Abstract
We have prepared human erythrocytes that contain exclusively α-nitrosyl hemoglobin (Hb), i.e., α(Fe-NO)2β(Fe-O2)2, by incorporating nitric oxide (NO) into erythrocytes in a well-controlled nitrosylation process. The amount of α(Fe-NO) corresponding to 50% of the total heme content of the erythrocytes and exclusive binding of NO to α-subunits of intraerythrocytic Hb were confirmed by EPR. Oxygenation experiments on the intraerythrocytic α-nitrosyl Hb over a wide range of pH showed that: (1) the oxygen affinity of cell-free and intraerythrocytic α-nitrosyl Hbs were much lower than native Hb in their respective environments; (2) the oxygenation characteristics of the intraerythrocytic α-nitrosyl Hb in the acidic range was similar to that of the cell-free α-nitrosyl Hb in the presence of 2,3-diphosphoglycerate; and (3) the apparent Bohr effect in the intraerythrocytic α-nitrosyl Hb was dramatically diminished. This can be due to a restricted variation in intraerythrocytic pH in the alkaline region and the presence and/or production of endogenous 2,3-diphosphoglycerate. By comparing oxygen saturation characteristics, it was found that the intraerythrocytic α-nitrosyl Hb, despite its halved oxygen carrying capacity, could deliver more oxygen than DPG-depleted erythrocytes under similar experimental conditions. This makes α-nitrosyl Hb-containing erythrocytes a promising candidate for blood transfusant.
| Abbreviations: | ||
| Hb | = | human adult hemoglobin; |
| NO | = | nitric oxide |
| DPG | = | 2,3-diphosphoglycerate. |
INTRODUCTION
In a previous study Citation[[1]], it has been demonstrated that α-nitrosyl hemoglobin (Hb), in which the two α-subunits are ligated with NO, despite the fact of being a half-ligated species is capable of binding oxygen reversibly to its α-subunits and yet shifting its quaternary conformation equilibrium between “R” and “T”-extreme. The latter is characterized by a dramatically decreased affinity for oxygen, lack of cooperativity, and virtually no response to organic phosphates, such as DPG or inositol hexaphosphate, when exposed to acidic pH conditions Citation[[1]]. The origin of this peculiar behavior was attributed to the NO-induced cleavage of the coordination bond between the NO-bound heme iron and the proximal His in the α-subunits Citation[[1]] We were, thence, interested in studying the oxygenation properties of this nitrosyl Hb derivative in its natural milieu, that is, inside the erythrocyte. To achieve this, it was necessary to complete the nitrosylation of exclusively the α-subunits of Hb within intact erythrocytes. In this paper we report the preparation protocol and oxygen binding characteristics of intraerythrocytic α-nitrosyl Hb, and its potential viability as a blood substitute.
MATERIALS AND METHODS
All reagents were of analytical grade.
Preparation of α-Nitrosyl Hb Within Intact Erythrocytes
Fresh blood (10 ml) was obtained by venipuncture using blood collection tubes containing EDTA as anticoagulant. After thorough mixing, the blood sample was resuspended in a 2-fold volume of chilled sucrose isotonic solution. This solution contained 250 mM sucrose, 5 mM KCl, 2 mM Na2PO4, 1 mM MgCl2·6H2O, and 10 mM glucose Citation[[2]] The mixture was centrifuged at 3,000 rpm for 10 min at 4°C. The supernatant and the buffy coat of white cells were then removed and discarded. The loosely packed erythrocytes were resuspended in the same isotonic solution and the procedure was repeated two to three more times. The final concentration of hemoglobin in the loosely packed erythrocytes is ca. 20 mM heme (or 5 mM tetrameric hemoglobin)
The washed erythrocytes were resuspended in a 2-fold volume of 0.15 M sodium-potassium phosphate buffer, pH 5.5, containing 10 mM glucose. The phosphate buffer was prepared by mixing 0.15 M Na2HPO4 and 0.15 M KH2PO4 to adjust the pH to 5.5. The red cell suspension was then transferred to a 300-ml Kjeldahl type flask with a rubber stopper to which two stainless-steel needles were previously inserted, one serving as a gas inlet and the other as gas outlet. Each of the two needles has attached a three-way stopcock. Deoxygenation of the erythrocyte suspension was achieved by flowing pure argon or nitrogen (grade 5). This process was carried out at room temperature with continuous but gentle stirring of the erythrocyte suspension. The progress of deoxygenation was followed by closely observing the change in color of the suspension. Once the internal oxygen pressure within the flask reached low levels, a solution containing sodium dithionite was added in a 2-fold excess in concentration over heme. This solution was prepared by dissolving sodium dithionite as 50 mg per ml of deaerated distilled water. Deaerated distilled water was conveniently prepared by bubbling pure argon or nitrogen into a 2-ml glass vial sealed with a silicone-rubber septum. After applying a slightly positive gas pressure into the vial, the appropriate volume of deaerated distilled water was removed with a gas-tight syringe by piercing the septum with a needle and then was injected into another vial previously filled with nitrogen or argon containing sodium dithionite. Once the appropriate volume of sodium dithionite solution was injected into the flask, the erythrocyte suspension was stirred gently and let stand on ice for about 10 minutes.
A 50 mg per ml of sodium nitrite solution was prepared freshly likewise the solution of sodium dithionite. An amount of 50% of nitrite equivalents with respect to the total equivalents of heme in the suspension was injected very slowly using a gas-tight syringe with a needle. Sodium nitrite reacts stoichiometrically and progressively with sodium dithionite to form nitric oxide, which binds to heme groups of hemoglobin. Since the isotonic buffer of the suspension was acidic (pH 5.5), the formation of α-nitrosyl form of hemoglobin derivative is promoted with time. Peroxide formation that eventually can interfere with following reactions was avoided by the previous removal of oxygen from the suspension prior the addition of sodium dithionite. Addition of large amounts of sodium nitrite into the suspension at once was avoided because the formation of fully nitrosylated Hb hampered the redistribution of NO towards the α-subunits. After allowing the reaction to proceed for several hours, the suspension was then quickly washed three times under anaerobic conditions with deaerated sucrose isotonic solution to remove excess of reagents and by-products. The thus obtained packed erythrocytes contained exclusively α-nitrosyl Hb, i.e., nitric oxide exclusively bound to heme groups of its α-subunits. Both concentration and nature of the nitrosyl derivative of Hb in the erythrocytes were determined by EPR.
Determination of Oxygen Equilibrium Curves
Oxygen binding to α-nitrosyl Hb containing erythrocytes were determined using an improved version of the Imai automatic device Citation[[3]], with specific modifications that allowed the spectroscopic monitoring of particulate samples such as suspension of erythrocytes. Light beam originating from a modified Hitachi 557 dual wavelength spectrophotometer (On-Line Instruments, Bogart, Georgia) was guided into the oxygenation cell using optical fiber. Scattered light was collected by a photomultiplier attached to the opposite side of the cell. Effective lightpath lengths were 6 mm. Changes in absorbance were monitored at 560 nm and the reference light was fixed at 497 nm. The oxygen concentration in the sample was monitored using an extra low-noise (S/N > 97 db) oxygen electrode (O2 Sensors, Malvern, Pennsylvania). The oxygen signal was then conditioned by a log amplifier (Current Designs, Philadelphia, Pennsylvania). Absorbance and oxygen concentration data were taken on-line and stored onto floppy disks for later processing. Measurements were carried out by suspending an equivalent of 60 μM heme of untreated erythrocytes or 120 μM heme of α-nitrosyl Hb erythrocytes, in 0.15 M sodium-potassium phosphate buffer at various pH values, 15°C. It should be noted that the intraerythrocytic concentration of Hb remained constant at ca. 20 mM heme.
EPR Spectroscopy
Total heme content in erythrocyte samples was calculated by EPR based on the intensity of iron-nitrosyl signal after full saturation of hemes with NO. This was achieved by suspending the erythrocytes in the appropriate buffer (usually, 100 mM bis-Tris propane, pH 7.4) and transferring to 3-mm precision-bore quartz EPR tubes using a syringe needle. A freezing/thawing cycle was repeated twice to assure complete cell breakage, followed by reduction with a small amount of sodium dithionite and addition of S-nitroso glutathione (NO donor), in slight excess. Intraerythrocytic α-nitrosyl Hb was determined without additional treatment. The intensity and shape of the hyperfine triplet around g = 2 was checked to assure that only α-subunits were ligated with NO, as previously discussed in Citation[[1]]. All measurements were carried out on a Varian X-band EPR spectrophotometer, model E109 (Palo Alto, California), integrated with a data acquisition system (Scientific Software Services, Normal, Illinois).
RESULTS
The decay in the concentration of α-nitrosyl Hb over time at different temperatures was investigated by measuring the decrease of the nitrosyl-heme EPR signal. It was found that the half-life times for the aerobic oxidation of α-nitrosyl Hb in solution at 15°C and 37°C were 7 h and 22 min, respectively. Corresponding values for normal Hb within erythrocytes could not be estimated because of the intraerythrocytic activity of reductase systems. It was concluded that at 15°C the amount of decomposed α-nitrosyl Hb produced for the extent of normal oxygenation experiments (about 1 h) was negligible and therefore the sample could be considered then relatively stable at this temperature. In consequence, all measurements were carried out at 15°C.
Oxygen equilibrium curves of normal and α-nitrosyl Hb-containing erythrocytes were determined within pH 5.8 and 8.2 and some representative results are shown in . Compared to untreated erythrocytes, intraerythrocytic α-nitrosyl Hb showed a decreased oxygen affinity and cooperativity. In fact, oxygen affinity of the α-nitrosyl Hb erythrocytes was comparable to early stages of ligation of untreated erythrocytes, and apparently was not much affected by pH. This is in contrast to what was observed in the case of α-nitrosyl Hb in solution Citation[[1]], as the oxygen affinity of the α-nitrosyl Hb in solution increased as pH was increased. In the case of intraerythrocytic α-nitrosyl Hb, the P50 values decrease only from 29.2 torr to 10.5 torr, despite an increase in pH from 5.8 to 8.2. Nevertheless, some cooperativity was observed within this pH range. Cooperativity index attained a peak at pH around 6.6 (nmax ∼ 1.3), and apparently corresponded to a transition between the curves obtained at both extreme pH values.
Figure 1. Hill plots for intraerythrocytic α-nitrosyl Hb (closed symbols) and untreated erythrocytes (open symbols) at pH 5.8 (A) and pH 7.4 (B). Experimental conditions were as follows: Treated and α-nitrosyl Hb-containing erythrocytes were suspended in 0.15 M sodium-potassium phosphate buffer of appropriate pH, in an amount equivalent to 60 μM heme (untreated erythrocytes) or 120 μM heme (α-nitrosyl Hb-containing erythrocytes). Temperature was 15°C. Deoxygenation was monitored using a dual wavelength spectrophotometer by tracing the optical absorbance increase at 577 nm referenced against 700 nm.
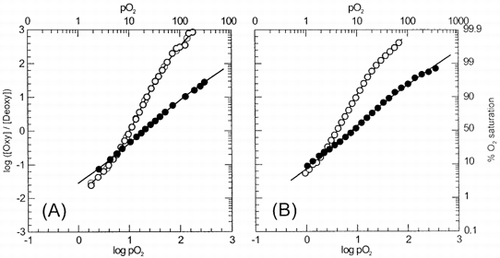
The Bohr effect of Hb derivatives is shown in . At pH 7 and below, intraerythrocytic α-nitrosyl Hb exhibited P50 values that were comparable to those for α-nitrosyl Hb in solution in the presence of DPG (data taken from Citation[[1]]). However, above neutral pH, the affinity for oxygen did not increase (i.e., no decrease in P50 value) as much as that observed in the case of α-nitrosyl Hb in the presence of DPG. The Bohr coefficient, ΔH+, determined as the change in oxygen affinity with pH variation around pH 7 for the intraerythrocytic α-nitrosyl Hb was only −.31 protons per binding site. This value is less than half of that determined for α-nitrosyl Hb in solution (−0.86) Citation[[1]]. The almost absence of change in oxygen affinity in the alkaline side indicates that the pH in the internal milieu of the erythrocyte remained practically invariable and was not affected by pH changes in the external milieu.
Figure 2. pH dependence of the oxygen affinity (as expressed by P50 values) of α-nitrosyl Hb and untreated Hb within erythrocytes (closed symbols) and in solution (open symbol). (•), intraerythrocytic α-nitrosyl Hb; (▪), untreated erythrocytes; (○), α-nitrosyl Hb (□), α- nitrosyl Hb + DPG, and native HbA (broken line). Compared to in solution conditions, Bohr effect is reduced in intraerythrocytic α-nitrosyl Hb (from –0.86 to –0.31 protons per binding site). Experimental conditions as in . Data for solution conditions were taken from Citation[[1]]
![Figure 2. pH dependence of the oxygen affinity (as expressed by P50 values) of α-nitrosyl Hb and untreated Hb within erythrocytes (closed symbols) and in solution (open symbol). (•), intraerythrocytic α-nitrosyl Hb; (▪), untreated erythrocytes; (○), α-nitrosyl Hb (□), α- nitrosyl Hb + DPG, and native HbA (broken line). Compared to in solution conditions, Bohr effect is reduced in intraerythrocytic α-nitrosyl Hb (from –0.86 to –0.31 protons per binding site). Experimental conditions as in FIGURE 1. Data for solution conditions were taken from Citation[[1]]](/cms/asset/3efbf5a6-6f58-4401-a5b6-38dc1d1bd2f8/ianb19_a_11116782_uf0002_b.gif)
DISCUSSION
Accurate determination of oxygen equilibrium curves of erythrocyte suspensions present several difficulties. One of these is the scattering of the incident light due to the particulate nature of the suspension. This technical problem was overcome in our system with the use of a dual wavelength spectrophotometer. By doing so, the noise due to light scattering was cancelled by referencing it to another light of different wavelength emerging from the same sample. On the other hand, the internal milieu in erythrocytes is variable and depends on the metabolic condition of the cells. It is well documented the fact that oxygen affinity of stored erythrocytes increase with time Citation[4-5]. This is due to a depletion in the intracellular content of DPG Citation[[6]].
It has been reported that exposure of erythrocytes to anaerobic conditions stimulates the synthesis of intracellular DPG Citation[[7]]. In the present study, we did not determine quantitatively whether new DPG was synthesized during the preparation of the sample or not. Nonetheless, some preliminary experiments indicated that erythrocytes previously incubated at physiological pH, under anaerobic conditions and in the presence of glucose showed an oxygenation curve shifted to the right with respect to the one taken prior deoxygenation. It is then likely that DPG synthesis was induced by removing oxygen from the sample. However, it is equally possible that if the intracellular pH was reduced as a consequence of the acidic conditions the erythrocytes were exposed during nitrosylation, or by formation of glycolysis catabolites as discussed below. On the other hand, the binding of already existent intracellular DPG to deoxyHb might have been enhanced. It is known that DPG exhibits a higher binding constant for deoxy over oxyHb Citation[[8]].
A low Bohr effect of the intraerythrocytic α-nitrosyl Hb might suggest how the intracellular enclosure provides Hb a constant environment. Although it was not determined, the intracellular pH seemed not to vary as much as the external milieu. Erythrocytes were equilibrated by repeated washing with the study isotonic buffer and then were suspended in a 50-fold volume of the same buffer for oxygenation measurements. This procedure could have been enough to change in some extent the intracellular pH, despite the high buffering capacity of Hb. Nevertheless, the oxygen binding behavior remained almost similar to that exhibited at pH 7, despite the fact that the pH of the extracellular medium was increased up to 8.2. It is known that there are several compensatory mechanisms by which the production of intracellular DPG can be regulated. Increase in intraerythocytic DPG levels induced by hypoxia and/or alkalosis have been reported Citation[[7]], Citation[[9]]. It is possible that as glycolytic pathways were activated inside erythrocytes, the internal milieu became acidic due to accumulation of lactate and pyruvate Citation[[10]].
During the preparation of intraerythrocytic α-nitrosyl Hb, no binding of NO to the β-subunits was observed when the erythrocytes were fresh. This is likely to be due to the presence of DPG within the cells, which promoted the binding of NO to α- rather than β-subunits. In addition, the oxygen binding characteristics of this derivative, such as reduced oxygen affinity and reduced cooperativity could have been caused by the presence of intracellular DPG, as revealed by the similarity of oxygen binding behavior of this derivative with α-nitrosyl Hb in the presence of DPG. It becomes clear that a variation in the intracellular content of DPG could alter dramatically the oxygenation properties of intraerythrocytic α-nitrosyl Hb, and thus affect the reproducibility of the oxygenation curves.
By placing Hb in an intracellular milieu, an efficient system that can reduce oxidized Hb (metHb) is also provided Citation[11-12]. As the oxygen tension decreases, the reductase systems within the erythrocytes become active. No metHb formation was detected during the preparation or measurement of samples. The presence of dithionite that was added to convert nitrite into NO by reduction could be also responsible for this. If some metHb was formed during the deoxygenation process, this might have been rapidly reduced. Reduction of nitrite by erythrocytes under anaerobic conditions and in the presence of methylene blue has been reported Citation[[13]]. Furthermore, in preliminary experiments (results not shown), we observed that α-15 NO Hb was formed inside erythrocytes when these were incubated in the presence of Na15NO2 under anaerobic conditions but in the absence of any additional reducing agent. EPR spectra revealed a double hyperfine structure around g = 2 that increased with time, indicating that the nitrogen of NO was indeed that provided as nitrite (common 14 NO will produced a typical triplet around the same g value). All these evidences seem to indicate that conditions can be maintained constant by placing Hb in an intracellular environment.
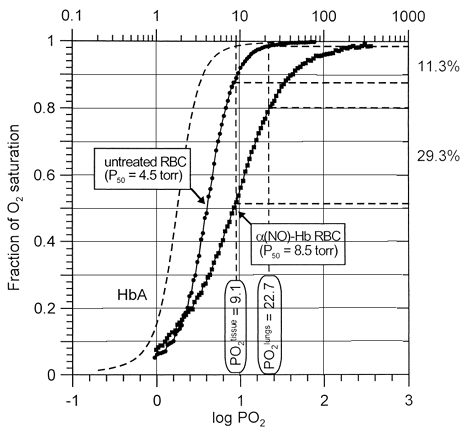
Typically each oxygen binding experiment takes about one hour to complete. Thus, measurements could not be conducted at 37°C without some sample decomposition taking place. For this reason, all measurements in this study were carried out at 15°C. Nevertheless, to obtain some physiological relevance from our data we could speculate about the oxygenation delivery performance of the α-nitrosyl Hb-containing erythrocytes. It is known that an increase in temperature will produce a shift to the right of the binding curves (approximately, P50 values double for an increment in temperature of 10°C Citation[[14]]. The oxygen delivery performances of untreated and NO-treated erythrocytes compared to that of free Hb are shown in . Accepted values for partial pressure of oxygen in lungs and tissue under physiological conditions are 100 and 40 torr, respectively Citation[[15]]. We could expect that hypothetical oxygen saturation curves maintain their relative shapes over temperature Citation[[8]]. Thus, these values after conversion to 15°C would be 22.7 and 9.1 torr, respectively. After depletion of intracellular DPG due to storage and aging, the oxygen delivery capacity of erythrocytes decreased to 11%. In contrast, the same erythrocytes after treatment with NO delivered about 29% of their total oxygen carrying capacity. In other words, the performance in oxygen delivery of α-nitrosyl Hb-contaning erythrocytes at pH 7.4 was more efficient than that of untreated DPG-depleted erythrocytes, notwithstanding the fact that the total carrying capacity in this Hb derivative was reduced by 50% (since both α-subunits are ligated with NO, only the β-subunits are able to carry oxygen. Thus, from the standpoint of oxygen delivery, erythrocytes containing α-nitrosyl Hb could become a viable substitute for normal blood transfusion. This procedure could be suitable for the rejuvenation of outdated blood, by simply altering the physicochemical properties of Hb in DPG-depleted erythrocytes to enhance the oxygen delivery capacity to tissues.
Figure 3. Oxygen delivery performance of untreated DPG-depleted (circles) and α-nitrosyl Hb-containing erythrocytes (squares) at pH 7.4 and 15°C, as revealed by comparing their oxygen dissociation curves. Normal Hb carries four molecules of oxygen per tetramer, whereas α-nitrosyll Hb half of this amount, since both the α-subunits are ligated with NO. While under normal conditions expired erythrocytes (2,3-DPG-depleted cells) unload about 11% of their total oxygen loading capacity going from lungs to tissues, α-nitrosyl Hb-containing erythrocytes deliver 29%. Data correspond to respective curves shown in .
ACKNOWLEDGMENTS
We are very much indebted to Prof. Cameron J. Koch (Department of Radiation Oncology, University of Pennsylvania) for donating the oxygen electrodes, and Mr. Ben Dugan for invaluable technical assistance on the implementation of the automatic oxygenation device. This work was supported by National Institutes of Health Grant HL 14508.
REFERENCES
- Yonetani T.Tsuneshige A., Zhou Y.-x., Chen X. Electron paramagnetic resonance and oxygen binding studies of alpha-nitrosyl hemoglobin. A novel oxygen carrier having NO-assisted allosteric functions. J. Biol. Chem 1998; 273: 20323–20333
- Tomoda A., Tsuda-Hirota S., Minakami S. Glycolysis of red cells suspended in solutions of impermeable solutes. Intracellular pH and glycolysis. J. Biochemistry 1977; 81: 697–701
- Imai K. Measurement of accurate oxygen equilibrium curves by an automatic oxygenation apparatus. Methods of Enzymology 1981; 76: 438–449, Academic Press, Ney York
- Valtis D. J., Kennedy A. C. Defective gas-transport function of stored red blood cells. The Lancet 1954; 1: 119–125
- Bunn H. F., May M. H., Kocholaty W., Shields C. E. Hemoglobin function in stored blood. J. Clin. Invest 1969; 48: 311–321
- Chanutin A. The effect of the addition of adenine and nucleosides at the beginning of storage on the concentrations of phosphates of human erythrocytes during storage in acid-citrate-dextrose and citrate-phosphate-dextrose. Transfusion 1967; 7: 120–132
- Oski F. A., Gottlieb A. J., Miller W. W., Delivoria-Papadopoulos M. The effects of deoxygenation of adult and fetal hemoglobin on the synthesis of red cell 2,3-diphosphoglycerate and its in vivo consequences. J. Clin. Invest 1970; 49: 400–407
- Imai K. Allosteric Effects in Haemoglobin. Cambridge Press, Cambridge 1982
- Duhm J., Gerlach E. On the mechanisms of the hypoxia-induced increase of 2,3-diphosphoglycerate in erythrocytes. Studies on rat erythrocytes in vivo and on human erythrocytes in vitro. Eur. J. Physiol. 1971; 326: 254–69
- Rörth M. Hemoglobin interactions and red cell metabolism. Ser. Haematol. 1972; 5: 1–104
- Gruener N., Cohen S. The reduction of methemoglobin in the human erythrocyte. Physiol. Chem. Phys 1973; 5: 375–380
- Kuma F. Properties of methemoglobin reductase and kinetic study of methemoglobin reduction. J. Biol. Chem 1981; 256: 5518–5523
- Kruszyna H.Kruszyna R.Smith R. P., Wilcox D. E. Red blood cells generate nitric oxide from directly acting, nitrogenous vasodilators. Toxicol. Appl. Pharmacol. 1987; 91: 429–438
- Bunn F. H., Forget B. G. Hemoglobin: Molecular, Genetic and Clinical Aspects. Saunders, Philadelphia 1986
- Ganong W. F. Review of Medical Physiology. Appleton & Lange, Norwalk, Conn. 1993