-Casein Changes the Structure of the Adsorbed Layer. Food Hydrocolloid. 2013, 32, 271–277. doi: 10.1016/j.foodhyd.2013.01.009.
- Phoon, P. Y.; Paul, L. N.; Burgner, N. J. W.; San Martin-Gonzalez, M. F.; Narsimhan, G. Effect of Cross-Linking of Interfacial Sodium Caseinate by Natural Processing on the Oxidative Stability of Oil-in-Water (O/W) Emulsions. J. Agr. Food Chem. 2014, 62, 2822–2829. doi: 10.1021/jf403285z.
- Dai, L.; Sun, C.; Wei, Y.; Mao, L.; Gao, Y. Characterization of Pickering Emulsion Gels Stabilized by Zein/Gum Arabic Complex Colloidal Nanoparticles. Food Hydrocolloid. 2018, 74, 239–248. doi: 10.1016/j.foodhyd.2017.07.040.
- Setiowati, A. D.; Saeedi, S.; Wijaya, W.; Van der Meeren, P. Improved Heat Stability of Whey Protein Isolate Stabilized Emulsions Via Dry Heat Treatment of Wpi and Low Methoxyl Pectin: Effect of Pectin Concentration, pH, and Ionic Strength. Food Hydrocolloid. 2017, 63, 716–726. doi: 10.1016/j.foodhyd.2016.10.025.
- Tavernier, I.; Patel, A. R.; Van der Meeren, P.; Dewettinck, K. Emulsion-Templated Liquid Oil Structuring with Soy Protein and Soy Protein: Κ-carrageenan Complexes. Food Hydrocolloid. 2017, 65, 107–120. doi: 10.1016/j.foodhyd.2016.11.008.
- Morales, R.; Martinez, M. J.; Pilosof, A. M. R. Caseinglycomacropeptide and Polysorbate Interactions Allow the Design of Smart Gelled Emulsions. Food Hydrocolloid. 2019, 93, 198–205. doi: 10.1016/j.foodhyd.2019.02.030.
- Mantelet, M.; Panouillé, M.; Boué, F.; Bosc, V.; Restagno, F.; Souchon, I.; Mathieu, V. Impact of Sol-Gel Transition on the Ultrasonic Properties of Complex Model Foods: Application to Agar/Gelatin Gels and Emulsion Filled Gels. Food Hydrocolloid. 2019, 87, 506–518. doi: 10.1016/j.foodhyd.2018.08.021.
- Feng, L.; Jia, X.; Zhu, Q.; Liu, Y.; Li, J.; Yin, L. Investigation of the Mechanical, Rheological and Microstructural Properties of Sugar Beet Pectin/Soy Protein Isolate-Based Emulsion-Filled Gels. Food Hydrocolloid. 2019, 89, 813–820. doi: 10.1016/j.foodhyd.2018.11.039.
- Xi, Z.; Liu, W.; McClements, D. J.; Zou, L. Rheological, Structural, and Microstructural Properties of Ethanol Induced Cold-Set Whey Protein Emulsion Gels: Effect of Oil Content. Food Chem. 2019, 291, 22–29. doi: 10.1016/j.foodchem.2019.04.011.
- Mao, L.; Roos, Y. H.; Miao, S. Study on the Rheological Properties and Volatile Release of Cold-Set Emulsion-Filled Protein Gels. J. Agr. Food Chem. 2014, 62, 11420–11428. doi: 10.1021/jf503931y.
- Geremias-Andrade, I. M.; Souki, N. P. D. B.; Moraes, I. C. F.; Pinho, S. C. Rheological and Mechanical Characterization of Curcumin-Loaded Emulsion-Filled Gels Produced with Whey Protein Isolate and Xanthan Gum. LWT. 2017, 86, 166–173. doi: 10.1016/j.lwt.2017.07.063.
- Lu, Y.; Mao, L.; Cui, M.; Yuan, F.; Gao, Y. Effect of the Solid Fat Content on Properties of Emulsion Gels and Stability of β-Carotene. J. Agr. Food Chem. 2019, 67, 6466–6475. doi: 10.1021/acs.jafc.9b01156.
- Dickinson, E.;. Emulsion Gels: The Structuring of Soft Solids with Protein-Stabilized Oil Droplets. Food Hydrocolloid. 2012, 28, 224–241. doi: 10.1016/j.foodhyd.2011.12.017.
- Zhuang, X.; Jiang, X.; Zhou, H.; Han, M.; Liu, Y.; Bai, Y.; Xu, X.; Zhou, G. The Effect of Insoluble Dietary Fiber on Myofibrillar Protein Emulsion Gels: Oil Particle Size and Protein Network Microstructure. LWT. 2019, 101, 534–542. doi: 10.1016/j.lwt.2018.11.065.
- Hou, J. J.; Guo, J.; Wang, J. M.; Yang, X. Q. Effect of Interfacial Composition and Crumbliness on Aroma Release in Soy Protein/Sugar Beet Pectin Mixed Emulsion Gels. J. Sci. Food Agr. 2016, 96, 4449–4456. doi: 10.1002/jsfa.7656.
- Wu, Y.; Wang, L.; Guo, B.; Ma, P. X. Interwoven Aligned Conductive Nanofiber Yarn/Hydrogel Composite Scaffolds for Engineered 3D Cardiac Anisotropy. ACS Nano. 2017, 11, 5646–5659. doi: 10.1021/acsnano.7b01062.
- Zhao, Y.; Alsaid, Y.; Yao, B.; Zhang, Y.; Zhang, B.; Bhuskute, N.; Wu, S.; He, X. Wood‐Inspired Morphologically Tunable Aligned Hydrogel for High‐Performance Flexible All‐Solid‐State Supercapacitors. Adv. Funct. Mater. 2020, 30, 1909133. doi: 10.1002/adfm.201909133.
- Nie, J.; Pei, B.; Wang, Z.; Hu, Q. Construction of Ordered Structure in Polysaccharide Hydrogel: A Review. Carbohyd. Polym. 2019, 205, 225–235. doi: 10.1016/j.carbpol.2018.10.033.
- Zhang, Z.; Yao, S.; Xie, S.; Wang, X.; Chang, F.; Luo, J.; Wang, J.; Fu, J. Effect of Hierarchically Aligned Fibrin Hydrogel in Regeneration of Spinal Cord Injury Demonstrated by Tractography: A Pilot Study. Sci. Rep-UK. 2017, 7, 40017. doi: 10.1038/srep40017.
- Wu, Z. L.; Gong, J. P. Hydrogels with Self-Assembling Ordered Structures and Their Functions. NPG Asia Mater. 2011, 3, 57–64. doi: 10.1038/asiamat.2010.200.
- Sano, K.; Ishida, Y.; Aida, T. Synthesis of Anisotropic Hydrogels and Their Applications. Angew. Chem. Int. Ed. 2018, 57, 2532–2543. doi: 10.1002/anie.201708196.
- Zhao, Z.; Fang, R.; Rong, Q.; Liu, M. Bioinspired Nanocomposite Hydrogels with Highly Ordered Structures. Adv. Mater. 2017, 29, 1703045. doi: 10.1002/adma.201703045..
- Ye, D.; Yang, P.; Lei, X.; Zhang, D.; Li, L.; Chang, C.; Sun, P.; Zhang, L. Robust Anisotropic Cellulose Hydrogels Fabricated via Strong Self-Aggregation Forces for Cardiomyocytes Unidirectional Growth. Chem. Mater. 2018, 30, 5175–5183. doi: 10.1021/acs.chemmater.8b01799..
- Hu, K.; Sun, J.; Guo, Z.; Wang, P.; Chen, Q.; Ma, M.; Gu, N. A Novel Magnetic Hydrogel with Aligned Magnetic Colloidal Assemblies Showing Controllable Enhancement of Magnetothermal Effect in the Presence of Alternating Magnetic Field. Adv. Mater. 2015, 27, 2507–2514. doi: 10.1002/adma.201405757.
- Liu, M.; Ishida, Y.; Ebina, Y.; Sasaki, T.; Hikima, T.; Takata, M.; Aida, T. An Anisotropic Hydrogel with Electrostatic Repulsion between Cofacially Aligned Nanosheets. Nature. 2015, 517, 68–72. doi: 10.1038/nature14060.
- Omidinia-Anarkoli, A.; Boesveld, S.; Tuvshindorj, U.; Rose, J. C.; Haraszti, T.; De Laporte, L. An Injectable Hybrid Hydrogel with Oriented Short Fibers Induces Unidirectional Growth of Functional Nerve Cells. Small. 2017, 13, 1702207. doi: 10.1002/smll.201702207..
- Antman-Passig, M.; Shefi, O. Remote Magnetic Orientation of 3D Collagen Hydrogels for Directed Neuronal Regeneration. Nano Lett. 2016, 16, 2567–2573. doi: 10.1021/acs.nanolett.6b00131.
- Le Ferrand, H.; Bolisetty, S.; Demirörs, A. F.; Libanori, R.; Studart, A. R.; Mezzenga, R. Magnetic Assembly of Transparent and Conducting Graphene-Based Functional Composites. Nat. Commun. 2016, 7, 12078. doi: 10.1038/ncomms12078.
- Ramón-Azcón, J.; Ahadian, S.; Estili, M.; Liang, X.; Ostrovidov, S.; Kaji, H.; Shiku, H.; Ramalingam, M.; Nakajima, K.; Sakka, Y.;, et al. Dielectrophoretically Aligned Carbon Nanotubes to Control Electrical and Mechanical Properties of Hydrogels to Fabricate Contractile Muscle Myofibers. Adv. Mater. 2013, 25, 4028–4034. doi: 10.1002/adma.201301300.
- Ahadian, S.; Ramón-Azcón, J.; Estili, M.; Liang, X.; Ostrovidov, S.; Shiku, H.; Ramalingam, M.; Nakajima, K.; Sakka, Y.; Bae, H.;, et al. Hybrid Hydrogels Containing Vertically Aligned Carbon Nanotubes with Anisotropic Electrical Conductivity for Muscle Myofiber Fabrication. Sci. Rep-UK 2014, 4, 4271. doi: 10.1038/srep04271.
- Dvir, T.; Timko, B. P.; Brigham, M. D.; Naik, S. R.; Karajanagi, S. S.; Levy, O.; Jin, H.; Parker, K. K.; Langer, R.; Kohane, D. S. Nanowired Three-Dimensional Cardiac Patches. Nat. Nanotechnol. 2011, 6, 720–725. doi: 10.1038/nnano.2011.160.
- You, J. O.; Rafat, M.; Ye, G. J.; Auguste, D. T. Nanoengineering the Heart: Conductive Scaffolds Enhance Connexin 43 Expression. Nano Lett. 2011, 11, 3643. doi: 10.1021/nl201514a.
- Morales, D.; Bharti, B.; Dickey, M. D.; Velev, O. D. Bending of Responsive Hydrogel Sheets Guided by Field-Assembled Microparticle Endoskeleton Structures. Small. 2016, 12, 2283–2290. doi: 10.1002/smll.201600037.
- Lu, Q.; Bai, S.; Ding, Z.; Guo, H.; Shao, Z.; Zhu, H.; Kaplan, D. L. Hydrogel Assembly with Hierarchical Alignment by Balancing Electrostatic Forces. Adv. Mater. Interfaces. 2016, 3, 1500687. doi: 10.1002/admi.201500687.
- Murata, K.; Haraguchi, K. Optical Anisotropy in Polymer–Clay Nanocomposite Hydrogel and Its Change on Uniaxial Deformation. J. Mater. Chem. 2007, 17, 3385. doi: 10.1039/b707570g.
- Wu, Z. L.; Sawada, D.; Kurokawa, T.; Kakugo, A.; Yang, W.; Furukawa, H.; Gong, J. P. Strain-Induced Molecular Reorientation and Birefringence Reversion of a Robust, Anisotropic Double-Network Hydrogel. Macromolecules. 2011, 44, 3542–3547. doi: 10.1021/ma200123u.
- Wang, B.; Torres-Rendon, J. G.; Yu, J.; Zhang, Y.; Walther, A. Aligned Bioinspired Cellulose Nanocrystal-Based Nanocomposites with Synergetic Mechanical Properties and Improved Hygromechanical Performance. ACS Appl. Mater. Inter. 2015, 7, 4595–4607. doi: 10.1021/am507726t.
- Millon, L. E.; Mohammadi, H.; Wan, W. K. Anisotropic Polyvinyl Alcohol Hydrogel for Cardiovascular Applications. J. Biomed. Mater. Res. Part B. Appl. Biomater. 2006, 79, 305–311. doi: 10.1002/jbm.b.30543.
- Lin, P.; Zhang, T.; Wang, X.; Yu, B.; Zhou, F. Freezing Molecular Orientation under Stretch for High Mechanical Strength but Anisotropic Hydrogels. Small. 2016, 12, 4386–4392. doi: 10.1002/smll.201601893.
- Downes, R.; Wang, S.; Haldane, D.; Moench, A.; Liang, R. Strain-Induced Alignment Mechanisms of Carbon Nanotube Networks. Adv. Eng. Mater. 2015, 17, 349–358. doi: 10.1002/adem.201400045.
- Shikinaka, K.; Koizumi, Y.; Shigehara, K. Mechanical/ Optical Behaviors of Imogolite Hydrogels Depending On their Compositions and Oriented Structures. J. Appl. Polym. Sci. 2015, 132, 41691. doi: 10.1002/app.41691.
- Chen, G.; Chen, L.; Wang, W.; Hong, F. F.; Zhu, M. Manufacture of a Novel Anisotropic Bacterial Nanocellulose Hydrogel Membrane by Using a Rotary Drum Bioreactor. Carbohyd. Polym. 2019, 211, 281–288. doi: 10.1016/j.carbpol.2019.01.072.
- Lin, S.; Liu, J.; Liu, X.; Zhao, X. Muscle-Like Fatigue-Resistant Hydrogels by Mechanical Training. Proc. National Academy Sci. 2019, 116, 10244. doi: 10.1073/pnas.1903019116.
- Si, Y.; Wang, L.; Wang, X.; Tang, N.; Yu, J.; Ding, B. Ultrahigh-Water-Content, Superelastic, and Shape-Memory Nanofiber-Assembled Hydrogels Exhibiting Pressure-Responsive Conductivity. Adv. Mater. 2017, 29, 1700339. doi: 10.1002/adma.201700339.
- Bai, H.; Polini, A.; Delattre, B.; Tomsia, A. P. Thermoresponsive Composite Hydrogels with Aligned Macroporous Structure by Ice-Templated Assembly. Chem. Mater. 2013, 25, 4551–4556. doi: 10.1021/cm4025827.
- Chau, M.; De France, K. J.; Kopera, B.; Machado, V. R.; Rosenfeldt, S.; Reyes, L.; Chan, K. J. W.; Förster, S.; Cranston, E. D.; Hoare, T.;, et al. Composite Hydrogels with Tunable Anisotropic Morphologies and Mechanical Properties. Chem. Mater 2016, 28, 3406–3415. doi: 10.1021/acs.chemmater.6b00792.
- Liu, T.; Huang, M.; Li, X.; Wang, C.; Gui, C.; Yu, Z. Highly Compressible Anisotropic Graphene Aerogels Fabricated by Directional Freezing for Efficient Absorption of Organic Liquids. Carbon. 2016, 100, 456–464. doi: 10.1016/j.carbon.2016.01.038.
- Zeng, X.; Ye, L.; Yu, S.; Sun, R.; Xu, J.; Wong, C. Facile Preparation of Superelastic and Ultralow Dielectric Boron Nitride Nanosheet Aerogels via Freeze-Casting Process. Chem. Mater. 2015, 27, 5849–5855. doi: 10.1021/acs.chemmater.5b00505.
- Choi, S.; Kim, J. Designed Fabrication of Super-Stiff, Anisotropic Hybrid Hydrogels via Linear Remodeling of Polymer Networks and Subsequent Crosslinking. J. Mater. Chem. B. 2015, 3, 1479–1483. doi: 10.1039/C4TB01852D.
- Wu, Z. L.; Kurokawa, T.; Sawada, D.; Hu, J.; Furukawa, H.; Gong, J. P. Anisotropic Hydrogel from Complexation-Driven Reorientation of Semirigid Polyanion at Ca2+ Diffusion Flux Front. Macromolecules. 2011, 44, 3535–3541. doi: 10.1021/ma2001228.
- Wu, Z. L.; Takahashi, R.; Sawada, D.; Arifuzzaman, M.; Nakajima, T.; Kurokawa, T.; Hu, J.; Gong, J. P. In Situ Observation of Ca2+ Diffusion-Induced Superstructure Formation of a Rigid Polyanion. Macromolecules. 2014, 47, 7208–7214. doi: 10.1021/ma501699d.
- Sano, K.; Igarashi, N.; Arazoe, Y. O.; Ishida, Y.; Ebina, Y.; Sasaki, T.; Hikima, T.; Aida, T. Internal Structure and Mechanical Property of an Anisotropic Hydrogel with Electrostatic Repulsion between Nanosheets. Polymer. 2019, 177, 43–48. doi: 10.1016/j.polymer.2019.05.064.
- Zhang, S.; Greenfield, M. A.; Mata, A.; Palmer, L. C.; Bitton, R.; Mantei, J. R.; Aparicio, C.; de la Cruz, M. O.; Stupp, S. I. A Self-Assembly Pathway to Aligned Monodomain Gels. Nat. Mater. 2010, 9, 594–601. doi: 10.1038/nmat2778.
- Wu, Z. L.; Kurokawa, T.; Liang, S.; Furukawa, H.; Gong, J. P. Hydrogels with Cylindrically Symmetric Structure at Macroscopic Scale by Self-Assembly of Semi-Rigid Polyion Complex. J. Am. Chem. Soc. 2010, 132, 10064–10069. doi: 10.1021/ja101969k.
- Zhou, J.; Du, X.; Gao, Y.; Shi, J.; Xu, B. Aromatic-Aromatic Interactions Enhance Interfiber Contacts for Enzymatic Formation of a Spontaneously Aligned Supramolecular Hydrogel. J. Am. Chem. Soc. 2014, 136, 2970–2973. doi: 10.1021/ja4127399.
- Ohtsuka, Y.; Seki, T.; Takeoka, Y. Thermally Tunable Hydrogels Displaying Angle-Independent Structural Colors. Angew. Chem. Int. Ed. 2015, 54, 15368–15373. doi: 10.1002/anie.201507503.
- Griffete, N.; Frederich, H.; Maître, A.; Ravaine, S.; Chehimi, M. M.; Mangeney, C. Inverse Opals of Molecularly Imprinted Hydrogels for the Detection of Bisphenol a and pH Sensing. Langmuir. 2012, 28, 1005–1012. doi: 10.1021/la202840y.
- Prince, E.; Alizadehgiashi, M.; Campbell, M.; Khuu, N.; Albulescu, A.; De France, K.; Ratkov, D.; Li, Y.; Hoare, T.; Kumacheva, E. Patterning of Structurally Anisotropic Composite Hydrogel Sheets. Biomacromolecules. 2018, 19, 1276–1284. doi: 10.1021/acs.biomac.8b00100.
- Le, X.; Lu, W.; Zhang, J.; Chen, T. Recent Progress in Biomimetic Anisotropic Hydrogel Actuators. Adv Sci. 2019, 6, 1801584. doi: 10.1002/advs.201801584.
- Barclay, T. G.; Day, C. M.; Petrovsky, N.; Garg, S. Review of Polysaccharide Particle-Based Functional Drug Delivery. Carbohyd. Polym. 2019, 221, 94–112. doi: 10.1016/j.carbpol.2019.05.067.
- Kouwer, P. H. J.; Koepf, M.; Le Sage, V. A. A.; Jaspers, M.; van Buul, A. M.; Eksteen-Akeroyd, Z. H.; Woltinge, T.; Schwartz, E.; Kitto, H. J.; Hoogenboom, R.;, et al. Responsive Biomimetic Networks From Polyisocyanopeptide Hydrogels. Nature 2013, 493, 651–655. doi: 10.1038/nature11839.
- Doyle, A. D.; Carvajal, N.; Jin, A.; Matsumoto, K.; Yamada, K. M. Local 3D Matrix Microenvironment Regulates Cell Migration through Spatiotemporal Dynamics of Contractility-Dependent Adhesions. Nat. Commun. 2015, 6, 8720. doi: 10.1038/ncomms9720.
- Ahmad, S.; Ahmad, M.; Manzoor, K.; Purwar, R.; Ikram, S. A Review On Latest Innovations in Natural Gums Based Hydrogels: Preparations & Applications. Int. J. Biol. Macromol. 2019, 136, 870–890. doi: 10.1016/j.ijbiomac.2019.06.113.
- Boido, M.; Ghibaudi, M.; Gentile, P.; Favaro, E.; Fusaro, R.; Tonda-Turo, C. Chitosan-Based Hydrogel to Support the Paracrine Activity of Mesenchymal Stem Cells in Spinal Cord Injury Treatment. Sci. Rep-UK. 2019, 9, 6402. doi: 10.1038/s41598-019-42848-w.
- Liu, J.; Tan, Y.; Zhang, H.; Zhang, Y.; Xu, P.; Chen, J.; Poh, Y.; Tang, K.; Wang, N.; Huang, B. Soft Fibrin Gels Promote Selection and Growth of Tumorigenic Cells. Nat. Mater. 2012, 11, 734–741. doi: 10.1038/nmat3361.
- Charoen, K. M.; Fallica, B.; Colson, Y. L.; Zaman, M. H.; Grinstaff, M. W. Embedded Multicellular Spheroids as a Biomimetic 3D Cancer Model for Evaluating Drug and Drug-Device Combinations. Biomaterials. 2013, 35, 2264–2271. doi: 10.1016/j.biomaterials.2013.11.038.
- Jeong, S.; Lee, J.; Shin, Y.; Chung, S.; Kuh, H. Co-Culture of Tumor Spheroids and Fibroblasts in a Collagen Matrix-Incorporated Microfluidic Chip Mimics Reciprocal Activation in Solid Tumor Microenvironment. Plos One. 2016, 11, e0159013–e0159013. doi: 10.1371/journal.pone.0159013.
- Truong, H. H.; de Sonneville, J.; Ghotra, V. P. S.; Xiong, J.; Price, L.; Hogendoorn, P. C. W.; Spaink, H. H.; van de Water, B.; Danen, E. H. J. Automated Microinjection of Cell-Polymer Suspensions in 3D Ecm Scaffolds for High-Throughput Quantitative Cancer Invasion Screens. Biomaterials. 2011, 33, 181–188. doi: 10.1016/j.biomaterials.2011.09.049.
- Szot, C. S.; Buchanan, C. F.; Freeman, J. W.; Rylander, M. N. 3D in Vitro Bioengineered Tumors Based on Collagen I Hydrogels. Biomaterials. 2011, 32, 7905–7912. doi: 10.1016/j.biomaterials.2011.07.001.
- Benton, G.; Arnaoutova, I.; George, J.; Kleinman, H. K.; Koblinski, J. Matrigel: From Discovery and Ecm Mimicry to Assays and Models for Cancer Research. Adv. Drug Deliver. Rev. 2014, 79–80, 3–18. doi: 10.1016/j.addr.2014.06.005.
- Chaudhuri, O.; Koshy, S. T.; Branco Da Cunha, C.; Shin, J.; Verbeke, C. S.; Allison, K. H.; Mooney, D. J. Extracellular Matrix Stiffness and Composition Jointly Regulate the Induction of Malignant Phenotypes in Mammary Epithelium. Nat. Mater. 2014, 13, 970–978. doi: 10.1038/nmat4009.
- Mredha, M. T. I.; Zhang, X.; Nonoyama, T.; Nakajima, T.; Kurokawa, T.; Takagi, Y.; Gong, J. P. Swim Bladder Collagen Forms Hydrogel with Macroscopic Superstructure by Diffusion Induced Fast Gelation. J. Mater. Chem. B. 2015, 3, 7658–7666. doi: 10.1039/c5tb00877h.
- Wang, L.; Zhang, L.; Qiu, S.; Liu, C.; Zhang, P.; Yin, L.; Chen, F. Rheological Properties and Structural Characteristics of Arabinoxylan Hydrogels Prepared from Three Wheat Bran Sources. J. Cereal Sci. 2019, 88, 79–86. doi: 10.1016/j.jcs.2019.05.003.
- Li, W.; Liu, L.; Tian, H.; Luo, X.; Liu, S. Encapsulation of Lactobacillus Plantarum in Cellulose Based Microgel with Controlled Release Behavior and Increased Long-Term Storage Stability. Carbohyd. Polym. 2019, 223, 115065. doi: 10.1016/j.carbpol.2019.115065.
- Qu, B.; Luo, Y. Chitosan-Based Hydrogel Beads: Preparations, Modifications and Applications in Food and Agriculture Sectors – A Review. Int. J. Biol. Macromol. 2020, 152, 437–448. doi: 10.1016/j.ijbiomac.2020.02.240.
- Li, Y.; Ma, Y.; Jiao, X.; Li, T.; Lv, Z.; Yang, C. J.; Zhang, X.; Wen, Y. Control of Capillary Behavior through Target-Responsive Hydrogel Permeability Alteration for Sensitive Visual Quantitative Detection. Nat. Commun. 2019, 10, 1036. doi: 10.1038/s41467-019-08952-1.
- Xiang, B.; He, K.; Zhu, R.; Liu, Z.; Zeng, S.; Huang, Y.; Nie, Z.; Yao, S. Self-Assembled DNA Hydrogel Based On Enzymatically Polymerized Dna for Protein Encapsulation and Enzyme/Dnazyme Hybrid Cascade Reaction. ACS Appl. Mater. Inter. 2016, 8, 22801–22807. doi: 10.1021/acsami.6b03572.
- Deshpande, S. R.; Hammink, R.; Das, R. K.; Nelissen, F. H. T.; Blank, K. G.; Rowan, A. E.; Heus, H. A. DNA-Responsive Polyisocyanopeptide Hydrogels with Stress-Stiffening Capacity. Adv. Funct. Mater. 2016, 26, 9075–9082. doi: 10.1002/adfm.201602461.
- Yao, C.; Tang, H.; Wu, W.; Tang, J.; Guo, W.; Luo, D.; Yang, D. Double Rolling Circle Amplification Generates Physically Cross-Linked Dna Network for Stem Cell Fishing. J. Am. Chem. Soc. 2020, 142, 3422–3429. doi: 10.1021/jacs.9b11001.
- Gómez-Mascaraque, G.; Martínez-Sanz, L.; Fabra, M.; López-Rubio, M. J. A. Development of Gelatin-Coated Ι-carrageenan Hydrogel Capsules by Electric Field-Aided Extrusion. Impact of Phenolic Compounds on Their Performance. Food Hydrocolloid. 2019, 90, 523–533. doi: 10.1016/j.foodhyd.2018.12.017.
- Gómez-Mascaraque, L. G.; Soler, C.; Lopez-Rubio, A. Stability and Bioaccessibility of Egcg within Edible Micro-Hydrogels. Chitosan Vs. Gelatin, a Comparative Study. Food Hydrocolloid. 2016, 61, 128–138. doi: 10.1016/j.foodhyd.2016.05.009.
- Huang, J.; Wang, Q.; Chu, L.; Xia, Q. Liposome-Chitosan Hydrogel Bead Delivery System for the Encapsulation of Linseed Oil and Quercetin: Preparation and in Vitro Characterization Studies. LWT. 2020, 117, 108615. doi: 10.1016/j.lwt.2019.108615.
- Zhang, Z.; Zhang, R.; McClements, D. J. Encapsulation of β-Carotene in Alginate-Based Hydrogel Beads: Impact on Physicochemical Stability and Bioaccessibility. Food Hydrocolloid. 2016, 61, 1–10. doi: 10.1016/j.foodhyd.2016.04.036.
- Facin, B. R.; Moret, B.; Baretta, D.; Belfiore, L. A.; Paulino, A. T. Immobilization and Controlled Release of β-Galactosidase from Chitosan-Grafted Hydrogels. Food Chem. 2015, 179, 44–51. doi: 10.1016/j.foodchem.2015.01.088.
- Lim, S.; Jung, G. A.; Muckom, R. J.; Glover, D. J.; Clark, D. S. Engineering Bioorthogonal Protein–Polymer Hybrid Hydrogel as a Functional Protein Immobilization Platform. Chem. Commun. 2019, 55, 806–809. doi: 10.1039/C8CC08720B.
- Sun, H.; Yang, H.; Huang, W.; Zhang, S. Immobilization of Laccase in a Sponge-Like Hydrogel for Enhanced Durability in Enzymatic Degradation of Dye Pollutants. J. Colloid Interfaces Sci. 2015, 450, 353–360. doi: 10.1016/j.jcis.2015.03.037.
- Wei, Z.; Volkova, E.; Blatchley, M. R.; Gerecht, S. Hydrogel Vehicles for Sequential Delivery of Protein Drugs to Promote Vascular Regeneration. Adv. Drug Deliver. Rev. 2019, 149–150, 95–106. doi: 10.1016/j.addr.2019.08.005.
- McClements, D. J.;. Recent Progress in Hydrogel Delivery Systems for Improving Nutraceutical Bioavailability. Food Hydrocolloid. 2017, 68, 238–245. doi: 10.1016/j.foodhyd.2016.05.037.
- McClements, D. J.;. Designing Biopolymer Microgels to Encapsulate, Protect and Deliver Bioactive Components: Physicochemical Aspects. Adv. Colloid Interface. 2017, 240, 31–59. doi: 10.1016/j.cis.2016.12.005.
- Joye, I. J.; Davidov-Pardo, G.; McClements, D. J. Nanotechnology for Increased Micronutrient Bioavailability. Trends Food Sci. Tech. 2014, 40, 168–182. doi: 10.1016/j.tifs.2014.08.006.
- Nile, S. H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives. Nano-Micro. Lett. 2020, 12, 45. doi: 10.1007/s40820-020-0383-9.
- McClements, D. J.;. The Future of Food Colloids: Next-Generation Nanoparticle Delivery Systems. Curr. Opin. Colloid Interface Sci. 2017, 28, 7–14. doi: 10.1016/j.cocis.2016.12.002.
- Lim, H.; Ho, K.; Surjit Singh, C. K.; Ooi, C.; Tey, B.; Chan, E. Pickering Emulsion Hydrogel as a Promising Food Delivery System: Synergistic Effects of Chitosan Pickering Emulsifier and Alginate Matrix on Hydrogel Stability and Emulsion Delivery. Food Hydrocolloid. 2020, 103, 105659. doi: 10.1016/j.foodhyd.2020.105659.
- Cooper, R. C.; Yang, H. Hydrogel-Based Ocular Drug Delivery Systems: Emerging Fabrication Strategies, Applications, and Bench-to-Bedside Manufacturing Considerations. J. Control. Release. 2019, 306, 29–39. doi: 10.1016/j.jconrel.2019.05.034.
- Zhu, K.; Yu, D.; Chen, X.; Song, G. Preparation, Characterization and Controlled-Release Property of Fe3+ Cross-Linked Hydrogels Based on Peach Gum Polysaccharide. Food Hydrocolloid. 2019, 87, 260–269. doi: 10.1016/j.foodhyd.2018.08.019.
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M. Construction of Self-Assembled Polyelectrolyte Complex Hydrogel Based on Oppositely Charged Polysaccharides for Sustained Delivery of Green Tea Polyphenols. Food Chem. 2020, 306, 125632. doi: 10.1016/j.foodchem.2019.125632.
- Mahinroosta, M.; Jomeh Farsangi, Z.; Allahverdi, A.; Shakoori, Z. Hydrogels as Intelligent Materials: A Brief Review of Synthesis, Properties and Applications. Mater. Today Chem. 2018, 8, 42–55. doi: 10.1016/j.mtchem.2018.02.004.
- Shewan, H. M.; Stokes, J. R. Review of Techniques to Manufacture Micro-Hydrogel Particles for the Food Industry and Their Applications. J. Food Eng. 2013, 119, 781–792. doi: 10.1016/j.jfoodeng.2013.06.046.
- Wu, B.; Degner, B.; McClements, D. J. Soft Matter Strategies for Controlling Food Texture: Formation of Hydrogel Particles by Biopolymer Complex Coacervation. J. Phys. Condens. Mater. 2014, 26, 464104. doi: 10.1088/0953-8984/26/46/464104.
- Chung, C.; Degner, B.; Decker, E. A.; McClements, D. J. Oil-Filled Hydrogel Particles for Reduced-Fat Food Applications: Fabrication, Characterization, and Properties. Innov. Food Sci. Emerg. 2013, 20, 324–334. doi: 10.1016/j.ifset.2013.08.006.
- Thompson, B. R.; Horozov, T. S.; Stoyanov, S. D.; Paunov, V. N. Structuring and Calorie Control of Bakery Products by Templating Batter with Ultra Melt-Resistant Food-Grade Hydrogel Beads. Food Funct. 2017, 8, 2967–2973. doi: 10.1039/C7FO00867H.
- Santos, M. D.; Ozaki, M. M.; Ribeiro, W. O.; Paglarini, C. D. S.; Vidal, V. A. S.; Campagnol, P. C. B.; Pollonio, M. A. R. Emulsion Gels Based on Pork Skin and Dietary Fibers as Animal Fat Replacers in Meat Emulsions: An Adding Value Strategy to Byproducts. LWT. 2020, 120, 108895. doi: 10.1016/j.lwt.2019.108895.
- Lucas-González, R.; Roldán-Verdu, A.; Sayas-Barberá, E.; Fernández-López, J.; Pérez-Álvarez, J. A.; Viuda-Martos, M. Assessment of Emulsion Gels Formulated with Chestnut (Castanea Sativa M.) Flour and Chia (Salvia Hispanica L) Oil as Partial Fat Replacers in Pork Burger Formulation. J. Sci. Food Agr. 2020, 100, 1265–1273. doi: 10.1002/jsfa.10138.
- Yang, X.; Gong, T.; Lu, Y.; Li, A.; Sun, L.; Guo, Y. Compatibility of Sodium Alginate and Konjac Glucomannan and Their Applications in Fabricating Low-Fat Mayonnaise-Like Emulsion Gels. Carbohyd. Polym. 2020, 229, 115468. doi: 10.1016/j.carbpol.2019.115468.
- Heck, R. T.; Saldaña, E.; Lorenzo, J. M.; Correa, L. P.; Fagundes, M. B.; Cichoski, A. J.; de Menezes, C. R.; Wagner, R.; Campagnol, P. C. B. Hydrogelled Emulsion From Chia and Linseed Oils: A Promising Strategy to Produce Low-Fat Burgers with a Healthier Lipid Profile. Meat Sci. 2019, 156, 174–182. doi: 10.1016/j.meatsci.2019.05.034.
- Bandyopadhyay, S.; Saha, N.; Brodnjak, U. V.; Saha, P. Bacterial Cellulose Based Greener Packaging Material: A Bioadhesive Polymeric Film. Mater. Res. Express. 2018, 5, 115405. doi: 10.1088/2053-1591/aadb01.
- Batista, R. A.; Espitia, P. J. P.; Quintans, J. D. S. S.; Freitas, M. M.; Cerqueira, M. Â.; Teixeira, J. A.; Cardoso, J. C. Hydrogel as an Alternative Structure for Food Packaging Systems. Carbohyd. Polym. 2019, 205, 106–116. doi: 10.1016/j.carbpol.2018.10.006.
- de Azeredo, H. M. C.;. Antimicrobial Nanostructures in Food Packaging. Trends Food Sci. Tech. 2013, 30, 56–69. doi: 10.1016/j.tifs.2012.11.006.
- Benito-Peña, E.; González-Vallejo, V.; Rico-Yuste, A.; Barbosa-Pereira, L.; Cruz, J. M.; Bilbao, A.; Alvarez-Lorenzo, C.; Moreno-Bondi, M. C. Molecularly Imprinted Hydrogels as Functional Active Packaging Materials. Food Chem. 2016, 190, 487–494. doi: 10.1016/j.foodchem.2015.05.128.
- Wang, L.; Rhim, J. Preparation and Application of Agar/Alginate/Collagen Ternary Blend Functional Food Packaging Films. Int. J. Biol. Macromol. 2015, 80, 460–468. doi: 10.1016/j.ijbiomac.2015.07.007.
- Oun, A. A.; Rhim, J. Carrageenan-Based Hydrogels and Films: Effect of ZnO and Cuo Nanoparticles on the Physical, Mechanical, and Antimicrobial Properties. Food Hydrocolloid. 2017, 67, 45–53. doi: 10.1016/j.foodhyd.2016.12.040.
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Bialik-Wąs, K.; Kijkowska, R.; Sobczak-Kupiec, A. Preparation and Cytotoxicity of Chitosan-Based Hydrogels Modified with Silver Nanoparticles. Colloids Surf. B. 2017, 160, 325–330. doi: 10.1016/j.colsurfb.2017.09.044.
- Baek, S.; Kim, D.; Jeon, S. L.; Seo, J. Preparation and Characterization of pH-Responsive Poly(N,N-Dimethyl Acrylamide-Co-Methacryloyl Sulfadimethoxine) Hydrogels for Application as Food Freshness Indicators. React. Funct. Polym. 2017, 120, 57–65. doi: 10.1016/j.reactfunctpolym.2017.09.003.
- Zhao, M.; Li, S.; Zhou, L.; Shen, Q.; Zhu, H.; Zhu, X. Prognostic Values of Excision Repair Cross-Complementing Genes mRNA Expression in Ovarian Cancer Patients. Life Sci. 2018, 194, 34–39. doi: 10.1016/j.lfs.2017.12.018.
- Devezeaux De Lavergne, M.; Strijbosch, V. M. G.; Van den Broek, A. W. M.; Van de Velde, F.; Stieger, M. Uncoupling the Impact of Fracture Properties and Composition on Sensory Perception of Emulsion-Filled Gels. J. Texture Stud. 2016, 47, 92–111. doi: 10.1111/jtxs.12164.
- Devezeaux, D. L. M.; van de Velde, F.; Stieger, M. Bolus Matters: The Influence of Food Oral Breakdown on Dynamic Texture Perception. Food Funct. 2017, 8, 464–480. doi: 10.1039/c6fo01005a.
- Sala, G.; de Wijk, R. A.; van de Velde, F.; van Aken, G. A. Matrix Properties Affect the Sensory Perception of Emulsion-Filled Gels. Food Hydrocolloid. 2008, 22, 353–363. doi: 10.1016/j.foodhyd.2006.12.009.
- Liu, K.; Stieger, M.; van der Linden, E.; van de Velde, F. Fat Droplet Characteristics Affect Rheological, Tribological and Sensory Properties of Food Gels. Food Hydrocolloid. 2015, 44, 244–259. doi: 10.1016/j.foodhyd.2014.09.034.
- Guo, Q.; Ye, A.; Lad, M.; Dalgleish, D.; Singh, H. The Breakdown Properties of Heat-Set Whey Protein Emulsion Gels in the Human Mouth. Food Hydrocolloid. 2013, 33, 215–224. doi: 10.1016/j.foodhyd.2013.03.008.
- Thompson, B. R.; Horozov, T. S.; Stoyanov, S. D.; Paunov, V. N. An Ultra Melt-Resistant Hydrogel From Food Grade Carbohydrates. RSC Adv. 2017, 7, 45535–45544. doi: 10.1039/c7ra08590g.
- Dickinson, E.;. Microgels — An Alternative Colloidal Ingredient for Stabilization of Food Emulsions. Trends Food Sci. Tech. 2015, 43, 178–188. doi: 10.1016/j.tifs.2015.02.006.
- Gonçalves, J. O.; Santos, J. P.; Rios, E. C.; Crispim, M. M.; Dotto, G. L.; Pinto, L. A. A. Development of Chitosan Based Hybrid Hydrogels for Dyes Removal from Aqueous Binary System. J. Mol. Liq. 2017, 225, 265–270. doi: 10.1016/j.molliq.2016.11.067.
- Gonçalves, J. O.; Da Silva, K. A.; Rios, E. C.; Crispim, M. M.; Dotto, G. L.; de Almeida Pinto, L. A. Single and Binary Adsorption of Food Dyes on Chitosan/Activated Carbon Hydrogels. Chem. Eng. Technol. 2019, 42, 454–464. doi: 10.1002/ceat.201800367.
- He, J.; Ni, F.; Cui, A.; Chen, X.; Deng, S.; Shen, F.; Huang, C.; Yang, G.; Song, C.; Zhang, J.;, et al. New Insight into Adsorption and Co-Adsorption of Arsenic and Tetracycline Using a Y-Immobilized Graphene Oxide-Alginate Hydrogel: Adsorption Behaviours and Mechanisms. Sci. Total Environ. 2020, 701, 134363. doi: 10.1016/j.scitotenv.2019.134363.
- Guo, X.; Xu, D.; Yuan, H.; Luo, Q.; Tang, S.; Liu, L.; Wu, Y. A Novel Fluorescent Nanocellulosic Hydrogel Based on Carbon Dots for Efficient Adsorption and Sensitive Sensing in Heavy Metals. J. Mater. Chem. A. 2019, 7, 27081–27088. doi: 10.1039/C9TA11502A.
- Gonçalves, J. O.; Da Silva, K. A.; Rios, E. C.; Crispim, M. M.; Dotto, G. L.; de Almeida Pinto, L. A. Chitosan Hydrogel Scaffold Modified with Carbon Nanotubes and Its Application for Food Dyes Removal in Single and Binary Aqueous Systems. Int. J. Biol. Macromol. 2020, 142, 85–93. doi: 10.1016/j.ijbiomac.2019.09.074.
- Zhang, W.; Song, J.; He, Q.; Wang, H.; Lyu, W.; Feng, H.; Xiong, W.; Guo, W.; Wu, J.; Chen, L. Novel Pectin Based Composite Hydrogel Derived from Grapefruit Peel for Enhanced Cu(Ii) Removal. J. Hazard. Mater. 2020, 384, 121445. doi: 10.1016/j.jhazmat.2019.121445.
- Wang, B.; Wang, B.; Wan, Y.; Zheng, Y.; Lee, X.; Liu, T.; Yu, Z.; Huang, J.; Ok, Y. S.; Chen, J.;, et al.. Alginate-Based Composites for Environmental Applications: A Critical Review. Crit. Rev. Environ. Sci. Tech.. 2019, 49, 318–356. doi:10.1080/10643389.2018.1547621.
- Hu, Z. H.; Omer, A. M.; Ouyang, X. K.; Yu, D. Fabrication of Carboxylated Cellulose Nanocrystal/Sodium Alginate Hydrogel Beads for Adsorption of Pb(Ii) from Aqueous Solution. Int. J. Biol. Macromol. 2018, 108, 149–157. doi: 10.1016/j.ijbiomac.2017.11.171.
- Zhang, W.; Wang, H.; Hu, X.; Feng, H.; Xiong, W.; Guo, W.; Zhou, J.; Mosa, A.; Peng, Y. Multicavity Triethylenetetramine-Chitosan/ Alginate Composite Beads for Enhanced Cr(Vi) Removal. J. Clean. Prod. 2019, 231, 733–745. doi: 10.1016/j.jclepro.2019.05.219.
- Yin, J.; Sun, W.; Song, X.; Ji, H.; Yang, Y.; Sun, S.; Zhao, W.; Zhao, C. Precipitated Droplets in-Situ Cross-Linking Polymerization Towards Hydrogel Beads for Ultrahigh Removal of Positively Charged Toxins. Sep. Purif. Technol. 2020, 238, 116497. doi: 10.1016/j.seppur.2019.116497.
- Balkız, G.; Pingo, E.; Kahya, N.; Kaygusuz, H.; Bedia Erim, F. Graphene Oxide/Alginate Quasi-Cryogels for Removal of Methylene Blue. Water, Air, Soil Pollut. 2018, 229, 1–9. doi: 10.1007/s11270-018-3790-5.
- Zhuang, Y.; Yu, F.; Ma, J.; Chen, J. Enhanced Adsorption Removal of Antibiotics from Aqueous Solutions by Modified Alginate/Graphene Double Network Porous Hydrogel. J. Colloid Interfaces Sci. 2017, 507, 250–259. doi: 10.1016/j.jcis.2017.07.033.
- Sun, Y.; Zhou, T.; Li, W.; Yu, F.; Ma, J. Amino-Functionalized Alginate/ Graphene Double-Network Hydrogel Beads for Emerging Contaminant Removal from Aqueous Solution. Chemosphere. 2020, 241, 125110. doi: 10.1016/j.chemosphere.2019.125110.
- Hao, L.; Wang, W.; Shen, X.; Wang, S.; Li, Q.; An, F.; Wu, S. A Fluorescent DNA Hydrogel Aptasensor Based on the Self-Assembly of Rolling Circle Amplification Products for Sensitive Detection of Ochratoxin A. J. Agr. Food Chem. 2020, 68, 369–375. doi: 10.1021/acs.jafc.9b06021.
- Zhao, M.; Wang, P.; Guo, Y.; Wang, L.; Luo, F.; Qiu, B.; Guo, L.; Su, X.; Lin, Z.; Chen, G. Detection of Aflatoxin B1 in Food Samples Based On Target-Responsive Aptamer-Cross-Linked Hydrogel Using a Handheld pH Meter as Readout. Talanta. 2018, 176, 34–39. doi: 10.1016/j.talanta.2017.08.006.
- Ma, Y.; Mao, Y.; Huang, D.; He, Z.; Yan, J.; Tian, T.; Shi, Y.; Song, Y.; Li, X.; Zhu, Z.;, et al. Portable Visual Quantitative Detection of Aflatoxin B1 Using a Target-Responsive Hydrogel and a Distance-Readout Microfluidic Chip. Lab Chip 2016, 16, 3097–3104. doi: 10.1039/C6LC00474A.
- Wang, Y.; Xie, T.; Yang, J.; Lei, M.; Fan, J.; Meng, Z.; Xue, M.; Qiu, L.; Qi, F.; Wang, Z. Fast Screening of Antibiotics in Milk Using a Molecularly Imprinted Two-Dimensional Photonic Crystal Hydrogel Sensor. Anal. Chim. Acta. 2019, 1070, 97–103. doi: 10.1016/j.aca.2019.04.031.
- Zhan, Y.; Zeng, Y.; Li, L.; Luo, F.; Qiu, B.; Lin, Z.; Guo, L. Ratiometric Fluorescent Hydrogel Test Kit for On-Spot Visual Detection of Nitrite. ACS Sens. 2019, 4, 1252–1260. doi: 10.1021/acssensors.9b00125.
- Nam, J.; Jung, I.; Kim, B.; Lee, S.; Kim, S.; Lee, K.; Shin, D. A Colorimetric Hydrogel Biosensor for Rapid Detection of Nitrite Ions. Sens. Actuators B Chem. 2018, 270, 112–118. doi: 10.1016/j.snb.2018.04.171.
- Gong, Z.; Wang, C.; Pu, S.; Wang, C.; Cheng, F.; Wang, Y.; Fan, M. Rapid and Direct Detection of Illicit Dyes on Tainted Fruit Peel Using a PVA Hydrogel Surface Enhanced Raman Scattering Substrate. Anal. Methods. UK 2016, 8, 4816–4820. doi: 10.1039/C6AY00233A.
- Liu, R.; Huang, Y.; Ma, Y.; Jia, S.; Gao, M.; Li, J.; Zhang, H.; Xu, D.; Wu, M.; Chen, Y.;, et al. Design and Synthesis of Target-Responsive Aptamer-Cross-Linked Hydrogel for Visual Quantitative Detection of Ochratoxin A. ACS Appl. Mater. Inter. 2015, 7, 6982–6990. doi: 10.1021/acsami.5b01120.
- Geng, Z.; Zhang, H.; Xiong, Q.; Zhang, Y.; Zhao, H.; Wang, G. A. Fluorescent Chitosan Hydrogel Detection Platform for the Sensitive and Selective Determination of Trace Mercury(Ii) in Water. J. Mater. Chem. A. 2015, 3, 19455–19460. doi: 10.1039/C5TA05610A.
- Huang, Y.; Wang, D.; Liu, W.; Zheng, L.; Wang, Y.; Liu, X.; Fan, M.; Gong, Z. Rapid Screening of Rhodamine B in Food by Hydrogel Solid-Phase Extraction Coupled with Direct Fluorescence Detection. Food Chem. 2020, 316, 126378. doi: 10.1016/j.foodchem.2020.126378.