Abstract
Spirulina subsalsa is immobilized with alginate, which increases the growth rate, chlorophyll content, phycocyanin content and nitrate reductase activity. Immobilized Spirulina subsalsa with alginate increases absorption of triphenyltin chloride (TPT). The phycocyanin of immobilized Spirulina subsalsa is more sensitive to TPT then free alga. The immobilization enhances the toxic effect of TPT on nitrate reductase activity of Spirulina subsalsa. Experimental results demonstrate that the immobilization of Spirulina subsalsa is feasible. Removal of TPT by immobilized Spirulina subsalsa reaches 68%. Biosorption mechanism of TPT by Spirulina subsalsa should be further studied.
INTRODUCTION
A variety of organotin compounds have been widely used as marine antifouling paints, agricultural pesticide, wood preservation and plastic stabilizers worldwide. The major source of organotin compounds to marine environment are antifouling paints, municipal and industrial wastewater and sewage sludge.Citation[1-2] Tributyltin concentrations in sediment over time are relatively constant.Citation[3-4] Its half-life is 2 to 4 years,Citation[[5]] even reachs 8 to 12 years.Citation[[6]] It is also worth to mention that phenyltins have high affinity to particulate matter and tend to enrich in sediment.Citation[[7]] It is due to tributyltin and triphenyltin both used as marine antifouling paints have long persistence in sediment which directly contaminate aquatic environment especially marine environment, and because both of them have high toxicity to aquatic organisms inhabiting in coastal areas including microalgae, phytoplanton, coastal fish and mussel, invertebrate, benthic organisms etc. Therefore among a variety of organotin compounds tributyltin and triphenyltin are of most concerns to the environmental scientists.
Immobilized microalgae have been studied for a variety of reason. If waste material containing Pb and Cd is passed through a system of immobilized cells of Chlorella, it will consume Pb and Cd at desired levels and thus free the waste water of heavy metals.Citation[[8]] Immobilized Chlorella vulgaris has great potentialities for removing nutrient from wastewater with high N concentration loading with a wide range of N/P.Citation[[9]]
According to the anti-radiation, anti-caducity and mutation,Citation[[10]] the Spirulina spp. lead the researchers to investigate all over the world. Spirulina spp. have been used to treat wastewater. Cadmium uptake by Spirulina maxima cells was more pronounced in living than in dead cells, with a maximum recovery of 47.63 mg Cd/g cells for living cells and 37.00 mg Cd/g cells for inactivated cells.Citation[[11]] Spirulina species have been used to treat tannery effluent.Citation[[12]]
In this paper, a series of experiments was carried out to investigate immobilized Spirulina subsalsa cell growth rates, chlorophyll a content, phycocyanin content, nitrate reductase activity in comparison with free cells. Work also was done to evaluate the kinetics of triphenlytin removal by immobilized Spirulina subsalsa.
MATERIALS AND METHODS
Routine Cultivation
Spirulina subsalsa was obtained form Institute for aquatic organism in Hubei, China and used in all studies. The alga was routinely cultured in 250 ml Erlenmeyer flask containing 100 ml Spirulina growth medium (CGM), containing (gl−1 distilled water): NaHCO3 8.40; NaNO3 2.50; K2SO4 1.00; CaCl2 0.04; K2HPO4 0.25; NaCl 5; EDTA 0.08; FeSO4·7H2O 0.01; MgSO4·7H2O 0.2.
Method of Immobilization
Spirulina subsalsa was centrifuged from culture medium and was stocked with 20 ml 2% (w/v) alginate which was autoclaved prior to use. Calcium algainate beads were formed according to the method of Zhang and Huang.Citation[[13]] 2.5 mm dia beads were formed by extrusion through a 10 ml hypodermic syringe. Beads were hardened in 0.3 M CaCl2 for 3–4 hours. Beads formed were washed with CGM or distilled water. Beads were added to 1500 ml sterile CGM in 3500 ml Erlenmeyer flasks and cultures were incubated under 16 hr light (2200±100 Lux)/8 hr dark photoperiod at 25±1°C. The TPT concentration in test water was 2 μg Sn/L.
Each test was replicated three times. Experiments lasted 8 days. Every two days, 120 ml test water and 1/10 beads of each Erlenmeyer flask were removed for analysis. At the same time 120 ml solution was removed form flasks containing alga in free suspension.
Algal growth was evaluated by completely dissolving beads in 120 ml 0.5 M NaH2PO4 (pH 6.5) and determining the solution absorbance at 560 nm. The dissolved solution was also used for determining the chlorophyll content, phycocyanin content and triphenlytin content in alga.
At the same time the 120 ml test water of control test was also to determine the algal growth, the chlorophyll content, phycocyanin content and triphenlytin content in alga and the nitrate reductase activity and triphenyltin concentration in solution.
The method used for determining alga growth rate, chlorophyll content, phycocyanin content, nitrate reductase activity has been published.Citation[[14]]
100 ml solution containing dissolved beads or control solution was centrifuged and alga was digested with tetramethylammonium-hydroxide solution for 1 hr at 100°C. The digested solution was put into a 250 mL separating funnel and was added to 5 mL n-hexane and 2 mL HCl. The funnel was shaken 300 times to extract TPT and was allowed to stand for 30 min. The organic phase was put into a color comparison tube. The organic solution was purged with N2 near to dry and stored in the refrigerator at 4°C.
TPT concentration measurements were made with an LS-5 luminescence spectrometer (Perkin–Elmer) equipped with 10 mm quartz cells. The slit widths of excitation and emission were 10 and 5 nm, respectively. The fix scale was 0.1. TPT extraction solution was added to 0.5 mL phthalandionehydrogen-potassium buffer solution (pH 4.0), 1.0 mL 10% Triton X-100 solution and 0.2 mL of 0.0025% morin methanol solution. The final volume of reaction solution was 5 mL. After 10 minutes, the fluorescent intensity of reaction solution was determined by emission scanning over range from 490 to 540 nm at 412 nm excitation wavelength.Citation[15-16] The observed values of fluorescent intensity of TPT peak were recorded to calculate TPT concentration. Standard curve was made with TPT standard solution.
RESULTS AND DISCUSSION
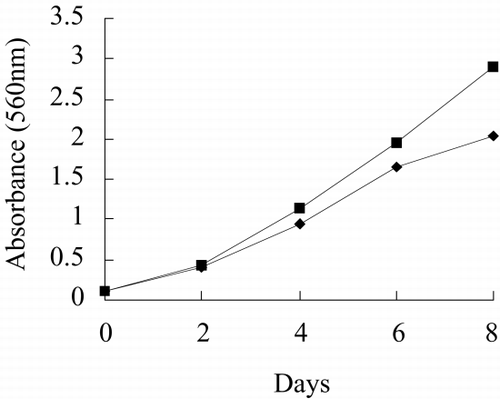
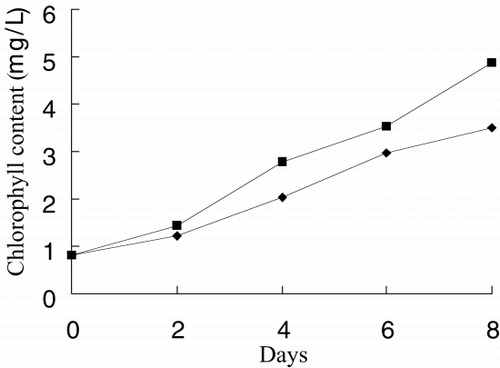
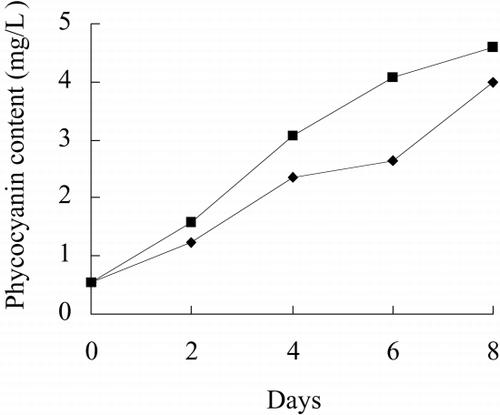
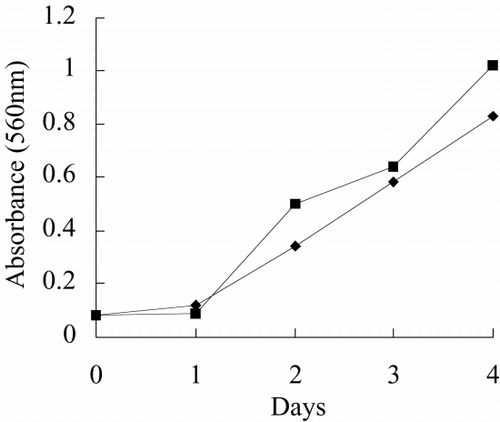
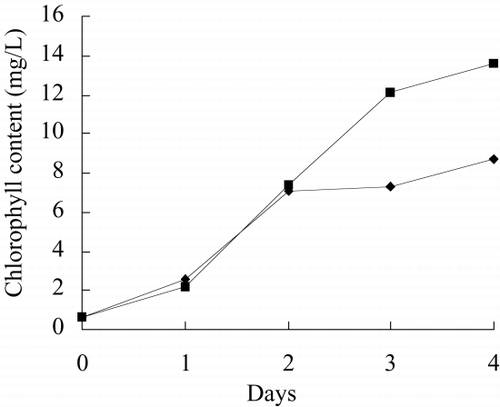
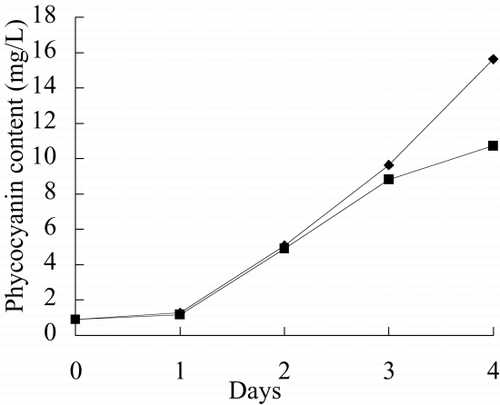
and show the photo of free culture and immobilized culture alga. It can be seen that the alga is long, twining together in free culture when the alga density is higher. shows that the long alga is broken down when immobilized The growth rate, chlorophyll content, phycocyanin content, nitrate reductase activity of alga in free culture and immobilized culture are shown in . According to , Immobilized alga grows faster than free alga. At the end of test, the biomass, chlorophyll content and phycocyanin content of immobilized alga are 1.14, 1.39 and 1.16 time of that of free alga, respectively. The nitrate reductase activity of immobilized alga is slightly lower than that of free alga, which suggests that immobilization has effect on physiological activity of alga. Meanwihile, it is showed that the nitrate reductase activity of immobilized alga has smaller fluctuation than that of free alga, which indicates that physiological function of immobilization alga is more stable.
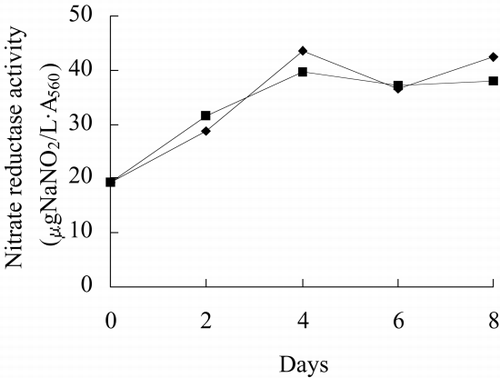
show growth rate, chlorophyll content, phycocyanin content, nitrate reductase activity, TPT concentration in test water and TPT content in alga in free culture and immobilized culture with 2 μg/L TPT. According to , at the end of the test, immobilized alga biomass is 1.23-fold of free alga, chlorophyll content of immobilized alga is 1.57-fold of free alga, phycocyanin content of immobilized alga is 68.7 percent of free alga. According to , at the end of test, the nitrate reductase activity of immobilized alga is 64.7 percent of free alga. At the beginning of test, nitrate reductase activity of immobilized and free alga sharply increases reaching a peak value and then sharply decreases. At the second day, nitrate reductase activity approaches stable, while nitrate reductase activity of immobilized alga shows fluctuating. The results of the study of toxicity of TPT to Spirulina subsalsaCitation[[14]] show that toxicity of TPT to chlorophyll content is higher than that to phycocyanin content, while effect of TPT on phycocyanin content is higher than that of TPT on chlorophyll content of immobilized Spirulina subsalsa in this study. This demonstrates that physiological activity of Spirulina subsalsa changes when immobilized, which leads to the appearance of different physiological characteristic between chlorophyll and phycocyanin, although chlorophyll and phycocyanin are photosynthetic pigment.
Figure 10. Nitrate reductase activity of immobilized Spirulina subsalsa with alginate for removing TPT.
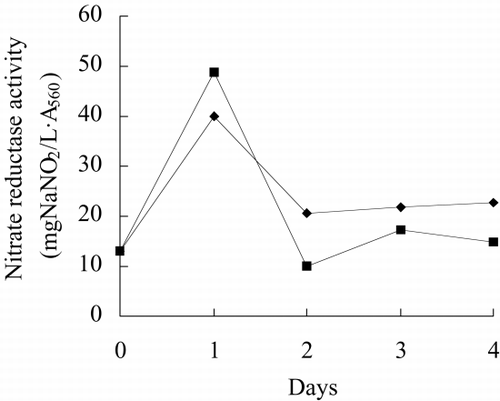
Figure 11. TPT concentration in test water of immobilized Spirulina subsalsa with alginate for removing TPT.
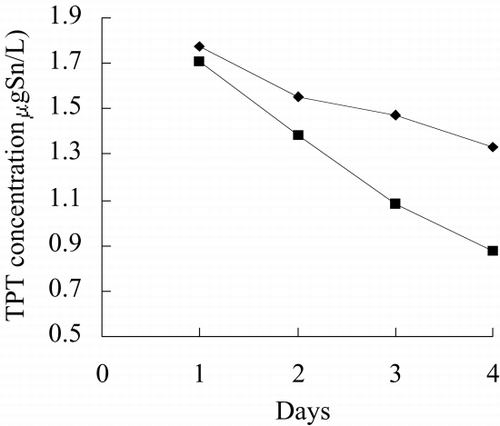
shows the TPT concentration of test water in free and immobilized culture. At the end of tests, TPT concentrations of test water in free suspension and immobilized are 67.8 and 64.4 percent of that at the beginning of test. This suggests that immobilized alga is more efficient than free alga to remove TPT from water.
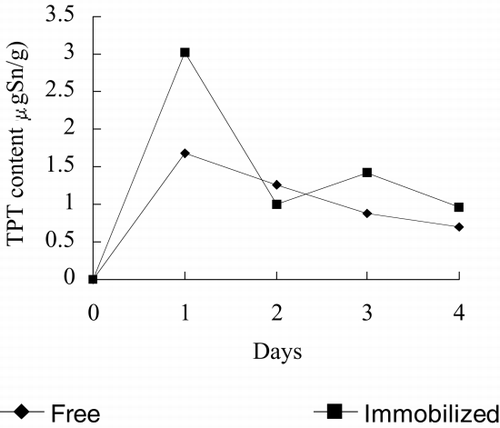
shows TPT content in free and immobilized alga. It can be seen that free or immobilized Spirulina subsalsa starts rapidly absorbing TPT at the beginning of test, both reaching a peak value at the first day. For immobilized Spirulina subsalsa, after the first day, TPT content decreases rapidly to the lowest value at the end of the second day, and then it reaches a constant value. For free Spirulina subsalsa, TPT decreases smoothly after the first day, and by the end of test, TPT content per gram immobilized Spirulina subsalsa is larger than that of free Spirulina subsalsa, which demonstrates that immobilized alga increases the absorption capability of TPT. TPT can also be adsorbed by the carrier (alginate), which increases TPT concentration nearby the immobilized alga, thus immobilized alga absorbs TPT easier than free alga. At the same time, under higher TPT concentrations, the effect of TPT on phycocyanin content and nitrate reductase activity is enhanced, therefore phycocyanin content and nitrate reductase activity of immobilized alga are lower than that of free alga.
In this study, it can be concluded that TPT removal efficiency by immobilized Spirulina subsalsa is higher than that of free Spirulina subsalsa which demonstrates that raise the capability of Spirulina subsalsa to absorb TPT. Meanwhile immobilization influences the physiological activity of Spirulina subsalsa. Studies should be further carried out to explore the mechanism of TPT biosorption by Spirulina subsalsa.
ACKNOWLEDGMENTS
This research was supported by the National Natural Sciences Foundation of China (grant no. 29777010).
REFERENCES
- Magure R. J., Chau Y. K., Bengert G. A., Hale E. S. Occurence of organotin compounds in Ontario lakes and rivers. Environ. Sci. Technol. 1982; 16: 698–701
- Fent K., Muller M. D. Occurrence of organotins in municipal water and sewage sludge and behavior in a treatment plant. Environ. Sci. Technol. 1991; 25: 489–493
- Krone C. A., Stein J. E., Varanasi U. Butyltin contamination of sediments and benthic fish from the East. Gulf and Pacific coasts of the United States. Environ. Monit. Assess. 1996; 40: 75–89
- Fent K. Ecotoxicology of organotin compounds. Crit. Rev. Toxcol. 1996; 26: 1–117
- Batley G. The Distibution and Fate of Tributyltin in the Marine Environment Chapter 5. Tributyltin: Case Study of an Environmental Contaminant, S. J. de Mora. Cambridge University Press. 1996; 139–166
- Astruc M., Lavigne R., Pinel R., Leguille F., Desauziers V., Ouevauviller P., Donard O.F. X. Speciation of Tin in Sediments of Arcachon Bay (France). Lewis Publ., ChelseaUSA 1990; 263–274, Ml48II8
- Fent K., Hunn J. Phenyltins in water, sediment and biota of freshwater marinas. Environ. Sci. Technol. 1991; 25: 956–963
- Srivastava P. S., Gupta D., Iqbal M. Trace Toxic Elem. Nutr. Health, Proc. Int. Conf. Health Dis.: Eff. Essent. Toxic Trace Elem., 4th, Meeting Date. 266–272
- Yan G. A., Yu T. X. Comparison of the effects of nitrogen concentration on nutrient removal, growth, and physiological characteristics of immobilized and free Chlorella vulgaris, Part I. Growth and nutrient removal. Toxicol. Environ. Chem., 58(1–4)63–74
- Chao Z. X. The effect of Spirulina spp. on the health of middle-aged and gerontism people. Oceanic Sci. 1994; 6: 32–34
- Da Costa A.C. A., De Franca F. P. Short communication: cadmium by Spirulina maxima: toxicity and mechanism. World J. Microbiol. Biotechnol. 1998; 14(4)579–581
- Rose P. D., Maart B. A., Dunn K. M., Rowswell R. A., Britz P. High rate algal oxidation ponding for the treatment of tannery effluents. Water Sci. Technol. 1996; 33(7, Waste Stabilization Ponds: Technology and Applications)219–227
- Li Z., Guolan H., Yu Y. Immobilization of microalgae for biosorption and degradation of butyltin chlorides. Artif. Cells, Blood Substitutes, Immobilization Biotechnol. 1998; 26(4)399–410
- Zhihui S., Guolan H. Toxicity of triphenyltin to Spirulina subsalsa. Bull. Environ. Contam. Toxicol. 2000; 64(5)723–728
- Yu T.-H., Arakawan Y. High-performance liquid chromatographic determination of dialkyltin homolognes using fluorescence detection. 1983; 258: 189–197
- Les E., Garcia Alonzo J. I. Determination of tributyltin ions in estuarine waters by high-performance liquid chromatography with fluorometric detection using morin in a micellar solution. Analyst (London) 1987; 112(11)1551–1554