Abstract
Reactive partially reduced oxygen species such as superoxide anion (O2−), hydrogen peroxide (H2O2) and hydroxyl radical (OH) are produced in aerobically growing organisms during normal cellular respiration. To provide an effective defense against these reactive species, many aerobic organisms have evolved a multienzyme defense which includes superoxide dismutase, catalase and peroxidase. The superoxide anion may cause appreciable cellular damage by oxidizing aminoacids or by causing DNA strand breakage. Catalase was covalently immobilized on activated methoxypolyethyleneglycol-5000 and catalase and PEG-catalase were encapsulated in erythrocyte. Enzyme activity, encapsulation yield and hemograme analysis were determined for each sample. The erythrocyte shape of the samples were investigated by using phase contrast microscopy.
INTRODUCTION
Catalase that is usually found as a tetrameric feriheme enzyme (EC 1.11.1.6) is one of the most extensively studied enzyme. It is widely distributed in nature and is found in all microorganisms as well as plants and animal cells Citation[1-2]. Catalase can be found in human tissues with its highest activity in the erythrocytes, liver and kidney, while its activity is very low in human serum. In tissues catalase is localized in the mitochondria and peroxisomes, while erythrocytes it can be found in soluble form. Its physiological function is not quite clear, but its protective role against free radicals from oxygen has been emphasized.
Aerobic metabolism entails the production of reactive oxygen species, even under basal condition, hence there is a continous requirement for inactivation of these reactive oxygen species. The steady-state of prooxidants and antioxidants may be disrupted. A disbalance in favor of the prooxidants and disfavoring the antioxidants, potentially leading to damage, has been called “oxidative stress” Citation[3-4] such damage may afflict all types of biological molecules, including DNA, lipids, proteins and cabohydrates. Thus oxidative stress may be involved in processes such as mutagenesis, carcinogenesis, membrane damage, lipid peroxidation, protein oxidation and fragmentation, as well as carbohydrate damage Citation[[5]].
All cell in eucaryotic organism contain powerful antioxidant enzymes. The three major antioxidant enzymes are superoxide dismutase, catalase and peroxidases. In addition, there are numerous specialized antioxidant enzymes reacting with and in general, detoxifying oxidant compounds. In direct antioxidant functions carried by enzymes are (a) the back up function and (b) the transport and elimination of reactive compounds Citation[[6]].
Erythrocytes posses many desirable properties of a suitable carrier. They are biodegradable and circulate throught circulatory system; large quantities of biologically active material can be encapsulated in relatively small volumes of these cells; they can be organ targeted within the reticuloendothelial system Citation[7-9]and they can be fused with living cells Citation[[10]]. Catalase and PEG-catalase were encapsulated in erythrocyte (1/1) (v/v) by using slow dialysis methods and some parameters were investigated.
MATERIALS AND METHODS
Chemicals
Methoxypolyethyleneglycol-5000, catalase were purchased from Sigma chemical; H2O2 was obtained Merck. All other chemicals and organic solvents were obtained from commonly used suppliess and were analytical grade or better.
Preparation of PEG-Catalase
MPEG-5000 was activated with cyanuric chloride and coupled to catalase by our previously study Citation[12-13]. The extent of conjugation was determined by measuring the number of reactive amino groups in the solution by TNBS methods Citation[[13]].
Enzyme Assay
Due to the absorption of H2O2 at 240 nm, the activity of free and PEG- catalase and encapsulated enzymes have been measured spectrophotometrically Citation[[16]]. We used 10.5 mM H2O2 solution as substrate which is prepared in phosphate buffer pH 7.4 50 mM. For the activity measurement of enzyme the reaction was begun by adding 0.02 ml of enzyme to the 3 ml of substrate in the cells. The absorbans values has determined each 10 seconds and the activity values were estimated by using first absorbance and eq (1);
Physical Characterization of Catalase and PEG-Catalase
Effect of pH and temperature on the activity of free and immobilized catalase were determined.
Preparation of Red Blood Cells
Human erythrocytes were isolated from freshly drawn, heparanized verous blood. Whole blood was centrifuged at 2000xg for 10 min. The serum and buffy- coat were removed and the packed cells were washed three times in 10 vol of phosphate buffer saline (10 mM pH 7.4, 0.15M NaCl, 10 mM glucose, 10 mM inosine) Citation[[13]] to remove unentrapped material.
Encapsulated Catalase and PEG-Catalase
Catalase and PEG-catalase encapsulated erythrocytes using slow dialysis procedure as described by Kruse et al. with optimization by our previously study Citation[[11]], Citation[[13]]. Percentage entrapment, percentage equlibration, cell yield, mean cell volume (McV), mean cell hemoglobin (McH), hematocrit (HcT), mean corpucular hemoglobin (McHc) were determined for each samples on a hematological analyser Celldyn 4000. The erythrocyte shape of the encapsulated samples were investigated by using phase contrast microscopy.
RESULT AND DISCUSSION
Catalase Immobilization on Activated PEG-5000
Catalase was covalently immobilized on activated PEG-5000. after immobilization total activities of each immobilized catalases were shown in .
Table I. Immobilization of Catalase on Activated PEG
Physical Characterization
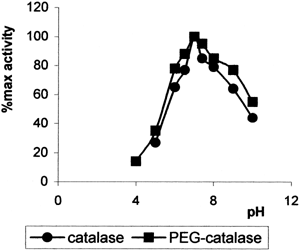
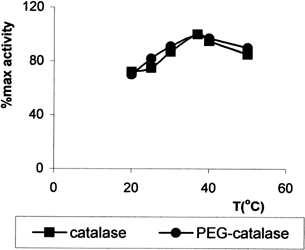
The effect of pH on the activity of immobilized catalase (1/3) and free catalase were studied in the different buffer solution of pH varying 4–10 (). The optimum pH was found to be the same pH 7.0 for both enzymes. Optimum temperature of immobilized and free catalase were found to be 37°C ().
Encapsulation of Catalase and PEG-Catalase
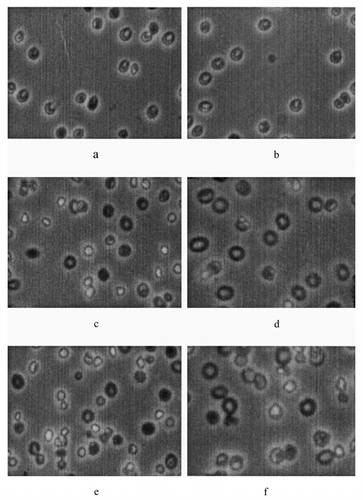
Catalase and PEG-catalase encapsulated within erythrocyte using different molar ratio. Encapsulation yield and the amount of encapsulated catalase were investigated. As is shown in and max. entrapment amount of catalase was given by using 2 mg/ml catalase concentration and percentage entrapment and the amount of encapsulated catalase are increased by using PEG as PEG is a hydrophobic molecule. Also, PEG protect the shape of erythrocyte but if catalase concentration was increased erythrocyte shape has been defect (f)
Table II. Encapsulation of Catalase in Erythrocyte
Table III. Encapsulation of PEG-catalase in Erythrocyte
Figure 3. The shape of erythrocyte samples (3a-1mg/ml catalase, 3b-1mg/ml PEGcatalase, 3c-2mg/ml catalase, 3d-1.9mg/ml PEGcatalase, 3e-5mg/ml catalase; magnification 3.3 × 10).
Several parameters of the erythrocytes submited to the procedure described above were investigated before and after encapsulation procedure. The hematologic parameters of encapsulated and control RBC from a typical preparation are presented in for catalase and for PEG-catalase. As is shown in and , there was a larger drop in McV and the decrease in McV for encapsulated mouse, bovine and human erythrocytes Citation[[19]], Citation[[13]]. With the reduction in the McV, there was an associated decrease in hemoglobin concentration. It appears that the lost of HgB and other cellular contents during the lysis step may account for the drop of the McV value of the encapsulated erythrocyte. We obtained max cell recovery using 2 mg/ml catalase and PEG-catalase and this recovery is very good. As is shown in and , using of PEG in enzyme encapsulation allowed to enter the catalase with the high loading capacity into the erythrocyte Citation[[13]].
Table IV. Hematologic Parameters of Encapsulated Catalase and Control RBC
Table V. Hematologic Parameters of Encapsulated PEG-Catalase and Control RBC
REFERENCES
- Nicholls P., G. R. The Enzymes 1963; 8: 147–225
- Bilinski T. Bulletin of the Polish Academy of Sciences Biological Sciences 1988; 36: 4–6
- Sies H. Angew. Chem. Int. Ed 1986; 25: 1058–1071
- Chance B., et al. Physiol. Rews 1979; 59: 527–605
- Halliwel B., Gutteridge J. M.C. Free Radicals in Biology and Medicine. (2nd edn.), Calerendon Press, Oxford 1989
- Sies H. Oxidative stress. Academic Press, London 1985
- DeLoach J. R. Biochim. Biophys. Acta 1977; 496: 507
- DeLoach J. R., Barton C. Am. J. Vet. Res. 1981; 42: 1971
- DeLoach J. R. Biblthca Haemat 1985; 51: 1–6
- Sprandel U., Way J. L. Erythrocytes as Drug Carriers in Medicine. Plenum Press, New York 1997
- Kruse C. A., et al. Biochim. Biophys. Acta 1981; 645: 339–345
- Hamarat Ş., Uslan A. H. Art.Cell. Blood Subst. Imm. Biotech 1996; 24(3)273–285
- Hamarat Baysal Ş., Uslan A. H. Art.Cell. Blood Subst. Imm. Biotech 2000; 28(3)263
- Gebicka L., et al. J. Radional Nucl. Chem. Artic 1987; 116(1)77–86
- Göth L. Enzyme 1989; 41: 91–199
- Chlu J. T., et al. J. Am. Chem. Soc 1989; 111: 7046–7050
- Lowen P. C. Can. J. Microbiol 1989; 35: 807–810
- Uslan A. H., Tarhan L. J. Fac. Sci. Ege Univ. Series A 1991; 14: 1
- Ebrahim A., Ryan W. L. Cytometry 1996; 25: 156–163