Abstract
Myasthenia Gravis (MG) is a neuromuscular disease often associated with thymic pathology due to neuromuscular transmission impairment by circulating antibodies directed against the cholinergic postsynaptic receptor on the neuromuscular junction (Anti‐AchR‐Ab). The treatment of MG includes cholinesterase inhibitors, steroids and thymectomy. Plasmapheresis can remove Anti‐AchR‐Ab but more recently plasmaperfusion (PP), a more specific apheresis for selective removal of noxious plasma components, has been developed.
Aim of the study: To study the effect of PP treatment, performed by using specific immunocolumns for Anti‐AchR‐Ab, on the clinical outcome of MG patients non‐responder to steroid therapy or thymectomy.
Matherials and Methods: We treated 8 patients suffering from severe MG by a cycle of 6 sessions of PP. We used columns containing triptophan as a specific ligand for Anti‐AchR‐Ab. In order to evaluate the effectiveness of treatment we used functional tests (muscular tests, respiratory function, electromyography) and laboratory tests (Anti‐AchR‐Ab; immunoglobulins, complement fractions, immunocomplexes).
Results: After one to three PP sessions, early clinical improvement in bulbar and respiratory symptoms were found in all patients and EMG showed improvement of neuromuscular transmission. Serum concentration of immunological markers decreased progressively and significantly during the treatment. Clinical improvements were progressive despite the tendency for Anti‐AchR‐Ab to reach initial values between one session and another. We observed no side effects due to the type of immunocolumns used.
Conclusions: Triptophan columns appear to be able to remove large quantities of Anti‐AchR‐Ab and immunological markers from plasma. Our experience shows that PP performed using triptophan columns in patients suffering from severe MG provides good clinical results, improving patients' outcome, without any risk linked to the procedure.
Introduction
In the past, several membrane‐filtration techniques have been developed to remove plasma or plasma constituents from whole blood during extracorporeal perfusion.
Plasmapheresis (PF) or plasma exchange (PE) — removal of the patient's plasma and replacement with normal plasma or suitable colloid — is nowadays widely used in the treatment of many diseases and immunologic disorders.
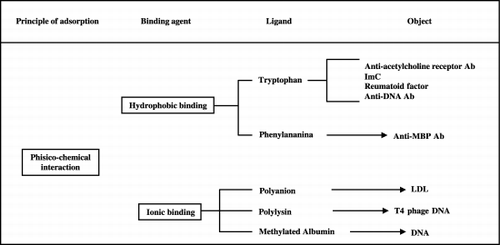
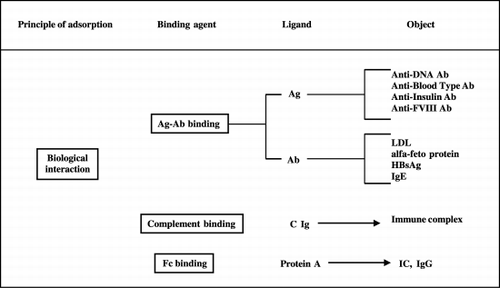
More recently plasmaperfusion (PP), a more specific apheresis for selective removal of noxious plasma components, has been developed (Yamavaki, [Citation1985]). Unlike PE, PP does not induce loss of essential blood factors and needs no fluid replacement (Malcheski et al., [Citation1986]). In PP, patient's plasma is previously separated from whole blood by a membrane plasmafilter, then it goes through immunoabsorbent columns containing some binder materials (ligands) that selectively “adsorbe” plasma toxic substances by specific physico‐chemical or biological interactions (Ross et al., [Citation2001]; Tagawa et al., [Citation1998]). After removal, purified plasma can be returned to the patient. and show the mechanisms by which PP could be of benefit to patients affected with different diseases.
There is clear evidence of the efficacy of apheretic techniques, especially PE, in the treatment of some serious neurological diseases. Since it has become apparent that circulating antibodies against the acetylcholine receptors (antiAchR‐Ab) at the neuromuscular junction are important in the patogenesis of myasthenia gravis (Vincent et al., [Citation2001]), removal of these antibodies by PE is found to be highly effective in ameliorating acute myasthenic weakness (Batocchi et al., [Citation2000]; Nakaji et al., [Citation2000]). In Guillain‐Barrè syndrome different pathogenetic factors may be involved, such as immune complexes (Hughes et al., [Citation1999]), demyelinating factors (Hadden and Gregson, [Citation2001]), or antibodies directed against peripheral nerve tissue (Yuki et al., [Citation2000]). Favorable responses have been reported using PE in acute and chronic Guillain‐Barrè syndrome (Haupt, [Citation2000]; Haupt et al., [Citation2000a]; Vedanarayanan and Chaudhry, [Citation2000]). Some immune factors are also involved in pathogenesis of multiple sclerosis (Bruck et al., [Citation2001]). Although there are many reports of patients with multiple sclerosis who appear marked clinical improvement from PE (De Andres et al., [Citation2000]; Takahashi et al., [Citation1997]), research on new pharmacological therapy goes on and many advances have been achieved (Steimnam, [Citation2001]; Wandinger et al., [Citation2001]). Finally, in chronic polyneuropathy, apheresis may be of benefit and good results have been obtained using PE combined with usual therapy (Nevo, [Citation1998]).
Myasthenia gravis (MG) is a disorder characterized by muscle weakness and abnormal faticability due to neuromuscular transmission impairment. Circulating antibodies directed against the acetylcholine receptors at the neuromuscular junction are the main cause of the transmission defect; such specific antibodies can be detected in 90% of MG patients (Palace et al., [Citation2001]). Evidence suggesting that immune factors are important in MG has led to attempts to treat this disease with timectomy, immunosoppressive therapy or plasma exchange. It is also possible to combine different kinds of treatment, such as PE and high dose of prednisone, in severe forms of MG, thus obtaining very good results despite the severity of the disease (Morosetti et al., [Citation1998]).
We studied the effects of PP on MG using specific immunocolumns for antiAchR‐Ab.
Methods
Eight patients affected with severe generalized MG were treated using Immunosorba IM T350 column by Asahi Medical Co., Tokio, Japan.
Plasma was previously separated using Plasmaflo AP 05H plasma filter by Asahi Medical Co., Tokio, Japan.
IM T350 contains microspheres of synthetic resin saturated with a polyvinyl alcohol gel in which triptophan is fixed as ligand. Such aminoacid is a specific ligand for antiAchR‐Ab probably because of a physico‐chemical interaction based on hydrophobic bond (Haupt et al., [Citation2000b]).
During each treatment the perfused plasma volumes were two litres and treatment times were about two hours per procedure. Blood flow rate through plasma separator was 100 ml/min, whereas plasma flow rate through IM T350 column was 20–30 ml/min. Heparin was used for anticoagulation. In six patients vascular access was obtained by a catheter in a femoral vein for afferent flow and a fistula needle in a peripheral vein as the efferent; two patients received treatment only by antecubital veins.
All patients had previously undergone thymectomy and were receiving chronic immunosoppressive therapy (prednisone or prednisone and azathioprine) (). They were selected from a large population of patients, because they were severely disabled despite treatment. All of them showed palpebral ptosis, diplopia, dysphonia, dysphagia and severe limb weakness. According to Osserman's classification, all were in the groups with life‐threatening signs (III or IV) and five were exhibiting ventilatory function impairment at the start of PP treatment.
Table 1. Patients' clinical profile
In order to evaluate the effectiveness of treatment we used: a quantified test for clinical signs and symptoms, the scores obtained in ten different muscle groups, according to prefixed standards, provide a general overview of the patient's condition (Sghirlanzoni et al., [Citation1984]); test of respiratory function; electromyographic (EMG) testing of repetitive supramaximal stimulation (RSS) — the radial nerve was stimulated at 3 Hz, recording from the extensor carpi brevis muscle) (AAEM, [Citation2001]); and before and after each PP the following laboratory tests were evaluated — AchR‐Ab titration (according to Lindstrom's method (Lindstrom, [Citation1977]), immune complexes (ImC) (according to Chia's method) (Chia et al., [Citation1979]), and immunoglobulins and complement fractions titrated by single radial immunodiffusion ().
Table 2. Evaluative test used
Immunosoppressive treatment remained unchanged during and after PP to avoid interference with evaluation of effectiveness of PP.
A cycle of six sessions of PP was used for each patients, with a 4–5 days interval. In two patients the working‐schedule was changed (): in patient 1 because of the excellent early clinical response, in patient 5 because of lack of superficial vein, resulting from the previous PE treatments and thrombosis of the femoral vein used. Changes in session intervals were needed in patient 4 and 6 because of severe respiratory failure requiring mechanical ventilation, because of food ingestion in one patient and a cholinergic crisis in the other.
Results
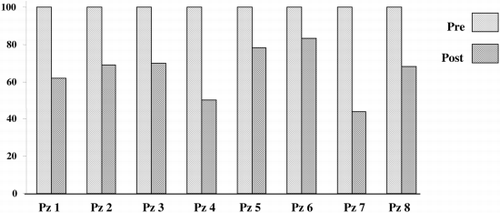
After one to three PP sessions, early clinical improvement in bulbar symptoms (dysphonia, dysphagia) and respiratory function has been found in all patients. Limb muscle strength, however, began to improve later on, especially in severely affected muscles. At the end of the treatment no patient showed respiratory weakness nor other vital functional impairment. A decrease in quantified test score was noted in all patients. Upon respiratory function testing, an increase in vital capacity and maximum voluntary ventilation, ranging between 20 and 100% was found. The most significant results were observed in the most severely affected patients.
EMGs showed improvement of neuromuscular transmission, with either an increase in amplitude of the first evoked muscle action potential or a reduction of the decremental response or both.
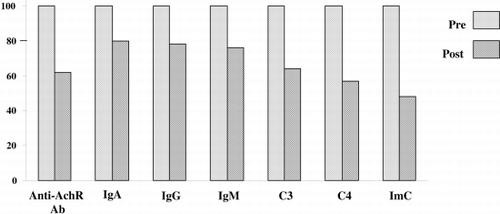
Serum concentrations of immunologic markers showed the following decreases (mean % ± DS): antiAchR‐Ab 36.47 ± 17.43; IgA 20.44 ± 11.26; IgG 21.24 ± 32.56; IgM 23.22 ± 11.40; C3 36.78 ± 10.15; C4 42.69 ± 14.82; ImC 55.82 ± 39.34 ( and ).
Figure 4. Decrease in serum concentration of immunological markers after all plasma perfusion sessions in all patients.
Routine laboratory tests, although not specific for MG, were carefully obtained to detect possible undesired effects of the procedure, as well as to assess its selectivity in removing blood components. The only significant finding was a decrease of fibrinogen (60 to 70% of the starting value) and of total protein content (0.5 to 1 g/dl) after each PP session.
In 6 of 8 patients the improvement continued after 10 months; in one patient the effects lasted 1 month, and in another one a severe relapse occurred after 10 months of benefit; this relapse was, however, successfully reversed by retreatment.
Only mild side effects were observed over the complete cycle of treatment: femoral phlebitis (one patient), femoral vein thrombosis (one patient) due to the cannula being left in situ for over 30 days; a short‐lived (24 hours) slight arthralgia with peripheral edema due to hemolysis in the extracorporeal circuit. These complications are common in all hemapheretic procedures and not specific for this technique.
Discussion
After PP treatment all patients showed both clinical improvement and decrease in antiAchR‐Ab concentrations. Moreover, clinical improvement was progressive despite the tendency for antiAchR‐Ab to reach its initial value between one session and another.
A second question concerns the mechanism of action of the procedure itself. Since tryptophan is a highly hydrophobic aminoacid, a selective binding to an unidentified hydrophobic site on the surface of the antibodies has been suggested, but further informations are needed to clarify this point.
No risk would be theoretically predicted for this procedure, however, even if tryptophan was released from the column into the circuit, because it is a normal blood component.
A separate immunoadsorbent column was used for each patient for the complete PP cycle. After each session the columns were repeatedly perfused with a glycerol solution, to make them ready for another PP, and sterilized with sodium azide. The columns so treated retained their efficiency, and no septic or febrile reaction arose. No patient showed clinical signs of immunodeficiency nor opportunistic infections have been found as effect of the combined PP and immunosoppressive drug treatment.
When compared to PE, PP on triptophan columns seems to have the same rationale and effectiveness, but fewer undesired side effects due to its selectivity. Although limited in number, we consider our resultsinteresting.
Conclusions
Triptophan columns, with their semiselective immunoadsorbent activity, appear to be able to remove large quantities of antiAchR‐Ab, immunocomplexes, immunoglobulins and complement fractions from plasma.
Our experience show that PP with these new immunoadsorbents for treatment of MG provides good clinical results without risks linked to the procedure, as well as contained management costs.
References
- AAEM Quality Assurance Committee. American Association of Electrodiagnostic Medicine. Literature review of the usefulness of repetitive nerve stimulation and single fiber EMG in the electrodiagnostic evaluation of patients with suspected myasthenia gravis or Lambert‐Eaton myasthenic syndrome. Muscle Nerve 2001; 24(9)1239–1247
- Batocchi A. P., Evoli A., Di Schino C., Tonali P. Therapeuthic apheresis in myasthenia gravis. Ther. Apher. 2000; 4(4)275–279
- Bruck W., Neubert K., Berger T., Weber J. R. Clinical, radiological, immunological and pathological findings in inflammatory CNS demyelination‐possible markers for an antibody‐mediated process. Mult. Scler. 2001; 7(3)173–177
- Chia D., Barnett E. V., et al. Quantitation and characterization of soluble immune complexes precipitated from sera by polyethylene glycol. Clin. Exp. Immunol. 1979; 37: 399–400
- de Andres C., Anaya F., Gimenez‐Roldan S. Plasma immunoadsorption treatment of malignant multiple sclerosis with severe and prolonged relapses. Rev. Neurol. 2000; 30(7)601–605
- Hadden R. D., Gregson N. A. Guillain‐Barre syndrome and Campylobacter jejuni infection. Symp. Ser. Soc. Appl. Microbiol. 2001; 30: 145S–154S
- Haupt W. F. Recent advances of therapeutic apheresis in Guillain Barrè syndrome. Ther. Apher. 2000; 4(4)271–274
- Haupt W. F., Birkmann C., van der Ven C., Pawlik J. Apheresis and selective adsorption plus immunoglobulin treatment in Guillain Barrè syndrome. Ther. Apher. 2000; 4(3)198–200
- Haupt W. F., Rosenow F., van der Ven C., Birkman C. Immunoadsorption in Guillain Barrè syndrome and myasthenia gravis. Ther. Apher. 2000; 4(3)195–197
- Hughes R. A., Hadden R. D., Gregson N. A., Smith K. J. Pathogenesis of Guillain‐Barre syndrome. J. Neuroimmunol. 1999; 100(1–2)74–97
- Lindstrom J. An easy for antibodies to human acetylcholine receptor in serum from patient with myastenia gravis. Clin. Immunol. Immunopathol. 1977; 7: 36–43
- Malcheski P. S., Horiuchi T., Usami M., Emura M., Nose J. Blood detoxification by membrane plasma filtration. Int. J. Art. Org. 1986; 9(5)349–354
- Morosetti M., Meloni C., Iani C., Caramia M., Galderisi C., Palombo G., Gallucci M. T., Bernardi G., Casciani C. U. Plasmapheresis in severe forms of myasthenia gravis. Artif. Organs 1998; 22(2)129–134
- Nakaji S., Oka K., Tanihara M., Takakura K., Takamori M. Development of a specific immunoadsorbent containing immobilized synthetic peptide of acetylcholine receptor for treatment of myasthenia gravis. Ther. Apher. 2000; 4(2)124–126
- Nevo Y. Childhood chronic inflammatory demyelinating polyneuropathy. Eur. J. Paediatr. Neurol. 1998; 2(4)169–177
- Palace J., Vincent A., Beeson D. Myasthenia gravis: diagnostic and management dilemmas. Curr. Opin. Neurol. 2001; 14(5)583–589
- Ross E. A., Branham M. L., Tebett I. R. High mass clearance of autoantibodies from a murine model of lupus nephritis by immunoadsorption using star‐configured polyethylene glycols. J. Biomed. Mater. Res. 2001; 55(1)114–120
- Sghirlanzoni A., Pelucchetti D., et al. Myasthenia gravis: prolonged treatment with steroids. Neurology 1984; 34: 170–174
- Steimnam L. Immunotherapy of multiple sclerosis: the end of the beginning. Curr. Opin. Immunol. 2001; 13(5)597–600
- Tagawa, et al. Ability to remove immunoglobulins and anti‐ganglioside antibodies by plasma exchange, DFP and immunoadsorption. J. Neurol. Sci. 1998; 157(1)90–95
- Takahashi I., Sawaishi Y., Takeda O., Enoki M., Takada G. Childhood multiple sclerosis treated with plasmapheresis. Pediatr. Neurol. 1997; 17(1)83–87
- Vedanarayanan V. V., Chaudhry V. Guillain Barrè syndrome: recent advances. Indian J. Pediatr. 2000; 67(9)635–646
- Vincent A., Palace J., Hilton‐Jones D. Myasthenia gravis. Lancet 2001; 357(9274)2122–2128
- Wandinger K. P., Sturzebecher C. S., Bielekova B., et al. Complex immunomodulatory effects of interferon‐beta in multiple sclerosis include the upregulation of T helper 1‐associated marker genes. Ann. Neurol. 2001; 50(3)349–357
- Yamavaki N. Artificial receptors and their clinical applications to blood purification therapy. J. Synt. Org. Chem. 1985; 43: 1024
- Yuki N., Susuki K., Hirata K. Ataxic form of Guillain‐Barr syndrome associated with anti‐GD1b IgG antibody. J. Neurol. Neurosurg. Psychiatry 2000; 69(1)136–137