ABSTRACT
This study meta-analyzed research examining relationships between diffusion tensor imaging and cognition following pediatric traumatic brain injury (TBI). Data from 14 studies that correlated fractional anisotropy (FA) or apparent diffusion coefficient/mean diffusivity with cognition were analyzed. Short-term (<4 weeks post-TBI) findings were inconsistent, but, in the medium to long term, FA values for numerous large white matter tracts and the whole brain were related to cognition. However, the analyses were limited by the diversity of brain regions and cognitive outcomes that have been examined; all in relatively small samples. Moreover, additional data are needed to investigate the impact of age and injury severity on these findings.
Traumatic brain injuries (TBIs) are a common cause of hospital visits and admissions in children and adolescents with the most vulnerable groups being very young children (aged 0–4 years), followed by older adolescents (aged 15–19 years) (Faul, Xu, Wald, & Coronado, Citation2010). These TBIs often result in a range of cognitive and neurological impairments (Babikian & Asarnow, Citation2009; Beauchamp et al., Citation2013). Diffuse axonal injury (DAI), caused by the rapid acceleration or deceleration of the head and brain (Wilde et al., Citation2010), is thought to be the main contributor to these cognitive impairments (Arfanakis et al., Citation2002). However, commonly used neuroimaging techniques—computed tomography (CT) and magnetic resonance imaging (MRI)—more effectively identify focal lesions (Lee & Newberg, Citation2005) than they do DAI (Arfanakis et al., Citation2002; Duckworth & Stevens, Citation2010); thereby limiting their usefulness for diagnostic, treatment, and prognostic purposes (Niogi & Mukherjee, Citation2010). Diffusion tensor imaging (DTI), on the other hand, is an MR technique that is designed to provide a more sensitive measure of DAI (Arfanakis et al., Citation2002) and is therefore likely to be more useful for predicting functional outcomes (Caeyenberghs et al., Citation2010b; Wozniak et al., Citation2007).
DTI measures the movement/diffusion of water molecules within the white matter of the brain (Assaf & Pasternak, Citation2008; Duckworth & Stevens, Citation2010), which is normally greatest parallel to nerve fibers. Physical obstructions within the axon, which vary in terms of their size, shape, composition, and spacing, influence the direction and amount of diffusion (Beaulieu, Citation2002). In myelinated axons, the main components (myelin, axonal membrane, microtubules, and neurofilaments) are all orientated longitudinally, which facilitates diffusion parallel to the length of the axon while hindering diffusion perpendicular to the length of the axon (Beaulieu, Citation2002). Brain damage that affects the orientation of axons and/or the surrounding myelin sheaths may alter the direction of the diffusion, while also increasing the total amount of diffusion because there are fewer intact structures constraining this movement (Niogi & Mukherjee, Citation2010). Several measures have been developed to capture these changes; some focus on the direction of the diffusion (anisotropy), the most common of which is fractional anisotropy (FA), and others on the amount/rate of diffusion, including the apparent diffusion coefficient (ADC) and mean diffusivity (MD) (Niogi & Mukherjee, Citation2010). Specifically, FA measures uniformity in the relative amount of diffusion in different directions, with values ranging between 0 (diffusion is the same in all directions) and 1 (diffusion only occurs only along a single orientation); when FA is large, it is generally assumed that the major direction of diffusion is parallel to the major axon in the voxel. ADC, on the other hand, measures the amount of diffusion, which is usually averaged over three orthogonal directions (X, Y, Z axes) (Niogi & Mukherjee, Citation2010). MD is very similar to the ADC and measures the average of the diffusivity values across the three axes/directions (Mueller, Lim, Hemmy, & Camchong, Citation2015). Indeed, ADC and MD values are similar when obtained from the same scanner, with the two terms often being used interchangeably in the literature (Yanagihara & Wang, Citation2014). Higher FA and lower ADC/MD values are generally interpreted to indicate white matter integrity, reflecting more consistent ordering of axons, greater myelination, and denser axons (Lebel et al., Citation2013).
Consistent with this, a recent meta-analysis found that, in the medium to long term, there are moderate to very large decreases in FA and comparable increases in ADC/MD in a large number of white matter tracts following pediatric TBI (children and adolescents aged under 18 years), reflecting decreased integrity in white matter microstructure (Roberts, Mathias, & Rose, Citation2014). Specifically, substantial changes were observed in the corpus callosum, internal capsule, uncinate fasciculus, longitudinal fasciculus and cingulate, as well as the frontal and temporal lobes. However, the opposite pattern was observed in the acute stages after a TBI, with moderate to large increases in FA and equivalent decreases in ADC observed in most regions, including: the whole brain, corpus callosum, corona radiata, cerebral peduncle, fornix, left cingulum, anterior thalamic radiation, right inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, subcallosal cortex, right thalamus, and left white matter. The latter findings possibly reflect early axonal swelling or edema, leading to a temporary increase in FA and decrease in ADC (Bigler & Bazarian, 2010; Gardner et al., 2012; Wu, Wilde, Bigler, Yallampalli, et al., Citation2010). In combination, the evidence suggests that DTI is sensitive to white matter changes following pediatric TBI, with the nature of these changes evolving over time, but its relationship to clinical outcomes also needs to be established.
To this end, Hulkower, Poliak, Rosenbaum, Zimmerman, and Lipton (Citation2013) reviewed research that has examined the relationship between DTI and cognition following TBI, but reported mixed findings, with positive, negative and inconsequential correlations all noted. Interestingly, however, this review combined data from studies that examined different levels of injury severity (mild, moderate, severe), pediatric and adult samples (aged 2–70 years) and all intervals post-TBI (acute to chronic); possibly contributing to the highly variable nature of their findings. Although acknowledging this variability, Hulkower et al. largely attributed it to the range of cognitive measures that were used, because few studies utilized the same measure. They also suggested that the mixed findings may reflect the range of injuries that were examined, particularly in studies of mild TBI, which may have included individuals who did not have any cognitive problems or who were assessed using tests that are not sensitive to subtle deficits. The fact that their findings were based on DTI data collected both in the short and long term, and from pediatric and adult samples, appears to have been overlooked.
Importantly, there are critical differences between pediatric and adult TBI (Pinto, Meoded, Poretti, Tekes, & Huisman, Citation2012)—due to differences in the brains, skulls, head size, and musculature of these groups—that provide a compelling case for considering them separately. More specifically, pediatric brains have a higher water content, which makes it softer and more susceptible to injury, and are less myelinated, making them more susceptible to shear injuries (for review see Pinto, Meoded, et al., Citation2012; Pinto, Poretti, Meoded, Tekes, & Huisman, Citation2012). Children’s skulls are also less rigid, their head size is larger in proportion to their body size, and their neck musculature is weaker but has to support a large/heavy head, relative to body size; all of which may contribute to differences in the injuries that are sustained and the associated outcomes. These differences are maximal at birth and then reduce with increasing age. For example, unlike body size, the intracranial volume of a 2-year-old is approximately 72% of that of an adult and by adolescence it is approximately 96% (Pinto, Poretti, et al., Citation2012). In addition, TBIs that are sustained during childhood can lead to cognitive impairments that may alter a child’s developmental trajectory; thereby having a greater impact than an equivalent injury in an adult (Hessen, Citation2010). The current study therefore focuses only on children and adolescents who have sustained a TBI.
Several studies have examined the relationship between cognitive functioning and DTI (FA, ADC/MD) following pediatric TBI. However, the number of studies examining the same brain regions using comparable cognitive measures is often small and the associated findings are often inconsistent. For example, correlations between FA values in the splenium (corpus callosum) and measures of attention/information processing range from no/small relationship in some studies (Adamson et al., Citation2013; Kurowski et al., Citation2009; Mayer et al., Citation2012) to medium or large positive relationship in others (Ewing-Cobbs et al., Citation2008; Treble et al., Citation2013; Wu, Wilde, Bigler, Li, et al., Citation2010). Moreover, imaging has been conducted in both the short (Mayer et al., Citation2012) and long term (Adamson et al., Citation2013; Ewing-Cobbs et al., Citation2008; Kurowski et al., Citation2009; Treble et al., Citation2013; Wu, Wilde, Bigler, Li, et al., Citation2010) after TBI; a difference that is very likely to affect the direction of the relationship between cognitive and DTI measures (Gardner et al., 2012). In combination, variability in the brain regions that have been examined, the measures that have been used to assess cognition, and the time at which the imaging is performed have made it difficult to determine whether, and to what extent, there is a relationship between DTI and cognitive outcomes. This, in turn, has limited our ability to evaluate the clinical utility of DTI for predicting cognitive outcomes after pediatric TBI.
Aims
The current study provides a meta-analytic review of research that has examined the relationship between DTI measures of white matter integrity (FA, ADC/MD) and cognitive performance in children and adolescents who have sustained a mild, moderate and severe TBIs. A key goal was to determine whether FA and ADC/MD measures obtained from DTI performed in the short term (≤4 weeks) and medium to long term (>4 weeks) predict cognitive outcomes, with a particular emphasis on the white matter tracts that have previously been reported to be most affected by pediatric TBI (Roberts et al., Citation2014). It was expected that better cognitive performance would be related to (a) lower FA and higher ADC/MD in the short term and (b) higher FA and lower ADC/MD values in the medium to long term.
Methods
Literature search
A comprehensive search of the literature was undertaken in order to identify research that has examined the relationship between DTI measures of white matter integrity and cognitive functioning in children and/or adolescents who had sustained a TBI. Seven electronic databases were searched (search performed December 2014): PubMed, PsycINFO (Ovid), CINAHL (EbscoHost), Scopus, Embase (Elsevier), Informit, and Web of Science. A broad range of search terms were used to cover: cognition/neurocognition/mental processes, TBI/brain injury/brain trauma, pediatric/child/adolescent, and DTI/DWI/diffusion MRI (see Appendix, for the specific search/logic grids).
A study was deemed eligible for inclusion if it met the following criteria: (a) the participants were children and/or adolescents (mean age <18) who had sustained a non-penetrating TBI; (b) cognitive functioning was assessed using objective tests (rating scales, parental questionnaires, and measures of health/emotional functioning were all excluded for current purposes); (c) it examined the relationship between DTI (FA, ADC/MD) and cognitive functioning; (d) it reported original data (excludes reviews); (e) the sample size was adequate for calculating correlations (n > 5); (f) it was published in a journal in English (excludes dissertations); and (g) correlations (Pearson r/Spearman rho or exact p-values) were provided between the cognitive and DTI measures.
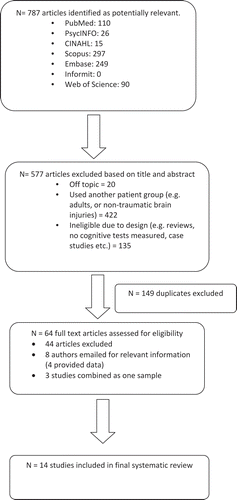
The literature search identified 787 potentially relevant studies (see for flow chart). Preliminary application of the inclusion criteria to the titles and abstracts of these papers, reduced this number to 210, 149 of which were duplicates. Full-text versions of the remaining 61 articles were then obtained and the inclusion criteria re-applied. A further 39 papers were excluded on this basis, with another eight reporting multivariate statistics (general linear models, ANOVAs) (Adamson et al., Citation2013; Liégeois et al., Citation2013; Mayer et al., Citation2012; Treble et al., Citation2013; Wilde et al., 2006; Wilde, Hunter, & Bigler, Citation2012; Wilde et al., Citation2010; Wozniak et al., Citation2007), rather than univariate correlations. The corresponding authors for these eight studies were subsequently contacted to request the relevant data. Of these, four provided the necessary data in the form of univariate correlations (Adamson et al., Citation2013; Mayer et al., Citation2012; Treble et al., Citation2013; Wozniak et al., Citation2007) and another replied, but was unable to assist (Wilde et al., Citation2012), leaving a total of 16 studies. Finally, all studies were checked for independence because meta-analyses assume that all samples are independent of one another. Three studies by Caeyenberghs and colleagues (Caeyenberghs et al., Citation2011, Citation2010a, Citation2010b) were combined and treated as one on this basis, leaving 14 independent studies from which data could be extracted and analyzed. The PRISMA statement (Moher, Liberati, Tetzlaff, & Altman, Citation2009), which outlines the Preferred Reporting Items for Systematic Reviews and Meta-Analyses and provides a 27-item checklist, was completed to ensure that the current meta-analysis met these guidelines (see Table A, supplementary material, for checklist).
Data collection and preparation
Demographic details (age, gender, handedness), injury details (Glasgow Coma Scale score/category, post-injury interval), MRI/DTI information (MRI magnet strength [Tesla: 1.5T or 3T], DTI metrics [FA, ADC, MD], region-of-interest [e.g., corpus callosum]), information relating to the cognitive tests (test name, cognitive domain, score type [no. correct, no. of errors, time]), and the data required for the calculation of the effect sizes (correlation coefficient/exact p-value) were extracted from each study. Cognitive tests were grouped into one of nine broad domains, based on the categories identified by Lezak, Howieson, Bigler, and Tranel (Citation2012): general cognition, verbal functions, executive functions, concept formation and reasoning, construction, motor function, memory, attention and information processing, and academic achievement (see Table B, supplementary material, for a list of the cognitive tests, the cognitive domain into which they were classified and studies using these tests). Where studies used multiple cognitive tests to assess the same cognitive domain (e.g., memory), the scores from these tests were averaged so that each study provided only one correlation for each domain (r scores transformed to Fisher’s Z, averaged and transformed back to an r; Borenstein, Hedges, Higgins, & Rothstein, Citation2009). Mean correlations cannot be calculated directly from r; r was therefore transformed to Fisher’s Zr and a mean Zr calculated, which was then converted back to r for ease of interpretation (Hedges & Olkin, Citation1985).
FA and ADC/MD data were analyzed separately, as were DTI data collected in the short and the medium to long term in order to assess changes in both the direction and rate of diffusion over time. There is considerable variation in the intervals that are used to define short and medium to long term within the TBI literature: we used 4 weeks to divide the study findings into short (≤4 weeks) and medium/long term (>4 weeks).
Effect size calculation and interpretation
Pearson’s r correlation coefficients (or Spearman’s rho) assessing the relationship between the cognitive and DTI measures were extracted for each region of interest (ROI) and study. Mean weighted effect sizes (rw) were calculated when multiple studies provided correlations between the same cognitive domain and ROI. The inverse variance was used to weight effect sizes when calculating means (Lipsey & Wilson, Citation2001). A positive r (or rw) indicates that higher FA values or lower ADC/MD values were associated with better cognitive performance, with r values of 0.1, 0.3 and 0.5 equating to small, medium, and large effects, respectively (Cohen, Citation1992). Probability (p) values were calculated to provide a measure of statistical significance, with a p value < .05 indicating that the true relationship between the cognitive and DTI measures in the pediatric TBI population differs significantly from zero. Ninety-five percent confidence intervals (95%CIs) were calculated to indicate the likely range of the true population effect. Calculations were performed using the Comprehensive Meta-Analysis program (CMA, Version 3; Borenstein, Hedges, Higgins, & Rothstein, Citation2005). A random-effects model was used because studies varied in terms of a number of methodological variables (e.g., age, TBI severity).
Finally, failsafe Ns (Nfs) were calculated to address the issue of publication bias (Lipsey & Wilson, Citation2001). This statistic provides a hypothetical measure of the number of unpublished studies with an effect size of zero that would need to exist in order to reduce a finding to a trivial effect (r < .1) and, therefore, call the current results into question (Orwin, Citation1983). The larger the Nfs, relative to the number of studies contributing to a result, the less likely it is that this number of unpublished studies with null findings would exist.
Ideally, all studies would use the same or comparable cognitive tests to assess specific cognitive domains (e.g., memory) and examine the same ROIs. Unfortunately, this was not the case, with very limited overlap in both the cognitive tests that were used and the brain regions that were examined by researchers. While meta-analyses are designed to pool data from multiple studies, it is not possible to determine the extent to which this is possible until all data have been collected. However, the provision of comparable data (r or rw, p, 95% CIs, Nfs statistics)—whether from individual or multiple studies—enables the findings in this emerging area to be directly compared and evaluated, which arguably represents an important advance in the research literature.
The inferences made throughout this meta-analysis are based on the aforementioned statistics. Specifically, we argue that we can be more confident that there is a relationship between cognitive functioning and white matter integrity—as measured by FA or ADC/MD—in children and adolescents following a TBI if the correlations are medium or larger in size (r > .3), statistically significant (p < .05), and have acceptable Nfs statistics (Nfs > Nstudies).
Quality of study reporting
All studies were evaluated against the “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) statement (Vandenbroucke et al., Citation2007), which contains a list of 32 items that should be detailed in all observational studies; thereby providing a means by which study reporting quality could be evaluated. All studies were individually evaluated to determine whether it provided information for each of the 32 items (present = 1, partial detail = 0.5, absent = 0). The percentage of studies that reported each item was also calculated (partial details excluded in this calculation).
Results
Participant and study characteristics
Altogether, the 14 studies provided data for a total of 358 participants, with many samples being relatively small in size (see for summary demographic data). Participants ranged in age from 7–15 years and, of those for whom there was demographic data, the majority were right-handed (88%), Caucasian (52%), or Hispanic (35%) males (67%). Notably, however, handedness and ethnicity were only reported by a limited number of studies (N = 6 and 4, respectively). Most studies were conducted in the United States (see also Table C, supplementary material, for details of individual studies).
Table 1. Demographic and imaging data.
Glasgow Coma Scale (GCS) scores were only reported for nine studies, with the mean falling in the moderate TBI category (mean = 9.8, SD = 2.9). However, all studies provided descriptive categorical information relating to injury severity, which indicated that most (n = 11) examined samples with mixed severity (mild, moderate and/or severe) and only three investigated patients with mild (n = 2) or severe (n = 1) TBI. Six studies examined moderate to severe TBI in combination with complicated-mild TBI, which was defined by a GCS score of 13–15 together with visible lesions on CT scans.
Most studies (n = 11) performed their DTI in the chronic phase after the TBI (mean = 1.4 years, SD = 1.6), with only three studies reporting findings in the short term (<4 weeks). Imaging and cognitive assessments were completed proximally for all studies except Babikian et al. (Citation2009), which performed imaging after an average of 6 days and cognitive testing after an average of 25 months post-injury. The data from this study were analysed with the other short-term data because DTI was performed acutely. The majority of studies used a 3 Tesla (3T) strength scanner (n = 10), with the remaining four using 1.5T, and most used a Phillips (n = 8) or Siemens (n = 4) brand scanner. The number of directions for DTI acquisition ranged from 3 to 45 (mean = 22, SD = 12), (see and Table D, supplementary material, for study-specific imaging details: scanner strength, brand, image acquisition details, software used for pre-/post-processing and analysis, ROI identification). Variability in DTI parameters and pre-processing/post-processing/analysis may have contributed to the heterogeneity of findings, but limited overlap and/or incomplete details prevented any analysis of these variables.
STROBE ratings of quality of study reporting
The reporting quality of the included studies varied widely, depending on what was being assessed (mean percent of studies meeting criteria for each item = 58%, SD = 45, range = 0–100%). Of the 30 items that are relevant here, 14 were reported by 86% (n = 12) or more of the studies, indicating acceptable reporting quality for many items. All studies provided sufficient information regarding their study rational and objectives, study design, definition and measurement of variables, sampling strategy, summary outcome data, key results, and generalizability (see Table E, supplementary material, for STROBE summary data). In contrast, many fewer studies provided information about the study location (14%), reasons for non-participation (7%), rationale for their sample size (7%), and how missing data were managed (7%). Moreover, limited information was provided about potential sources of bias (29%) and sample representativeness was difficult to evaluate due to a lack of information about participant selection (14%). Thus, while many key aspects were reported, it is not possible to determine from the available data whether the study participants are broadly representative of children with TBI.
Cognitive domains and regions of interest
As seen in , a variety of different cognitive domains and a very large number of ROI (n = 44) were examined, both in the short and medium to long term, using FA and/or ADC/MD. However, most cognitive domains were only examined by single studies, as were most ROIs; with attention/information processing being the only cognitive domain, and the corpus callosum being the only ROI, to be examined more widely (see for summary details and Table C, supplementary material, for specific study details). In addition, most studies focussed on FA, rather than ADC/MD, in the medium to long term (FA: n = 10, ADC/MD: n = 3 studies), with many fewer ROIs examined in the short term and none by more than one study (FA: n = 2, ADC/MD: n = 2 studies). Above all, highlights the breadth of the existing research and available data—in terms of cognitive domains, ROIs, DTI measures (FA vs. ADC/MD) and timing (short vs. medium to long term)—which is at the expense of depth (overlap), consequently the current findings should be viewed as preliminary, providing an empirical basis by which to focus future research.
Table 2. Summary information relating to the cognitive domains and regions of interest assessed in the short and medium to long term.
Relationship between DTI and cognition after TBI
Overall, there were 31 noteworthy findings across a range of cognitive functions, as indicated by moderate-to-large (r < −.3 or r > .3) and significant (p < .05) correlations that were unlikely to be undermined by any publication bias (Nfs > Nstudies). – summarize these findings, with Tables F and G in the supplementary material documenting the complete set of findings, including the smaller effect sizes (r > −.3 to r < .3). With only two exceptions (short-term FA: concept formation and corpus callosum [body]; short-term ADC: memory and left cingulate; see ), the direction of the relationship between FA or ADC/MD and cognition for the various ROIs (–)—in both the short and medium to long term—was consistent with expectations (short term: negative r between FA and cognition, positive r between ADC and cognition; medium to long term: positive r between FA and cognition, negative r between ADC/MD and cognition). Moreover, in total, there were 64 medium to large effects, 61 of which were in the predicted direction. However, only 48% were statistically significant, probably due to the small sample sizes.
Table 3. Pearson r effect sizes (moderate-to-large only) measuring the relationship between FA/ADC and cognitive function in specific ROI in the short term (<4 weeks) post-TBI.a
Table 4. Pearson r effect sizes (moderate-to-large only) measuring the relationship between FA and cognitive function in specific ROI in the medium to long term (>4 weeks) post-TBI.a
Table 5. Pearson r effect sizes (moderate-to-large only) measuring the relationship between ADC/MD and cognitive function in specific ROI in the medium to long term (>4 weeks) post-TBI.a
Given the large number of findings that needed to be consolidated—combined with the complexity caused by the assessment of FA or ADC/MD in both the short and medium to long term, and the examination of nine different cognitive domains and 44 ROIs—only the most noteworthy (r < −.3 or r > .3, p < .05, Nfs > Nstudies) are summarized as follows:
Contrary to prediction, better concept formation was predicted by higher ADC/MD (short term) in the corpus callosum (body) ().
Better executive function was predicted by lower FA (short term) in the cerebral peduncle and internal capsule ().
Better memory was predicted by higher ADC (short term) in the left cingulate () and, higher FA (medium to long term) in the left cingulate and right uncinate fasciculus () and lower ADC/MD (medium to long term) in the left frontal lobe and left uncinate fasciculus ().
Better academic achievement was predicted by higher FA (medium to long term) of the corpus callosum (splenium, isthmus) ().
Better attention and information processing was predicted by higher FA (medium to long term) in the composite/whole brain, corpus callosum (total, splenium, isthmus) (); and lower ADC/MD (medium to long term) in the left frontal lobe and left cingulate ().
Better construction was predicted by higher FA (medium to long term) in the corpus callosum (genu-anterior) () and lower ADC/MD (medium to long term) in the frontal lobes (left and right) and corpus callosum (anterior) ().
Better general cognition was predicted by higher FA (medium to long term) of the corpus callosum (isthmus) ().
Better motor function was predicted by higher FA (medium to long term) in the optic radiation, cortico-spinal tract, posterior thalamic radiation, composite whole brain, medial lemniscus, cerebellum, superior peduncle, corpus callosum (splenium, isthmus, and body) ().
To summarize, in the short term, contrary to predictions, better concept formation was significantly related with higher FA in the corpus callosum (body). However, consistent with predictions, better executive function was significantly related with lower FA in two white matter tracts—the cerebral peduncle and internal capsule. Noteworthy findings for ADC in the short term were also contrary to prediction, with better memory being related to higher ADC in the left cingulate.
In the medium to long term, better cognitive functioning was consistently related to higher FA values. More specifically, attention/information processing, academic achievement, construction, and general cognition were linked to higher FA values in the corpus callosum. Memory functions were more related to other large white matter tracts, such as the cingulate and uncinate fasciculus, and attention/information processing was additionally related to composite/whole brain FA. In addition, motor functions were consistently related to higher FA values across a range of large white matter tracts and the composite/whole brain.
Finally, in the medium to long term, better cognitive function was related to lower ADC/MD values in several ROIs. More particularly, attention/information processing, construction and memory were all related to ADC/MD in the frontal lobes; construction was related to ADC/MD in the corpus callosum; and memory was related to ADC/MD in the cingulate.
Discussion
Ashwal, Tong, Obenaus, and Holshouser (Citation2010) have suggested that newer imaging techniques, such as DTI, may provide a more sensitive marker of white matter injury than conventional MRI and CT; proposing that they may also better predict cognitive impairments following TBI. This study is the first to systematically synthesize the data from studies that have examined the relationship between DTI findings and tests of cognitive function in children with TBI in order to evaluate the predictive value of DTI. It is also the first study to examine the extent to which these changes vary between the short and medium to long term.
We analyzed data from 14 studies that correlated FA or ADC/MD with measures of cognitive functioning; the latter being grouped into one of nine broad cognitive domains (general cognition, verbal functions/language skills, executive, concept formation and reasoning, construction, motor function, memory, attention/information processing, academic achievement). In all, the data were collected from 358 participants (229 males, 112 females), with a mean age of 13.5 years (SD = 1.9).
One of the most striking findings of this meta-analysis is that there was very limited overlap in the cognitive domains and brain regions (ROIs) that were examined by these 14 studies. In addition, most studies focused on the medium to long term after a TBI, with only three studies assessing the relationship between DTI and cognition performed in the short term. There was also significant heterogeneity in terms of the severity of brain injury, with most studies including participants that had mild or moderate to severe TBIs, without providing data for these subgroups. This variation, combined with the small samples, suggests that the current findings should be viewed as preliminary. Arguably, we can place the greatest confidence in those correlations (between FA or ADC/MD and cognitive functioning) that were medium to large in size (r < −.3 or >.3), statistically significant (p < .05) and had acceptable Nfs statistics (Nfs > Nstudies); consequently the discussion will be confined to these findings.
In the first 4 weeks (short term) after a TBI, the findings were not consistent, with both positive and negative relationships reported between FA and cognitive function. In the short term after TBI, and contrary to prediction, we found a strong positive relationship between FA in the corpus callosum (body) and concept formation, with the relationship accounting for over 56% of the variance. This finding contrasted with the large negative relationship between FA in both the cerebral peduncle and internal capsule and executive function, which accounted for between 30% and 36% of the variance. It is not clear why the direction of these relationships differ, but it may be related to the fact that the contributing study (Mayer et al., Citation2012) examined children with mild TBIs 2 weeks post-injury; at which time there may be some early resolution of cognitive and physiological changes (Hung et al., Citation2014). This is also consistent with the finding by this same study that their TBI and healthy controls performed comparably in these cognitive domains. However, it is equally important to note that these results were based on a single small-scale study (N = 16); consequently, they await replication.
There were also inconsistencies in the relationship between cognition and ADC/MD in the short term. Although memory was positively related to ADC of the left cingulate, as predicted, all other correlations between cognition and ADC were negative. Once again, these differences may reflect the timing of the DTI, with the former resulting from imaging performed on a mild TBI sample after a mean of three days post-injury (range 1–6 days) (Wu, Wilde, Bigler, Yallampalli, et al., Citation2010) and the latter being from a mild, moderate and severe TBI sample that was scanned after a mean of 6 days (range 1–16 days) (Babikian et al., Citation2009).
Thus, the findings from DTI performed within the first 4 weeks after a TBI are mixed and suggest that additional data are needed to draw firm conclusions about the direction of the relationship between DTI and cognition. Specifically, a finer-grained analysis of the changes that occur during this first month (i.e., comparing acute and post-acute) is now needed, ideally using data from studies that have performed DTI on multiple occasions with the same sample. Based on the available data, the corpus callosum, cerebral peduncle, and internal capsule would appear to be the most promising white matter tracts for continued research, as are complex cognitive functions, such as concept formation and executive functioning.
Turning to the medium to long term, there were many more studies that examined the relationship between DTI and cognitive functioning in the months and years after pediatric TBI. Consistent with this, more regions and cognitive domains were examined, but the findings consistently showed that higher FA and lower ADC/MD was associated with better cognitive performance. In fact, there were medium to large and significant correlations between FA or ADC/MD and most cognitive domains. Specifically, cognition (academic achievement, attention/information processing, construction, general cognition, memory) was positively related with FA in one or more of the following regions: the whole brain, corpus callosum, uncinate fasciculus, and cingulate. Thus, better cognition was related to higher FA values, reflecting more uniform water diffusion due to intact white matter tracts. Moreover, better fine motor functioning was related to higher FA in a large number of regions, including the whole brain composite, which is consistent with Wilde, Hunter and Bigler’s (Citation2012) suggestion that DTI measures of global white matter may provide the best predictor of cognitive outcomes. However, it is unclear whether this holds true for the other cognitive outcomes, as composite brain measures were only reported for attention/information processing and motor function.
Although the findings consistently indicate that there is a positive relationship between cognitive functioning and intact white matter (higher FA) post-TBI, it is important to note that there were only three instances where more than two studies, with over 100 participants (n = 141–164), examined the same cognitive function and ROI (attention/information processing and the splenium, body and genu of the corpus callosum). Thus, given the limited overlap between studies, our conclusions are largely based on data obtained from one to two studies and, frequently, small samples (N = 9–56).
Finally, many fewer studies examined the relationship between ADC/MD and cognition in the medium to long term (N = 3), with the most noteworthy findings consistently indicating a negative relationship. That is, consistent with predictions, lower ADC/MD values in the frontal lobe, corpus callosum and cingulate—reflecting less diffusion and intact fibers—predicted better cognitive function. In particular, medium to large and significant relationships were found between: attention/information processing and ADC/MD in both the left frontal lobe and left cingulate; construction and ADC/MD in both frontal lobes and the anterior corpus callosum; and memory and the left frontal lobe and left uncinate fasciculus. However, caution is again warranted as the findings were largely based on single studies and small samples. In particular, the relationship between attention/information processing and cognitive function was based on a single small study (N = 6, Wilde et al., Citation2011); consequently, it needs to be replicated.
Limitations of the study
First, as with all meta-analyses, it is possible that there are studies that were not captured by the current searches. Every attempt was made to minimize the likelihood of this happening by conducting broad searches of seven different data-bases and by calculating Nfs statistics, which effectively determine how many “missing” studies (either due to publication bias or failures in the search procedures) would be needed to render a finding inconsequential. Although we did not correct our p values to take into account multiple analyses, we focused solely on moderate to large effects in order to reduce the likelihood of false positives. Second, of the studies that were identified, some failed to provide the required data—either in the original publication or upon written request—and were therefore necessarily excluded from this analysis; thereby limiting the final sample.
Third, although STROBE ratings of study reporting quality indicated that many items were reported to an acceptable standard, researchers consistently failed to report a number of aspects of their study design, including participant selection, making it difficult to determine whether the children who were included in this meta-analysis were broadly representative of children who sustain TBIs. Clear details relating to the study recruitment processes and participant retention rates are recommended to address this limitation. Similarly, details of missing data and how these were managed should also be included because it is likely that children who were unable to complete the study were either more severely injured or had pre-injury behavioral or cognitive difficulties, potentially leading to a biased sample.
Fourth, studies varied in the pre-processing of data, the software packages that were used to analyse the DTI data, and how ROIs were derived. Different approaches to processing and correction—including motion correction, which is a particular issue with pediatric participants—can affect the FA and MD/ADC data, and may have contributed to the heterogeneous findings (Jones & Cercignani, Citation2010). Unfortunately, there was insufficient data to analyze the impact of these variables on our findings, but these are issues that warrant consideration and highlight the need for studies to provide this information.
Finally, the studies examined many different brain regions and used a wide variety of different tests to assess a range of cognitive functions/domains. This meant that, although covering a broad area, there was limited overlap/depth in the research findings. This, in turn, highlights the need for research that uses common measures of cognitive functioning and assesses key ROIs using DTI (e.g., corpus callosum, cingulate, uncinate fasciculus, frontal lobe, whole brain); consistent with a recent recommendation to use common outcome measures in pediatric TBI research (McCauley et al., Citation2012).
Implications for practice and research
A recent meta-analysis, which compared the DTI findings of children who had sustained a TBI with healthy controls, identified substantial decreases in FA and/or increases in ADC/MD in the medium to long term following TBI; particularly in the corpus callosum, internal capsule, uncinate fasciculus, longitudinal fasciculus and cingulate, as well as in the frontal, and temporal lobes (Roberts et al., 2014). With two exceptions (longitudinal fasciculus, temporal lobes), the current study found that these same ROIs were all related to cognition in the medium- to long- term, suggesting that not only are these regions most affected by TBI, but they are also related to cognitive functioning. Unfortunately, the existing research is largely cross-sectional, with DTI and cognitive function assessed contemporaneously.
Clinically, of greatest interest is whether DTI obtained in the acute period predicts longer-term cognition and recovery. Overall, the current findings suggest that there is likely to be significant clinical value in using DTI to predict cognitive functioning, particularly in the medium to long term post-TBI (>4 weeks), by which time there is a consistent relationship between cognitive functioning and FA and ADC/MD values in numerous large white matter tracts, as well as the whole brain. Longitudinal research is therefore now needed to examine whether DTI successfully predicts later cognitive functioning, using those ROIs that the current meta-analysis identified to focus this work. This research should employ designated cognitive tests (McCauley et al., Citation2012) and focus on those ROIs that show the greatest changes in DTI metrics after pediatric TBI and are also related to cognition when assessed contemporaneously (corpus callosum, internal capsule, uncinate fasciculus, longitudinal fasciculus, cingulate, frontal lobe; Roberts et al., 2014).
In addition, age and severity of injury should be considered in order to advance our understanding of how these variables impact on DTI and the relationship between DTI and cognition. In the case of injury severity, preliminary data suggest that the relationship between DTI and cognition may be weak following mild TBI in children (Mayer et al., Citation2012). This is broadly consistent with the findings of a recent study of adults, which reported that early white matter changes following mild TBI were no longer evident at 90 days (Narayana et al., Citation2015), suggesting that injury severity and timing of the DTI may be important variables to consider.
Conclusions
FA and ADC/MD measures obtained in the medium to long term consistently predict cognitive outcomes assessed contemporaneously following pediatric TBI. Lower FA and higher ADC/MD in the medium to long term—both of which are thought to indicate decreased white matter integrity—predicted poorer performance on a wide range of cognitive functions. DTI of the large white matter tracts—such as the corpus callosum, uncinate fasciculus, and cingulate—and the brain, as a whole, were most predictive of cognitive functioning when using FA. Similarly, DTI of the frontal lobe, uncinate fasciculus, cingulate and corpus callosum were most predictive of cognitive outcomes when using ADC/MD. More research is needed to examine the relationship between DTI and cognition in the short term following pediatric TBI, and to evaluate the potential for DTI performed in the acute period to predict longer-term cognitive outcomes, if it is to be used for clinical purposes.
On-line Supplementary Materials
Download MS Word (69.2 KB)Supplemental material
Supplemental material for this article can be accessed at www.tandfonline.com/hdvn.
References
- References marked with an asterisk indicate studies included in the meta-analysis.
- *Adamson, C., Yuan, W., Babcock, L., Leach, J. L., Seal, M. L., Holland, S. K., & Wade, S. L. (2013). Diffusion tensor imaging detects white matter abnormalities and associated cognitive deficits in chronic adolescent TBI. Brain Injury, 27, 454–463. doi:10.3109/02699052.2012.750756
- Arfanakis, K., Haughton, V. M., Carew, J. D., Rogers, B. P., Dempsey, R. J., & Meyerands, M. E. (2002). Diffusion tensor MR imaging in diffuse axonal injury. American Journal of Neuroradiology, 23, 794–802.
- Ashwal, S., Tong, K. A., Obenaus, A., & Holshouser, B. A. (2010). Advanced neuroimaging techniques in children with traumatic brain injury. In V. Anderson & K. O. Yeates (Eds.), Pediatric traumatic brain injury: New frontiers in clinical and translational research (pp. 68–93). Cambridge, UK: Cambridge University Press.
- Assaf, Y., & Pasternak, O. (2008). Diffusion Tensor Imaging (DTI)-based white matter mapping in brain research: A review. Journal of Molecular Neuroscience, 34, 51–61. doi:10.1007/s12031-007-0029-0
- Babikian, T., & Asarnow, R. (2009). Neurocognitive outcomes and recovery after pediatric TBI: Meta-analytic review of the literature. Neuropsychology, 23, 283–296. doi:10.1037/a0015268
- *Babikian, T., Tong, K. A., Galloway, N. R., Freier-Randall, M. C., Obenaus, A., & Ashwal, S. (2009). Diffusion-weighted imaging predicts cognition in pediatric brain injury. Pediatric Neurology, 41, 406–412. doi:10.1016/j.pediatrneurol.2009.06.002
- Beauchamp, M. H., Beare, R., Ditchfield, M., Coleman, L., Babl, F. E., Kean, M., … Anderson, V. (2013). Susceptibility weighted imaging and its relationship to outcome after pediatric traumatic brain injury. Cortex, 49, 591–598. doi:10.1016/j.cortex.2012.08.015
- Beaulieu, C. (2002). The basis of anisotropic water diffusion in the nervous system—A technical review. NRM in Biomedicine, 15, 435–455. doi:10.1002/nbm.782
- Bigler, E. D., & Bazarian, J. J. (2010). Diffusion tensor imaging: A biomarker for mild traumatic brain injury? Neurology, 74, 626–627. doi:10.1212/WNL.0b013e3181d3e43a
- Borenstein, M., Hedges, L., Higgins, J., & Rothstein, H. (2005). Comprehensive meta-analysis (version 3.3.070). Englewood, NJ: Biostat.
- Borenstein, M., Hedges, L. V., Higgins, J. P. T., & Rothstein, H. R. (2009). Introduction to meta-analysis. Chichester, UK: John Wiley & Sons.
- *Caeyenberghs, K., Leemans, A., Geurts, M., Linden, C. V., Smits-Engelsman, B. C. M., Sunaert, S., & Swinnen, S. P. (2011). Correlations between white matter integrity and motor function in traumatic brain injury patients. Neurorehabilitation and Neural Repair, 25, 492–502. doi:10.1177/1545968310394870
- *Caeyenberghs, K., Leemans, A., Geurts, M., Taymans, T., Linden, C. V., Smits-Engelsman, B. C. M., … Swinnen, S. P. (2010a). Brain-behavior relationships in young traumatic brain injury patients: DTI metrics are highly correlated with postural control. Human Brain Mapping, 31, 992–1002. doi:10.1002/hbm.20911
- *Caeyenberghs, K., Leemans, A., Geurts, M., Taymans, T., Linden, C. V., Smits-Engelsman, B. C. M., … Swinnen, S. P. (2010b). Brain-behavior relationships in young traumatic brain injury patients: Fractional anisotropy measures are highly correlated with dynamic visuomotor tracking performance. Neuropsychologia, 48, 1472–1482. doi:10.1016/j.neuropsychologia.2010.01.017
- Cohen, J. (1992). A power primer. Psychological Bulletin, 112, 155–159. doi:10.1037/0033-2909.112.1.155
- Duckworth, J. L., & Stevens, R. D. (2010). Imaging brain trauma. Current Opinion in Critical Care, 16, 92–97. doi:10.1097/MCC.0b013e3283374900
- *Ewing-Cobbs, L., Prasad, M. R., Swank, P., Kramer, L., Cox, C. S., Jr., Fletcher, J. M., … Hasan, K. M. (2008). Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: Relation to neurobehavioral outcomes. Neuroimage, 42, 1305–1315. doi:10.1016/j.neuroimage.2008.06.031
- Faul, M., Xu, L., Wald, M. M., & Coronado, V. G. (2010). Traumatic brain injury in the United States: Emergency Department visits, hopitalizations and deaths 2002–2006. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control.
- Gardner, A., Kay-Lambkin, F., Stanwell, P., Donnelly, J., Williams, W. H., Hiles, A.,…Jones, D. K. (2012). A systematic review of diffusion tensor imaging findings in sports-related concussion. Journal of Neurotrauma, 29(16), 2521–2538. doi:10.1089/neu.2012.2628
- Hedges, V., & Olkin, I. (1985). Statistical methods for meta-analysis. Orlando, FL: Academic Press.
- Hessen, E. (2010). Very long-term neuropsychological and behavioral consequences of mild and complicated mild TBI: Increased impact of pediatric versus adult TBI. In V. Anderson & K. O. Yeates (Eds.), Pediatric traumatic brain injury: New frontiers in clinical and translational research (pp. 118–144). Cambridge, UK: Cambridge University Press.
- Hulkower, M. B., Poliak, D. B., Rosenbaum, S. B., Zimmerman, M. E., & Lipton, M. L. (2013). A decade of DTI in traumatic brain injury: 10 years and 100 articles later. American Journal of Neuroradiology, 34, 2064–2074. doi:10.3174/ajnr.A3395
- Hung, R., Carroll, L. J., Cancelliere, C., Côté, P., Rumney, P., Keightley, M., … Cassidy, J. D. (2014). Systematic review of the clinical course, natural history, and prognosis for pediatric mild traumatic brain injury: Results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Archives of Physical Medicine and Rehabilitation, 95, S174–191. doi:10.1016/j.apmr.2013.08.301
- Jones, D. K., & Cercignani, M. (2010). Twenty-five pitfalls in the analysis of diffusion MRI data. NMR in Biomedicine, 23, 803–820. doi:10.1002/nbm.1543
- *Kurowski, B., Wade, S. L., Cecil, K. M., Walz, N. C., Yuan, W., Rajagopal, A., . . . Holland, S. K. (2009). Correlation of diffusion tensor imaging with executive function measures after early childhood traumatic brain injury. Journal of Pediatric Rehabilitation Medicine, 2, 273–283. doi:10.3233/PRM-2009-0093
- Lebel, C., Shaywitz, B., Holahan, J., Shaywitz, S., Marchione, K., & Beaulieu, C. (2013). Diffusion tensor imaging correlates of reading ability in dysfluent and non-impaired readers. Brain & Language, 125, 215–222. doi:10.1016/j.bandl.2012.10.009
- Lee, B., & Newberg, A. (2005). Neuroimaging in traumatic brain imaging. NeuroRx, 2, 372–383. doi:10.1602/neurorx.2.2.372
- *Levin, H. S., Wilde, E. A., Chu, Z., Yallampalli, R., Hanten, G. R., Li, X., … Hunter, J. V. (2008). Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. Journal of Head Trauma Rehabilitation, 23, 197–208. doi:10.1097/01.HTR.0000327252.54128.7c
- Lezak, M. D., Howieson, D. B., Bigler, E. D., & Tranel, D. (2012). Neuropsychological assessment (5th ed.). New York, NY: Oxford University Press.
- Liégeois, F. J., Mahony, K., Connelly, A., Pigdon, L., Tournier, J., & Morgan, A. T. (2013). Pediatric traumatic brain injury: Language outcomes and their relationship to the arcuate fasciculus. Brain and Language, 127, 388–398. doi:10.1016/j.bandl.2013.05.003
- Lipsey, M. W., & Wilson, D. B. (2001). Practical meta-analysis. Thousand Oaks, CA: Sage.
- *Mayer, A. R., Ling, J. M., Yang, Z., Pena, A., Yeo, R. A., & Klimaj, S. (2012). Diffusion abnormalities in pediatric mild traumatic brain injury. Journal of Neuroscience, 32, 17961–17969. doi:10.1523/JNEUROSCI.3379-12.2012
- McCauley, S. R., Wilde, E. A., Anderson, V. A., Bedell, G., Beers, S. R., Campbell, T. F., … Yeates, K. O. (2012). Recommendations for the use of common outcome measures in pediatric traumatic brain injury research. Journal of Neurotrauma, 29, 678–705. doi:10.1089/neu.2011.1838
- *McCauley, S. R., Wilde, E. A., Bigler, E. D., Chu, Z., Yallampalli, R., Oni, M. B., … Levin, H. S. (2011). Diffusion tensor imaging of incentive effects in prospective memory after pediatric traumatic brain injury. Journal of Neurotrauma, 28, 503–516. doi:10.1089/neu.2010.1555
- Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & The PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Medicine, 6, e1000097. doi:10.1371/journal.pmed.1000097
- Mueller, B. A., Lim, K. O., Hemmy, L., & Camchong, J. (2015). Diffusion MRI and its role in neuropsychology. Neuropsychology Review, 25, 250–271. doi:10.1007/s11065-015-9291-z
- Narayana, P. A., Yu, X., Hasan, K. M., Wilde, E. A., Levin, H. S., Hunter, J. V., … McCarthy, J. J. (2015). Multi-modal MRI of mild traumatic brain injury. NeuroImage: Clinical, 7, 87–97. doi:10.1016/j.nicl.2014.07.010
- Niogi, S. N., & Mukherjee, P. (2010). Diffusion Tensor Imaging of mild traumatic brain injury. Journal of Head Trauma Rehabilitation, 25, 241–255. doi:10.1097/HTR.0b013e3181e52c2a
- Orwin, R. G. (1983). A fail-safe N for effect size in meta-analysis. Journal of Educational Statistics, 8, 157–159. doi:10.2307/1164923
- Pinto, P. S., Meoded, A., Poretti, A., Tekes, A., & Huisman, T. A. G. M. (2012). The unique features of traumatic brain injury in children. Review of the characteristics of the pediatric skull and brain, mechanisms of trauma, patterns of injury, complications, and their imaging findings—Part 1. Journal of Neuroimaging, 22, e1–e17. doi:10.1111/j.1552-6569.2011.00688.x
- Pinto, P. S., Poretti, A., Meoded, A., Tekes, A., & Huisman, T. A. G. M. (2012). The unique features of traumatic brain injury in children. Review of the characteristics of the pediatric skull and brain, mechanisms of trauma, patterns of injury, complications, and their imaging findings- Part 2. Journal of Neuroimaging, 22, e18–e41. doi:10.1111/j.1552-6569.2011.00690.x
- Roberts, R. M., Mathias, J. L., & Rose, S. E. (2014). Diffusion tensor imaging (DTI) findings following pediatric non-penetrating TBI: A meta-analysis. Developmental Neuropsychology, 39(8), 600–637. doi:10.1080/87565641.2014.973958
- *Tasker, R. C., Westland, A. G., White, D. K., & Williams, G. B. (2010). Corpus callosum and inferior forebrain white matter microstructure are related to functional outcome from raised intracranial pressure in child traumatic brain injury. Developmental Neuroscience, 32, 374–384. doi:10.1159/000316806
- *Treble, A., Hasan, K. M., Iftikhar, A., Stuebing, K. K., Kramer, L. A., Cox, C. S., … Ewing-Cobbs, L. (2013). Working memory and corpus callosum microstructural integrity after pediatric traumatic brain injury: A diffusion tensor tractography study. Journal of Neurotrauma, 30, 1609–1619. doi:10.1089/neu.2013.2934
- Vandenbroucke, J. P., von Elm, E., Altman, D. G., Gøtzche, P. C., Mulrow, C. D., Pocock, S. J., … Egger, M.; for the STROBE Initiative. (2007). Strengthening the reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Medicine, 4, e297. doi:10.1371/journal.pmed.0040297
- Wilde, E. A., Chu, Z., Bigler, E. D., Hunter, J. V., Fearing, M. A., Hanten, G.,…Levin, H. S. (2006). Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. Journal of Neurotrauma, 23(10), 1412–1426. doi:10.1089/neu.2006.23.1412
- Wilde, E. A., Hunter, J. V., & Bigler, E. D. (2012). A primer of neuroimaging analysis in neruorehabilitation outcome research. NeuroRehabilitation, 31, 227–243. doi:10.3233/NRE-2012-0793
- *Wilde, E. A., Newsome, M. R., Bigler, E. D., Pertab, J., Merkley, T. L., Hanten, G., … Levin, H. S. (2011). Brain imaging correlates of verbal working memory in children following traumatic brain injury. International Journal of Psychophysiology, 82, 86–96. doi:10.1016/j.ijpsycho.2011.04.006
- Wilde, E. A., Ramos, M. A., Yallampalli, R., Bigler, E. D., McCauley, S. R., Chu, Z., … Levin, H. S. (2010). Diffusion tensor imaging of the cingulum bundle in children after traumatic brain injury. Developmental Neuropsychology, 35, 333–351. doi:10.1080/87565641003696940
- *Wozniak, J. R., Krach, L., Ward, E., Mueller, B. A., Muetzel, R., Schnoebelen, S., … Lim, K. (2007). Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: A diffusion tensor imaging (DTI) study. Archives of Clinical Neuropsychology, 22, 555–568. doi:10.1016/j.acn.2007.03.004
- *Wu, T. C., Wilde, E. A., Bigler, E. D., Li, X., Merkley, T. L., Yallampalli, R., et al. (2010). Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Developmental Neuroscience, 32, 361–373. doi:10.1159/000317058
- *Wu, T. C., Wilde, E. A., Bigler, E. D., Yallampalli, R., McCauley, S. R., Troyanskaya, M., … Levin, H. S. (2010). Evaluating the relationship between memory functioning and cingulum bundles in acute mild traumatic brain injury using diffusion tensor imaging. Journal of Neurotrauma, 27, 303–307. doi:10.1089/neu.2009.1110
- Yanagihara, T. K., & Wang, T. J. C. (2014). Diffusion-weighted imaging of the brain for glioblastoma: Implications for radiation oncology. Applied Radiation Oncology, Dec., 5–13.
Appendix: Logic grids used in database searches
Databases to search: PubMed, PsycINFO, Scopus, Embase, Web of Science, CINAHL, Informit
Table A1. Logic grid for PubMed.
Table A2. Logic grid for PsycINFO.
Table A3. Logic grid for Scopus.
Table A4. Logic grid for Embase.
Table A5. Logic grid for Web of Science.
Table A6. Logic grid for CINAHL.
Table A7. Logic grid for Informit (health databases).