INTRODUCTION
Having characterized some of the biophysical properties of artificial cells, we are now in a position to examine their biological properties. Whether in solution or imbedded in membranes of intracellular organelles, enzymes in nature are located most abundantly within biological cells. While retained within the cells and protected from extracellular environments, enzymes act efficiently on permeant substrates entering through the cell membranes by diffusion or special transport mechanisms.
Initially, I was interested in studying an artificial system of cellular dimensions in which individual microdroplets of erythrocyte hemolysate with its hemoglobin and complex enzyme systems are enveloped in spherical ultrathin polymer membranes (Fig. 2) (Chang, 1957). In this way, the enveloped proteins are retained within cell-like microscopic dialysis bags and prevented from coming into direct contact with the extracellular environment. Permeant external molecules, like oxygen and carbon dioxide, can diffuse rapidly across the enclosing membrane to interact with the enclosed erythrocyte hemolysate. Additional enzyme systems can also be added to the red blood cell hemolysate to be enclosed in artificial cells. Thus enzymes could be enclosed in artificial cells by first dissolving or suspending them in the hemolysate solution and then proceeding as described for the preparation of artificial cells. It should be emphasized that the preparation of stable artificial cells is facilitated by the presence of a high concentration of protein in the aqueous phase. A small fraction of the protein takes part in the formation of the cell membrane by cross-linking or by coacervation. The osmotic pressure of the colloid also helps to retain the turgor of the artificial cell membranes, which would otherwise tend to collapse in an aqueous environment. Thus, dilute enzyme preparations can be most conveniently enclosed in artificial cells if they are first added to the hemoglobin solution or hemolysate. Only in a very few cases can enzyme solutions (e.g. Sigma type V urease 0.15 gm/ml) be microencapsulated inside artificial cells without the addition of further protein. In these cases, the membranes are usually not well formed, even though a high concentration of the enzymes has been used. Another point which should be emphasized is that the chemicals used in the interfacial polymerization procedure may inactivate some types of enzymes, e.g. catalase and uricase, but have no marked effects on other types of enzymes, e.g. urease and asparaginase. The interfacial precipitation procedure, on the other hand, did not have any major adverse effect on all the enzymes tested so far, for instance, carbonic anhydrase, catalase, trypsin, uricase, urease, and asparaginase. However, in the case of the interfacial precipitation procedure, the physicochemical properties of the solution to be encapsulated are extremely important. One of these is the pH of the solution. For example, when 1.8 gm of urease (Sigma type II powder, 1720 units/gm) was dissolved in 10 ml of hemolysate containing tris buffer (0.08 M), the high acidic content of Sigma type III urease lowered the pH of the final solution to 6.2. The collodion membrane artificial cells prepared from this solution were very fragile and easily ruptured. On the other hand, if the pH was maintained at 9 by the use of a high concentration of tris buffer (0.48 M), the artificial cells prepared had much stronger membranes.
Besides enzymes and hemolysate, cell extracts, cell homogenates, or insolubilized enzymes have been added to the hemolysate and then enclosed in the artificial cells. Some of the enzymes and proteins enclosed in artificial cells are summarized in . Enzymes can be microencapsulated, then stabilised by treatment with glutaraldehyde (Chang. 1971d).
Table 2. Enzymes and Proteins Enclosed in Artificial Cells
MODEL SYSTEM
Artificial Cells Containing Urease
Urease, which catalyzes the hydrolysis of urea, is a globulin with a molecular weight of 480,000. It is unstable in solution, losing 50 percent of its activity in 24 hours at 37°C, but is more stable in the presence of 2 percent gum arabic or 5 percent egg albumin and very stable in the insolubilized form. Its substrate, urea, is the major diffusible nitrogenous constituent of the body fluid, and the interesting possibility of a demonstration of an in vivo action of urease enclosed in artificial cells has prompted the investigation of the efficiency of this system (Chang, 1965).
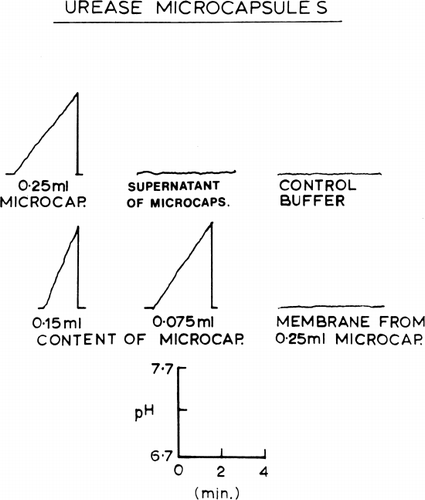
Urease dissolved in hemolysate was enclosed in nylon membrane artificial cells by the methods described in the previous section. After washing with phosphate buffer to remove any broken artificial cells, urease activities were analysed by the procedure of Van Slyke and Archibald (1944), as the rate of rise of pH of a buffered urea solution. The result is shown in . Five Sumner units of the enzyme enclosed in artificial cells had an activity corresponding to that of 1.83±0.10 Sumner units of enzyme in free solution. Thus the activity of the artificial cell urease was about 37 percent of the activity of the same amount of enzyme in free solution. It also shows that a sample of buffer in contact with its own volume of urease-loaded artificial cells for 12 hours acquires no measurable enzymatic activity, showing that the enzyme does not leak to a significant degree out of the stored nylon membrane artificial cells (Chang, 1965). In another set of experiments, nylon membrane artificial cells containing urease were ruptured in a cell homogenator (glass-pistal). The homogenate was recovered and the membranes washed three times with phosphate buffer. No measurable urease activity was found in the isolated artificial cell membranes. The urease activity was all in the homogenates. Since the enclosed urease did not leak out of the artificial cells and since the membranes of the artificial cells did not contain any measurable enzyme activity, it appears that in order for the urea to be acted on by the enclosed enzyme, it has to diffuse into the artificial cells. Further experiments were done to test the effect of artificial cell diameter on the activity of the enclosed enzyme. In the case of the smaller artificial cells (27.1μ±11.9μ), the rate of enzymatic conversion was about 39 percent of the same amount of enzyme activity in free solution. In the case of the larger artificial cells (89.8μ±26.0μ), the activity was about 21 percent of the activity in free solution.
Figure 29. Urease activity, measured as rate of rise of pH of urea-buffer medium. No pH change in the presence of supernatant from a 50% suspension of artificial cells stored for 12 hours. The artificial cells are homogenized and separated into the membrane fraction and homogenate fraction. Nearly all the enzyme activity is located in the membrane-free homegenate.
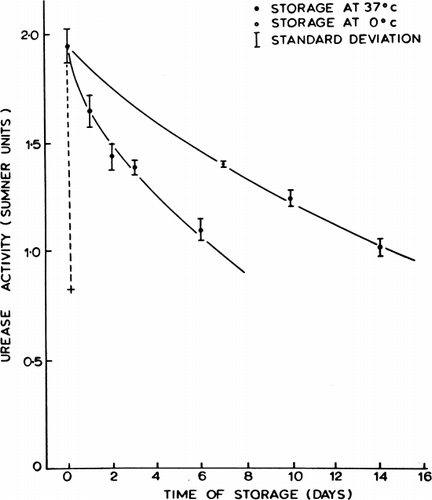
Additional experiments were done to test the stability of the urease enclosed in artificial cells (Chang, 1965). Urease encapsulated alone without the presence of hemolysate lost its activity rapidly with a half-time of three hours when stored at 37°C. When urease was enclosed together with hemolysate in artificial cells, the stability of the enclosed enzyme was greatly improved. This is shown in . It was found that the half-time of the hemolysate-stabilized microencapsulated urease was about one week when kept at 37°C and two weeks when kept at 4°C. Urease enveloped in collodion membrane artificial cells or BHC-collodion membrane artificial cells also showed similar in vitro activities.
Figure 30. Stability of urease enclosed in nylon membrane artificial cells, stored in phosphate buffer at pH 6.7. Continuous lines represent activity after storage at 0°C (upper line) and at 37°C (lower line) for enzyme encapsulated with hemolysate. Discontinuous line represents activity after storage at 37°C for enzyme encapsulated without hemolysate. (From Chang, 1965.)
Site of In Vivo Introduction
In order to study the in vivo action of artificial cells, a site has to be selected for their in vivo introduction. Except for blood cells, other cells are bathed in interstitial fluid, an ultrafiltrate of blood plasma. Peritoneal fluid is an ultrafiltrate of blood plasma (Maurer, et al., 1940). Small molecular weight substances exchange rapidly between the peritoneal blood vessels and the peritoneal fluid in the peritoneal cavity (Courtice and Simmonds, 1954). In addition to the demonstrated efficient exchanges between blood plasma and peritoneal fluid, the peritoneal cavity has the potential capacity to hold a large volume of artificial cells. Thus it appears that the peritoneal cavity would be an ideal experimental site for the in vivo introduction of artificial cells (Chang, 1965).
Effects and Fates of Injected Artificial Cells
Before the in vivo action of artificial cells could be investigated, a knowledge of the toxicity and fate of intraperitoneally injected artificial cells would assist in the interpretation of the results. Nylon membrane artificial cells were chosen for these studies for the following reason: Teflon® and silicone rubber, though extremely inert biologically, are not semipermeable to water or to aqueous solutes. Nylon, though it causes some tissue reactions in the sheet or block form, is nearly inert when used as a suture material.
Since particles the size of erythrocytes are removed from the peritoneal cavity, we have used larger nylon membrane artificial cells of 100μ mean diameter prepared as described. After washing to remove Tween 20, the enclosed hemoglobin was tagged with sodium chromate Cr 51. Male rats of average weight 130 gm were divided into six groups. The animals of five groups were each given 1 ml intraperitoneally of the 25 percent suspension of nylon membrane artificial cells. Each rat of the sixth group received 1 ml of the supernatant from the suspension. All the animals remained active and healthy, without significant changes in weight, abdominal tenderness or rigidity, or alteration of bowel movements. On opening the abdomens, there were no signs of inflammation in any abdominal structure, except for one case in which all the artificial cells had been injected accidentally into a small pocket of omentum resulting in some local inflammation and fibrosis. In all other animals, larger individual nylon membrane artificial cells and small aggregates could be seen loosely sticking to the abdominal wall or omentum and could easily be picked up with fine forceps, leaving no signs of fibrosis or inflammation at the sites. After the first week, the artificial cells were well dispersed over the whole peritoneal cavity, but at the second week and thereafter there was some tendency for them to be found in larger numbers in the upper part of the peritoneal cavity, where many of them were located at the upper surface of the liver and spleen just below the diaphragm. None were observed on the peritoneal surface of the diaphragm. No significant radioactivity was detected in the lung, liver, spleen, lymph nodes, blood, or saline wash fluid. Recovered radioactivity was associated with the artificial cells or small aggregates of artificial cells which stuck loosely to the abdominal structures.
A dog was given intraperitoneal injections of nylon membrane artificial cells at weekly intervals for three weeks. It was then followed for one year. The dog continued to be active, gained weight, and showed no signs of abdominal tenderness or rigidity. Examination after one year showed no fibrosis or chronic inflammation in the peritoneal cavity.
In Vivo Action of Artificial Cells Containing Enzymes
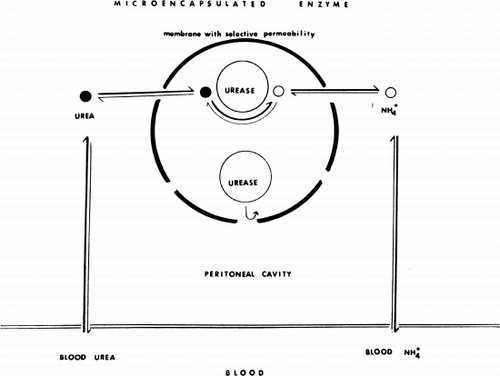
Artificial cells containing urease were tested (Chang and MacIntosh, 1964b, Chang, 1965). The principle is shown diagrammatically in . The enzyme urease does not leak out from the artificial cells. The size of the artificial cells (100μ average diameter) prevents the artificial cells from leaving the peritoneal cavity. Thus, for the urease in the artificial cells to have significant in vivo activity, its substrate, urea, would have to diffuse into the peritoneal cavity from the blood and then across the artificial cell membrane to be acted upon by the enclosed enzyme. The product, ammonium carbonate, would have to diffuse in the reverse direction to raise the blood ammonia level. Thus, the in vivo activity of the enclosed enzyme could be assessed by following the blood ammonia levels.
Figure 31. Schematic representation of the action of intraperitonally injected artificial cells loaded with urease. (From Chang, 1965.)
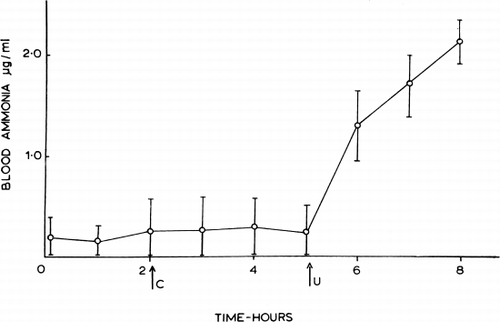
The results obtained from anesthetized dogs are shown in . Control artificial cells containing red blood cell hemolysate (0.25 ml/kg) injected intraperitoneally produced no significant changes in the arterial blood levels of the animals. However, after the intraperitoneal injection of artificial cells containing both hemolysate and urease (100 Sumner units in 0.25 ml/kg), the arterial blood ammonia levels rose. At the end of the experiments, three to four hours after the injection of the artificial cells containing urease, the blood amonia level was still rising. No significant changes were observed in blood pressure, electrocardiogram, or respiration. As a test for leakage of protein from the injected artificial cells, the enclosed hemoglobin was tagged with Cr 51. No radioactivity was detectable in the circulating blood.
Figure 32. Effect of urease-loaded artificial cells on blood ammonia in dogs anesthetized with Nembutal; the graph summarizes data from 3 experiments (mean±SD). C=injection of control artificial cells (no urease, 0.25 ml/kg). U=injection of urease-loaded artificial cells (0.25 ml and 100 Sumner units/kg). (From Chang, 1965.)
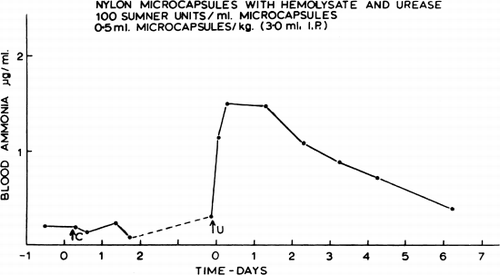
The changes in blood ammonia obtained in an unanesthetized dog are shown in . The intraperitoneal injection of the control artificial cells had no significant effect on either the blood ammonia level or the general state of the dog. When the artificial cells containing urease were injected (0.5 ml containing 50 Sumner units/kg), there was likewise no immediate effect on the behavior of the animal. Later, corresponding to the peak of the blood ammonia level, the animal became more sedated and walked with an unsteady gait. Twenty-four hours later, even though the blood ammonia level remained high, the animal appeared to be perfectly normal and has remained so up to one year after the injection. It is seen that the in vivo activity of the urease in the artificial cells, as indicated by the blood ammonia level, has declined slowly with a half-time of two to three days. This decrease in activity is not entirely related to the decrease in enzyme activity when stored at 37°C (half-time of about seven days); it could be due to the coating of the artificial cells by protein, fibrin, or phagocytes, thus reducing the permeability of substrates across the membrane.
Figure 33. Effect of artificial cells on blood ammonia in an unanesthetized dog. C=control artificial cells (0.5 ml/kg). U=urease-loaded artificial cells (0.5 ml and 50 Sumner units/kg). In this test, the urease preparation (NBC) was stabilized by the addition of hemolysate. (From Chang, 1965.)
The results of these experiments show conclusively that urease in the artificial cells when injected intraperitoneally can act efficiently on endogenous urea, converting it into ammonia. Only a small proportion of the body's urea was changed to ammonia in these tests, but it must be remembered that the intact liver was simultaneously reconverting ammonia to urea. In addition, the in vivo action of urease appeared to rise steeply with enzyme dosage. The greater effectiveness of the higher dosage indicates that the rate-limiting factor for ammonia formation under the conditions of these tests was the amount of enzyme present in the peritoneal cavity rather than the rate of transfer of urea or ammonia across the peritoneal membrane.
These results led us to suggest that artificial cells containing enzymes might be useful for enzyme replacement therapy (Chang, 1964, 1965; Chang and MacIntosh, 1964b). In this form, the enzyme, while acting on external permeant substrate, cannot leak out of the artificial cells to become involved in allergic or immunological reactions (Fig. 2).