Abstract
We prepared microspheres of Vincristine Sulfate (VCR) through drying‐from‐oil method, then mixed the microspheres into 0.7% collagen swelling solution to prepare emulsion, spread the emulsion on plate to form film by frozen‐dry method. The film was cross‐linked and sterilized, then planted into the site of tumor and expected to release at steady speed. We measured the release of VCR in vivo and in vitro by HPLC. The results demonstrated that VCR controlled release films release at approximate steady speed in 15 days.
Introduction
Tumour is a type of common and frequently‐occuring ailment, which was detrimental to human's health and life. At present, chemical therapy still is the main method to cure the tumour. For chemical therapy, some curative effects can be found in general administration, but there still exists that not enough drug concentration at the focus of tumour and relatively heavy toxic and side effects in general administration. We prepared the controlled anti‐neoplastic drug delivery system with the carrier of biodegradable collagen, then plant it into the body, conceived that it can attain a controlled release purpose, and increase the local drug concentration, enhance the curative effects of the neoplastic drug. On the other hand, for its features of low‐dosage and slow‐release, the film can be expected to achieve the goal that ease its general toxic and side effects, improve the patients' endurance to the anti‐neoplastic drug. The controlled Vincristine Sulfate (VCR) release film can be expected to supply a new method to cure malignant tumour, such as acute and chronic leukaemia, malignant lymphosarcoma, little cell lung cancer, and breast cancer (Pridum et al., [Citation1993]; Citation[[Zheng]).
Materials
VCR, Pharmaceutical factory of Hangzhou Minsheng; Swelling collagen solution(solid content 1.39%), Institute of Biomedical Engineering, Chinese Academy of Medical Science; Malonic acid, Shanghai Nanxiang Reagent Factory of China; Acetone, PLGA, Span 85, Liquid paraffin, Hexane, Analytic pure; Methanol acetonitrile, Chromatographic pure; Double distilled water; 714 HPLC system, Gilson Medical Electronics; SPD‐6AV changeable wavelength ultraviolet detector; AUTOSCIENCE vacuum pump; PB solution: pH = 3, 0.04 mol/l phosphate buffer solution.
Methods and Results
Preparation of VCR Microspheres
Dissolve 1 g PLGA with 10 ml acetone, then put 50 mg VCR in it; mix 4.5 g span‐85 into 100 ml liquid paraffin, place it in 10°C water bath, drop PLGA solution into liquid paraffin and increase water temperature to 53 degrees C slowly, keep stirring for 1 h at this temperature, then get VCR microspheres by suction filtration, wash microspheres with hexane, then dry it in decompression circumstance, the microspheres are round and tough, particle diameter is between 100 and 600 μm (Imbert‐Marcille et al., [Citation1995]; Lotan, [Citation1995]). is the distribution of diameter of VCR microspheres.
Table 1. Distribution of diameter of VCR microspheres
Preparation of VCR Film
Dissolve 50 g (1.39%) swelling collagen solution with 0.3%(w/v) malonic acid, get 100 ml 0.7%(w/v) swelling collagen solution; then put 0.5 g microspheres which were prepared as the above method, 0.025 g Span‐85 and 0.1 g tween‐80, into the 0.7% swelling collagen solution, stir at 300 rpm till the emulsion is homogeneous; put the liquid evenly on the polytetrafluoroethylene plate and frozen‐dry it, then cross‐link it with 0.3% formaldehyde solution, wash it with water, frozen‐dry it again.
Determination of Release of VCR Film
HPLC Conditions
Stainless stell C18 5 μm chromatography column, 150 mm × 4.6 mm; mobile phase: methanol/PB solution (55/45); flow speed: 1.0 ml/min; detection wavelength 254 nm; injection volume: 20 μl (Van Belle, [Citation1992]; Xu et al., [Citation1996]).
Chromatography Behavior of VCR and Standard Curve
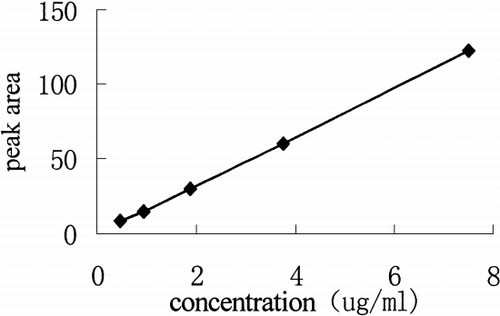
Weigh 0.75 mg VCR precisely, dissolve it in mobile phase, prepare standard solution whose concentration are 7.5 μg/ml, 3.75 μg/ml, 1.875 μg/ml 0.9375 μg/ml and 0.46875 μg/ml, then determine their absorption spectrum, we can get the linear relation from peak area (Y) and concentration (X):
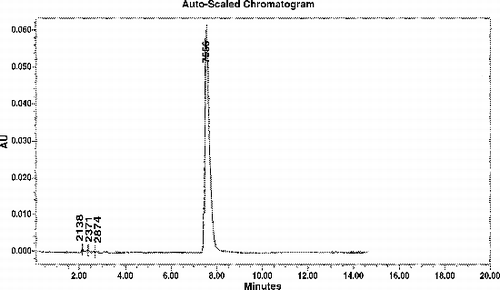
is the chromatography behavior, is the standard curve of VCR. VCR retention time is 7.5–7.6 minutes.
Release of VCR Film
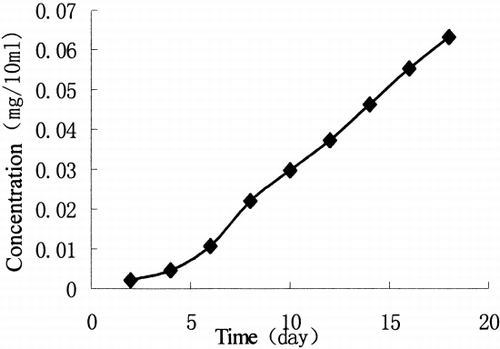
Put 1 × 1 cm2 VCR film into 15 ml phosphate buffer solution (pH 7.4, 0.1 mol/L, 37 degrees C) to release, vibrate at the speed of 72 times per minute. Every 48 h, we take out 1 ml release solution to determine and add 1 ml fresh PB solution into it. The samples were filtrated by Millipore filter before injection into HPLC. According to the peak area of 20 μl release solution, we can get the concentration of the release solution. is the release curve of VCR film in vitro.
Test of Recovery and Repetition of HPLC
Weigh 0.5 mg VCR precisely, dissolve it with mobile phase, VCR concentration in the solution is 0.05 μg/ml. Add the solution (100 μl) into the control release solution (without VCR), then determine it by HPLC. Determine the concentration of 0.05 μg/ml VCR solution 5 times by HPLC to define the repetition of this method. is the result of recovery and repetition test.
Table 2. Test of recovery and repetition
The Lower Limit of Determination of the Method
Inject 20 μl standard solution into HPLC, we can determine the lower limit is 10 ng/ml at limit of the sensitivity of HPLC.
Discussions and Conclusions
Controlled release system is a dosage form by which the drug is released to specific organ for therapeutic action at constant speed (zero order or approach to zero order speed), it developed very fast and was applied in clinic therapy in recent years. There are many advantages in controlled release system, such as longer effective time of drug action, convenient use without frequent administration. Compared with the slow release system, its releasing is uniform and steady, so it will result in little fluctuation in the blood drug and low side effects (Jalil and Nixon, [Citation1990]).
Several respects should be considered in designing controlled release system, such as drug's physical and chemical property, pharmacokinetics, pharmacological and physiological property. Although collagen has good degradable property in vivo, preparing film just mixing collagen with drug, drug is still able to diffuse from collagen film and not release following the collagen's degradation strictly. What's more, the degradation rate of collagen is not very constant, so simply mixing drug with collagen will not accomplish controlled release.
We prepare VCR microspheres by drying‐from‐oil method, then mix it with collagen to prepare film, this operation not only utilize gradual degradable property of collagen in vivo, but also the volume of microspheres is so large that it can not diffuse easily, and the drug will be released slowly from the microspheres. So we can expect to prepare an ideal drug controlled‐release system through the method.
There are still many steps and details in the technology of preparing microspheres which could affect the biodegradation of microspheres and release of drug, such as the content proportion of drug to materials, solubility of the materials, homogeneous degree of drug in material solution, stirring speed and temperature of the whole system, the content proportion of swelling collagen solution to microspheres and cross‐linking degree of film. We decided the optimum coating technology with orthogonal design by changing the conditions of preparing microspheres, observing the structure of microspheres and its release property in vitro.
VCR has good stability in mobile phase solution, there is only a tiny peak of decomposed product before the VCR peak 48 hours later, it doesn't affect the determination of VCR. It was reported that the stability of VCR in aqueous is familiar to our result, VCR will decompose little in 50 hours, and it has good repetition of determination. For decreasing the error, it demands that we determine the standard substance and sample as soon as possible.
At present, there are still some questions to be resolved better, the key is to decide the drug content with more effective and lower toxicity, another question to be resolved is that we should find a method to guide the drug act to the target region. Drug or bio‐materials binding to immunological antibody will be the perspective controlled release system.
Acknowledgment
This work was supported by National Project Committee for Important Science and Technology Item.
References
- Imbert‐Marcille B. M., Berreur M., Thedrez P., et al. Effects of interferon gamma and tumor necrosis factor alpha association in intraperitoneal radioimmunotherapy: studies on an in vitro micrometastasis model. Anti‐Cancer Drug Des. 1995; 10: 491–505
- Jalil R., Nixon J. R. J. Microencapsulation 1990; 7: 245
- Lotan R. Retinoids and their receptors in modulation of differentiation development, and prevention of head and neck cancer. Differentiation Therapy, S. Vaxman. Chall. Mod. Med., 1995; 10: 303
- Pridum N., Karrer K., Sun Y., . Adjuvant chemotherapy after surgery with curative intent for SCLC. Adjuvant Therapy of Cancer VII, S. Salmon, et al. JB Lippincott Co., Phildelphia 1993; 132–137
- Van Belle S.J. P. Determination of vinca alkaloids in mouse tissue by high performance liquid chromatography. J. Chromatogr. 1992; 578: 223
- Xu S., Deng D., Cai J. Determination of Ipriflavone by RP‐HPLC. Chin. J. Pharmaceutical Analysis 1996; 16(2)81–83
- Zheng J. Long‐effect pharmaceutics and controlled release system. Pharmaceutics, N. Xi, X. Gu. People's Health Publish House