Abstract
In our system, urease/AlaDH have been encapsulated within erythrocytes by using slow dialysis methods. Urea is decomposed into ammonia and bicarbonate and the ammonia released is converted into alanine by reacting pyruvate under the catalytic action of AlaDH. It is very important for our that products are formed quickly but the ammonia is not connected definetely. For this aim, urease/AlaDH we encapsulated using different enzyme activity ratio (0.5:1.5; 0.5:2.5; 0.25:1.25 U/U urease/AlaDH). The activities of enzyme systems, encapsulation yield, McV, McH, and McHc were measured for each sample. Investigated results suggest that loaded enzyme systems can be used as potential carrier systems for the removal of high levels of urea from blood.
Introduction
Throughout the last decades it has been widely demostrated that human and animal erythrocytes (red blood cells, RBC) can be properly engineered to behave as cariers for a large variety of active drugs (Citation[1-5]. These transport cell systems have thus been proposed for dissemination of active therapeutic compounds throughout the human body. The major advantages of this specific strategy are the in vivo protection of the drugs from premature degradation as well as a widespread distribution of these drugs throughout the circulatory system. Moreover, this strategy avoids immunological reactions of the organisms to the drug (Citation[[1]], Citation[[2]]).
Exogenous molecules can be inserted into erythrocytes by dialyzing or by diluting the suspension of erythrocytes and exogenous compound with a hypotonic solution and lowering the osmotic concentration of the medium surrounding the cells. As a result of this, erythrocytes become swollen, and numerous holes of sufficient size are formed on the cell membrane. Intracellular components such as enzymes, protein, and drugs, which are impermeant solutes at normal conditions, can enter the cells. When isotonicity is restored, the openings on the cell membrane close and some of the exogenous substance is trapped within the erythrocytes (Citation[3-6].
Many researchers have encapsulated exogenous substances in erythrocytes from different mammals and man by various modifications of the above procedure (Citation[7-10a], Citation[10b-15].
The aim of this research was to determine the ability of removal of urea level in erythrocyte-Urease/AlaDH system.
MATERIALS AND METHODS
Chemicals
Urease (type III), alaninedehydrogenase (AlaDH), pyruvate, nicotineamidedinucleotide (NADH), were obtained from Sigma Chemical; phenol, urea, and dinitrophenilhydrazin were purchased from Merck Darmstad. All other chemicals and organic solvent were obtained from commonly used supplies analytical grade or better.
Encapsulation
Human blood was drawn from healthy donors by venipuncture into heparanized tubes. Blood was centrifuged (1000 g× 10 min) and plasma as well as buffy coat removed. RBC were washed three times in 10 vol of phosphate buffer saline (10 mM pH 7.4; 0.15 M NaCl, 10 mM glucose and 10 mM inosine)(PBS) (Citation[[16]], Citation[[17]]). After the last wash, the cell suspension was centrifuged to obtain packet RBCs.
Urease and AlaDH were encapsulated within erythrocytes as described by Kruse et al., with optimization by our previous study (Citation[[16]]) using different urease/AlaDH ratios (0.5:1.5; 0.25:1.25; 0.5:2.5 U/U Urease/AlaDH).
After encapsulation, the suspension washed three times with PBS buffer and centrifuged 2,000 g for 5 min and collected centrifugate. Urease and AlaDH were investigated in washed water so encapsulation yield were determine.
Different RBC suspensions were prepared from the original sample: native RBCs in the absence (control), dialyzed/resealed–annealed RBCs in the absence (unloaded) and presence, of enzyme system (loaded).
RBC count (cell/l), hematocrit (HcT,%), mean cell volume (McV,fl), mean hemoglobin (McH, pg), and mean cell hemoglobin concentration (McHc, g/dl) were measured with a hematology analyzer system (Celldyn 4000).
Enzyme Activities
The ammonia formed is estimated according to Berthelot methods (Citation[[17]], Citation[[19]]). Urease activity was calculated by using standart curve (0–10.5 μmol/ml NH 4 +).
AlaDH activity was determined as described by Sidney and Kaplan (Citation[[16]], Citation[[17]]). AlaDH was assayed by monitoring initial rates of NADH oxidation at 340 nm in a Jasco spectrophotometer.
After encapsulation, three different tubes were prepared for determination of percentage entrapment, enzyme system activity, and ability of alanine synthesis:
Blank: 100 μl natural erythrocyte and 2.9 ml 10 mM citrate phosphate dextrose buffer (CPD) pH 7.6 (Citation[[16]], Citation[[17]]).
Control: 100 μl erythrocyte including PEG-urease/PEG-AlaDH enzyme system, 10 μl urea (0.417 μmol/ml) and 2.89 ml CPD buffer.
Sample: 100 μl erythrocyte including enzyme system, 200 μl pyruvate (8.34 μmol/ml), 50 μl NADH (0.846 μmol/ml), 10 μl urea (0.417 μmol/ml) and 10 mM pH 7.6 CPD buffer in a final volume 3.0 ml.
All tubes incubated 37°C at different time (5, 10, 15, 20, 30, 45, 60, and 90s)and after each incubation time, the samples were centrifuged at 2000×g for 5 min and remove erythrocytes and store centrifugate at 4°C for determination the level of pyruvate and ammonia. Pyruvate was assayed by dinitrophenilhydrozon methods as described by Aras (standart curve 0–30 μg pyruvate/ml) (Citation[[18]]).
RESULT AND DISCUSSION
In urease/AlaDH enzyme system, urea is decomposed into NH 3 and HCO 3 − and the ammonia released is converted into alanine by reacting pyruvate under the catalytic action of AlaDH. It is very important for us that the products are formed quickly but the ammonia is not connected definitely. For this aim, the activity of the enzyme system were investigated for each sample. The success of the system will be enable the renal patients with diabetes mellitus.
Encapsulation Yield
Urease/AlaDH were encapsulated within erythrocytes using 0.5:1.5; 0.5:2.5; 0.25:1.25 U/U (urease/AlaDH) ratio. Encapsulation yield and the amount of encapsulated enzyme are given in .
The encapsulation parameters and erythrocyte index are shown in . These include cell recovery and erythrocyte indexes for the RBC suspensions. Although we obtained a cell recovery range is 87.20% during encapsulation, the cell recovery is 81.60% after encapsulasion and a percentage of hemoglobin release is 12.61%. There is a drop in McV and McHc. These result suggest that the cell preparate has some stomatocytes.
The results for 0.25:1.25 U/U sample are shown in . The cell recovery range is 53.06% and a percentage of hemoglobin release is 36.57% in 0.5:2.5 sample. Drop in McV, McH and McHc show that carrier erythrocytes are smaller than normal cells.
Table 1. The Encapsulation of Urease/AlaDH Enzyme System
Table 2. The Encapsulation Parameters and Erythrocyte Index
Ammonia Assay
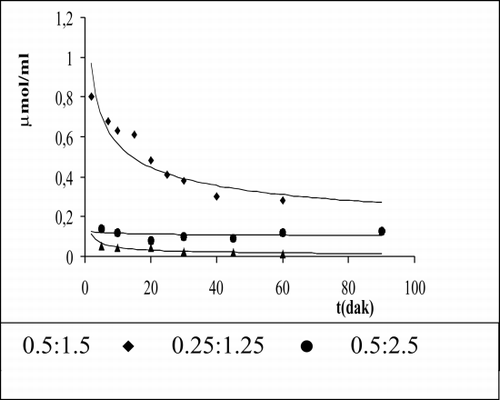
The levels of ammonia for each enzyme ratio assemble are given in . In 0.5:1.5 enzyme activity ratio, ammonia was investigated as 0.3 μmol/ml in the medium, showing that AlaDH activity is inefficient. The investigated ammonia is very in 0.5:2.5 and 0.25:1.25 enzyme activity ratio.
Pyruvate Assay
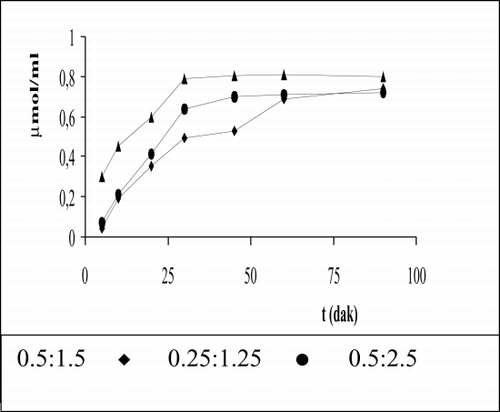
The measurement of pyruvate is given in for each sample. As is shown in consumption of pyruvate is 0.69 μmol/ml at 60 min for 0.5:1.5 enzyme activity ratio. The transform of beginning of urea level into alanine is 82.7% for 0.5:1.5, 85.0% for 0.25:1.25, and 97% for 0.5:2.5. These results correspond with investigated ammonia levels for these enzyme ratios.
REFERENCES
- Magnani M., Rossi L., Casabıanca A., Fraternale A., Schıavano G., Brandı G., Mannello F., Piedımonte G. Adv. Exp. Med. Biol., M. Magnani, J. DeLoach. Plenum Press, New York 1992; 326: 239–245
- DeLoach J., Way J. L. Carrier and Bioreactor Red Blood Cells for Drug Delivery and Targeting, J. DeLoach, J. L. Way. 1. Elsevier Science Ltd. Pergamon, Great Britain 1994
- Ebrahim A., Ryan W. L. Cytometry 1996; 25: 156–163
- Sprandel U., Way J. L. Erythrocytes as Drug Carriers in Medicine, U. Sprandel, J. L. Way. Plenum Press, New York 1997
- Garın M. I., Lopez R. M., Luque J. Cytokine 1997; 9: 66–71
- Garın M. I., Lopez R. M., Sanz S., Pınılla M., Luque J. Pharm. Res. 1996; 869–874
- Sanz S., Lizano C., Luque J., Pınilla M. Life Sci. 1999; 65(26)2781–2789
- Beutler E., Dale G. L., Guinto E., Kuhl W. Proc. Natl. Acad. Sci. U.S.A. 1977; 74: 4620–4623
- Dale G. L., Villacorte D. G., Beutler E. Biochem. Med. 1977; 18: 220–225
- DeLoach J. R., Ihler G. M. Biochem. Biophys. Acta 1977; 496: 136–145
- DeLoach J. R., Peters S., Pinkard O., Glew R., Ihler G. M. Biochem. Biophys. Acta 1977; 496: 507–515
- Ihler G. M., Glew R. H., Schnune F. W. Proc. Natl. Acad. Sci. U. S. A. 1973; 70: 2663–2666
- Ihler G., Lantzy A., Purpura J., Glew R. H. J. Clin. Invest. 1975; 56: 595–602
- Thorpe S. R., Fiddler M. B., Desnick R. J. Pediatr. Res. 1975; 9: 918–923
- Updike S. J., Wakamiya R. T., Lightfoot E. N. Science 1976; 193: 681–683
- Zimmerman U., Pilwat G., Esser B. J. Clin. Chem. Clin. Biochem. 1978; 16: 135–144
- Hamarat Baysal Ş, Uslan A. H. Artif. Cells, Blood Substitutes, Immobilization Biotechnol. 2000; 28(3)263–261
- Hamarat Baysal Ş, Uslan A. H. Artif. Cells, Blood Substitutes, Immobilization Biotechnol., in press
- Aras K., Erşen G. Klınik Biyokimya, Ankara Üniversitesi Diş Hekimliği Fakülte Yayınları, Sayı 1975; 2: 245
- İmren H. Üre tayini, Klinik Tanıda Laboratuvar, Beta yayınevi 1985; 716–723