Abstract
In type 1 diabetes an absolute deficiency of insulin secretion requires exogenous insulin supply to guarantee the patient's life avoiding ketoacidotic coma and to prevent the chronic complications of diabetes. In order to obtain a more physiological replacement therapy different approaches have been pursued since the early 70s to create an artificial wearable pancreas able to deliver insulin according to the blood glucose values as determined by continuous monitoring. Four components are considered essential for the realisation of an artificial pancreas: the sampling system, the glucose sensor, the mathematical models and the related algorithms for the calculation of the insulin doses and the infusion system for the insulin delivery. At present the still unsolved issues are mainly represented by the availability of reliable continuous glucose monitor and control algorithms, while the new technologies allow for the miniaturisation of the system.
Introduction
Diabetes Mellitus is a chronic disease characterised by an alteration of glucose metabolism due to an absolute ((type 1)) or relative ((type 2)) insulin deficiency. In type 1 diabetes, an autoimmune process leads to the β‐‐cells destruction with a progressive and complete loss of insulin secretion. The consequent metabolic alterations require exogenous insulin supply to guarantee the patient's life avoiding ketoacidotic coma. The second goal of insulin replacement is the prevention of the chronic complications of the disease. Several studies ((Diabetes Control and Complications Trial Research Group, [Citation1993]; UK Prospective Diabetes Study Group, [Citation1998])) have shown that the optimisation of metabolic control can reduce or minimise the occurrence of these complications. In recent years different degree of intensified insulin therapy were applied in order to reach near normalisation of metabolic control. Good results were obtained with the multiple daily insulin injections regime combined with dose adjustment according to the blood glucose home monitoring. However a strict glycaemic control secondary to an intensified therapy was often associated with an increased risk of hypoglycaemia ((Diabetes Control and Complications Trial Research Group, [Citation1993])).
The major problem of insulin therapy in type 1 diabetes is the impossibility to ensure a real physiological replacement of the lost β‐‐cell function; in fact in normal individuals a basal insulin secretion is delivered in the portal system of the liver and is maintained throughout the day with peaks in correspondence of meals, according to blood glucose levels and to a number of other hormonal and neurological signals and. None of these mechanisms is preserved in patients on exogenous insulin therapy. During the last thirty years an alternative approach for the insulin replacement was explored by different groups in order to create a more physiological β‐‐cell substitution: the utilisation of a device for continuous insulin infusion whose delivery rate is controlled by the glycaemic values determined through a continuous monitoring system (()). The first, and still not equalled, example of artificial pancreas, the Biostator, was developed in the early 70s by Albisser et al. (([Citation1974])). The Biostator, a macro bedside device, consisted of a system for continuous venous sampling through a double lumen catheter, an electrode for continuous glucose monitoring, a software for the management of the collected data and a system of pumps for continuous feed‐‐back insulin infusion and glucose or glucagons supply in case of hypoglycaemia. Following the Biostator experience, three main area of research where investigated: 1)) the miniaturisation of the system to create a wearable device for daily life use, 2)) the continuous glucose monitoring and 3)) the metabolic model for the control algorithms.
Figure 1. Overall architecture of an endocrine artificial pancreas. The artificial endocrine pancreas consists of a system for continuous glucose sampling, an electrode for continuous glucose monitoring, a software for the management of the collected data and a system of pumps for continuous feed‐‐back insulin infusion and glucose supply in case of hypoglycaemia.
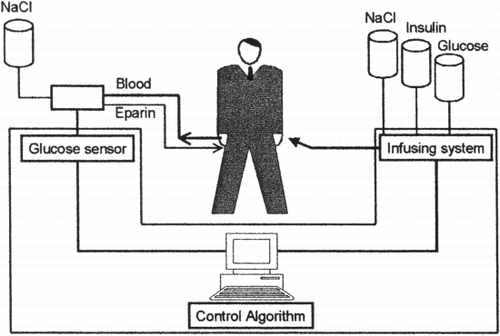
Our article will give an overview on the state of the art on the research for the realisation of the artificial pancreas and especially it will be focused of the glucose continuous monitoring techniques that still represent the mayor challenge for the realisation of such a device.
Continuous Glucose Monitoring
The realisation of an instrument for continuous blood glucose measurement was one of the mayor topic in diabetes research since the 70s and different approaches were explored up to now from the first animal implanted sensor, developed and tested by Chang et al. (([Citation1973])). The utilisation in clinical practice requires that a system for in vivo glucose continuous monitoring fits the following basic requirements: generates reliable measurements, is stable for long term use and is minimally invasive.
The latter requirement is most important to guarantee an appropriate compliance of the patients. Three different methods are actually under development: the glucose‐‐sensor devices totally or partially implanted in the subcutaneous tissue, the extraction of glucose from interstitial fluid and the totally non invasive methodology based on spectroscopic techniques ((Pickup et al., [Citation1999])).
The implanted glucose sensor devices can be divided in two main subgroups, the transcutaneous needle‐‐type enzyme electrodes and the totally implanted sensor.
The transcutaneous needle‐‐type glucose sensor (()) is based on the glucose oxidase immobilization on the tip of a tube that consists of a platinum wire melted in an oxygen//natural gas flame to form a ball ((the anode)) and of a steel tube stained with silver ((the catode)) ((Shichiri et al., [Citation1988])). The glucose oxidase in the presence of glucose and oxygen catalyses a reaction that generates gluconic acid and hydrogen peroxide; when loaded with a 0–6 V voltage the sensor measures the generated hydrogen peroxide. At the end of the process, the sensor generates a current that is proportional to the glucose concentration. The system was initially tested by Sichiri e coll in three pancreatectomised dogs ((Shichiri et al., [Citation1982])). They demonstrated that the system had a good linearity within a wide range of blood glucose measurements ((0–500 mg//dl)), that was not affected by temperature and that the generated glucose measurements were strictly correlated with the intravenous control measurements even if a 15%% reduction and a delay of 2–5 minutes was registered. The major problem related to this type of glucose sensor is the acute local inflammatory reaction, affecting the glucose and oxygen concentration in the interested area resulting in inappropriate results.
Figure 2. Transcutaneous needle‐‐type glucose sensor. The sensor is based on the glucose oxidase immobilization on the tip of a tube that consists of a platinum wire ((the anode)) and of a steel tube stained with silver ((the catode)) The glucose oxidase in the presence of glucose, oxygen and of a 0–6 V volt current catalyses a reaction that generates gluconic acid and hydrogen peroxide; the sensor measures the generated hydrogen peroxide. ((Adapted from Shichiri et al. (([Citation1988])).))
![Figure 2. Transcutaneous needle‐‐type glucose sensor. The sensor is based on the glucose oxidase immobilization on the tip of a tube that consists of a platinum wire ((the anode)) and of a steel tube stained with silver ((the catode)) The glucose oxidase in the presence of glucose, oxygen and of a 0–6 V volt current catalyses a reaction that generates gluconic acid and hydrogen peroxide; the sensor measures the generated hydrogen peroxide. ((Adapted from Shichiri et al. (([Citation1988])).))](/cms/asset/4cd27b1d-1e89-413a-8cc4-228bf2c20b79/ianb19_a_11116852_uf0002_b.gif)
Totally implanted sensors were primarily developed to reduce the variability of glucose measurements obtained with the transcutaneous ones. They can be based on an enzyme electrode, using glucose oxidase, or on the so called artificial receptors that are based primarily on the molecular imprinting or on the near infrared fluorescence. The abiotic glucose receptors based on molecular imprinting, use the property of macromolecules such as monomers, to interact with an analogue of the analyte. This reaction generate a template that is used to create, through polymerisation, a polimer that can be used to bind and thus measure glucose ((Chen et al., [Citation1997])).
The second group of artificial receptors are based on fluorescent molecules that can interact with glucose or glucose derivatives. The binding of glucose to specific protein labelled with fluorophores, alters protein conformation and modifies fluorescence ((Marvin and Hellinga, [Citation1998]; Tolosa et al., [Citation1997])).
An alternative approach is the extraction of glucose from the subcutaneous interstitial fluid for external evaluation. Three different techniques are actually used to extract glucose: the microdialysis, the microperfusion and the reverse iontophoresis (()).
Figure 3. Minimally invasive sampling techniques. Three different minimally invasive sampling techniques for continuous glucose sampling that are based on the extraction of glucose from the interstitial fluid for an external determination. A)) Microdyalisis, B)) Open flow microperfusion and C)) reverse iontophoresis. ((Adapted from Hashigughi et al. (([Citation1994])), Trajanoski et al. (([Citation1997])), and Pickup (([Citation2000])).))
![Figure 3. Minimally invasive sampling techniques. Three different minimally invasive sampling techniques for continuous glucose sampling that are based on the extraction of glucose from the interstitial fluid for an external determination. A)) Microdyalisis, B)) Open flow microperfusion and C)) reverse iontophoresis. ((Adapted from Hashigughi et al. (([Citation1994])), Trajanoski et al. (([Citation1997])), and Pickup (([Citation2000])).))](/cms/asset/4002dddd-63fa-484b-98ab-8f126914a232/ianb19_a_11116852_uf0003_b.gif)
The microdialysis was the first and most studied of the three techniques; it is based on a microdialysis catheter inserted in a probe. The hollow fiber probe is perfused with an isotonic saline solution that is transported at a constant rate ((controlled by a microroller pump)), through an inlet cannula to the tip of the probe. At that site the perfusate is exchanged, through a dialysis membrane with the dialysate that is collected via an outlet cannula. The dialysate can be then analysed through different techniques even if the most used is the enzyme electrodes one? Hashigughi et al. (([Citation1994])) tested the system in 5 healthy volunteers and in eight diabetics by continuous monitoring of glucose in the abdominal subcutaneous tissue for about 8 days. The results showed a strict correlation between interstitial and blood glucose levels ((r == 0.99)) in a wide range of glucose concentrations ((1.7–27.8 mM)); the time delay between the two compartment was of about 6.9 minutes during blood glucose rise and of about 8.8 minutes during blood glucose decrease. The system was stable for four days and then needed to be recalibrated in all the individuals.
More recently a different technique has been developed to gain access to the interstitial glucose: the open flow microperfusion ((Trajanoski et al., [Citation1997])). A double‐‐lumen catheter with micro‐‐perforations ((120 holes of about 0.5 mm diameter created with excimer laser)) on its tip is inserted in the subcutaneous tissue. A peristaltic pump regulates the continuous perfusion of the catheter and the collection of the interstitial fluid ensuring a partial equilibrium between the perfusate and the interstitial fluid. The measure of glucose from interstitial fluid requires a calibration that can be done with different methodology: zero flow rate, no net flux, recirculation procedures and ionic reference and suction technique. Schaupp et al. (([Citation1999])) have tested the system in 25 volunteers in basal conditions ((after 12 h of overnight fast)) and in 5 volunteers during an hyperglycaemic clamp comparing interstitial to blood glucose measurements. In both the condition interstitial glucose measurements were lower than the blood glucose ones and the adoption of a recovery technique for calibration allowed to obtain a better correspondence between the two measures resulted in a significant correspondence of the modifications of the glucose concentrations in the two compartment under dynamic conditions.
A third approach to extract glucose from the interstitial fluid is the reverse iontophoresis ((Pickup, [Citation2000])). A low voltage electric current is applied to the intact skin creating a flux of charged ions between two electrodes that determines a movement of glucose and other neutral species to the cathode by convective flow. A sensor electrode is associated with the cathode to measure the interstitial glucose using glucose oxidase. This technique was implemented in the GlucoWatch Biografer ((Cygnus Inc., Redwood City, CA, USA)), an automatic glucose reader for frequent blood glucose measurements ((one determination every 20 minutes)). This instrument was recently tested by Gark et al. (([Citation1999])) against fingerstick blood glucose measurements both in controlled ((outpatient clinic)) and uncontrolled ((home setting)) conditions in type 1 diabetic patients. The resulting values were highly correlated with capillary measurements in both conditions ((r == 0.90 and r == 0.85 respectively)) when a time delay of around 20 minutes was considered between the two compartment. It was also investigated the correspondence of two different GlucoWatch Biographers applied simultaneously to the same patient; the data collected showed a significant correspondence between the two measurements series ((r == 0.94)) indicating that the system is effective and safe for discrete continuous glucose monitoring.
The last group of glucose sensing devices are the completely non invasive techniques. They comprise the near infrared spectroscopy, the light scattering and the photoacustic spectroscopy. The potential advantage of these techniques is enormous due to their absolute absence of invasivity, but they are still not reliable and very few studies have been performed up to now for the definition of their clinical use ((Arnold, [Citation1996]; Heinemann and Schmelzeisen‐‐Redekeron, [Citation1998])).
Modelling and Control Algorithms
Data deriving from continuous glucose monitoring need to be processed and interpreted in order to drive a feed back insulin infusion. A number of mathematical models of glucose metabolism and control algorithms have been developed to guarantee an automatic control on a moment‐‐to‐‐moment basis of the glucose–insulin relationship. The control algorithms could be implemented in a completely automatic controller ((closed‐‐loop systems)) or in semi‐‐closed loop system requiring an external intervention to modify the advice for the insulin delivery taking into consideration not only blood glucose values, but also information on diet, exercise, etc.
The original algorithms for insulin adjustment were based only on the absolute values of blood glucose resulting in a poor outcome ((Nomura et al., [Citation1984])). Better results were subsequently obtained by the dynamic control algorithms also considering of the entity of the variations of glucose concentration both in term of amplitude and speed of change; a further increment was then achieved with the development of self adaptive algorithms and of expert systems.
In the early '80 Shichiri and Kawamori (([Citation1985])) have developed a wearable artificial pancreas that was based on a double control algorithm: one was regulated by the absolute glucose value while the other was regulated by the variation of blood glucose levels. In that experience insulin was infused directly in the veins with a scarce possibility to be implemented routinely.
The clear need to infuse insulin in subcutaneous tissue for a clinical daily use lead to the development of more complex algorithms modelling the pharmacokinetics of insulin infused subcutaneously. For that purpose the same authors developed a three‐‐compartments model consisting of the local subcutaneous depot, the insulin local degradation and the insulin absorption ((Asakawa et al., [Citation1987])). The model showed a good capability to manage insulin infusion obtaining fair results in term of metabolic control; however it was not able to cover special situations altering insulin sensitivity such as hypoglycaemias or intercurrent illness. A specific algorithm that models insulin sensitivity was developed based on a self‐‐adaptative function able to detect changes of insulin sensitivity and to adjust the glucose–insulin relation accordingly ((Kawamori et al., [Citation1980])).
The limit of the algorithms based on subcutaneous both glucose measurement and insulin infusion is that they can be effective in fasting conditions but they cannot manage the postprandial state with the same efficacy without an external intervention. The efforts for further improvements could be represented by the development of semi‐‐closed control loop systems able to elaborate additional data in respect to the blood or interstitial glucose measurements. The manual introduction by the patient of information related to the meals ((time of assumption, consistency, composition, etc.)) could allow to generate advice on the consistency of the bolus and on the rate for the basal infusion rate in the following hours.
Infusion Systems
The last component of an artificial pancreas is the infusion system for the insulin administration. Different companies have developed insulin pumps during the last thirty years; actually the different models available on the market are reliable, safe and easy to manage for a large routine use, so that they do not represent a limiting step in the process of development of the artificial pancreas. Questions could however arise about the site of infusion; in fact in order to reproduce a more physiological replacements the possibility to infuse insulin intraperitoneally was largely investigated in the last years.
The subcutaneous infusion in fact has three principal point of weakness: it fails to reestablish the physiological portal‐‐peripheral gradient, generates peripheral hyperinsulinism and fails to mimic the rapid rise and decline of plasma insulin after meals as can be observed in healthy subjects. Moreover the continuous subcutaneous infusion presents other disadvantages: 1)) the use of regular insulin infused continuosely in microamounts expose the patient to the risk of rapid metabolic deterioration up to severe ketoacidosis in case of interruption of insulin delivery due to pump malfunction or to occlusion of the infusion catheter, 2)) local inflammatory processes caused by the presence of the needle could affect the absorption of the insulin, 3)) insulin precipitation or aggregation in the syringe, and 4)) psychological rejection of the pump ((Hoffman and Ziv, [Citation1997])).
The intraperitoneal administration offers some advantages in respect to the subcutaneous infusion, like the absorbtion of a relevant percentage of the infused insulin in the portal system with consequent lower peripheral insulin levels, reduced risk of hypoglycaemia and lower variability. Moreover the peritoneal absorption does not deteriorate also after long term administration using the implanted device. Despite this advantages, actually the intraperitoneal administration is poorly utilised due the more relevant technical problems than during the subcutaneous infusion. In fact several experiences have shown an higher instability of the system with respect to the external pumps due to battery depletion, catheter occlusion ((13–27%% year)), pump failure, insulin aggregation, infusion rate slowdowns and backflows ((Hoffman and Ziv, [Citation1997])).
Conclusions
The possibility of developing an artificial organ to replace a lost function could be a great opportunity for a more physiological approach to the treatment of different disease where the pathology is associated with the impairment of a specific function such as in type 1 diabetes in which the primary defect is the insulin deficiency.
During the last thirty years several attempts were made to create a usable artificial pancreas but so far the artificial organ was only applied for scientific purposes. In fact several problems encountered during its development such as primarily the dimensions of the different systems, the realisation of reliable monitoring devices and decision algorithms have prevented its use in routine clinical practice.
Some barriers, such as the miniaturisation of the systems, have been superated by the advancement of the technologies but several others problems ((monitoring devices and algorithms)) still represent a barrier for the realisation of a real wearable artificial pancreas.
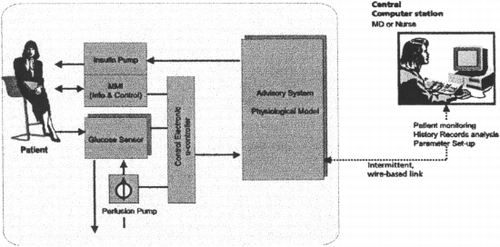
More scientific research and development efforts will thus be necessary to overcome the existing problems in the next future, as in the ADICOL ((Advanced Insulin Infusion system using a Control Loop)) project founded by the European Union within the 5th Framework for the Information Technologies ((ADICOL ((Advanced Insulin infusion using a Control Loop)) Project, [Citation1999])). The aim of the ADICOL international Consortium is to develop a prototype of a feed‐‐back controlled personal insulin dosing system (()) based on the newest biosensor technology, sophisticated software simulation tools and new micro‐‐dosing means. In order to reach the target two parallel line of research will be conducted during the duration of the project. The first one focused on the development of a reliable device for the continuous blood glucose monitoring and the second for the realisation of the computer model for the insulin infusion control. Two different model will be employed in the system of which one will control the basal insulin infusion in fasting condition realising a real closed‐‐loop and the other will take care of the meals driving insulin infusion, both boluses and basal rate, in post‐‐prandial periods.
Figure 4. Adicol overall architecture. The system consists of a glucose sensor for continuous glucose measurements, a physiological model of glucose metabolism in humans for the interpretation of the collected data and for the generation of the advice on insulin therapy and an infusing device for insulin administration.
References
- ADICOL ((Advanced Insulin infusion using a Control Loop)) Project. 5th EC Framework Programme. IST—Information Society Technologies. 1999, Project No.: IST‐‐1999‐‐14207
- Albisser A. M., Leibel B. S., Ewart T. G., Davidovac Z., Botz C. K., Zingg W., Schipper H., Gander R. Clinical control of diabetes by the artificial pancreas. Diabetes 1974; 23: 397–404
- Arnold M. A. Non‐‐invasive glucose monitorino. Curr. Opin. Biotechnol. 1996; 7: 46–49
- Asakawa N., Saito Y., Yamasaki Y., Kawamori R., Shichiri M. Validation of closed‐‐loop subcutaneous insulin infusion algorithm. Application of subcutaneous insulin absorption kinetics. Diabetes Res. 1987; 5: 193–198
- Chang K. W., Alsenberg S., Soeldner J. S., Hiebert J. M. Validation and bioengineering aspects of an implantable glucose sensor. Trans. Am. Soc. Artif. Intern. Organs 1973; 19: 352–360
- Chen G., Guan Z., Chen C.‐‐T., Fu L., Sundaresan V., Arnold F. A glucose sensing polymer. Nat. Biotechnol. 1997; 15: 354–357
- Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long‐‐term complications in insulin‐‐dependent diabetes mellitus. N. Engl. J. Med. 1993; 322: 977–986
- Gark S. K., Potts R. O., Ackerman N. R., Fermi S. J., Tamada J. T., Chase H. P. Correlation of fingerstick blood glucose measurements with GlucoWatch Biographer glucose results in young subjects with type 1 diabetes. Diabetes Care 1999; 22(10)1708–1714
- Hashigughi Y., Sakakida M., Nishida K., Uemura T., Kajiwara K., Shichiri M. Development of a miniaturized glucose monitoring system by combining a needle‐‐type glucose sensor with microdyalisis sampling method. Diabetes Care 1994; 17(5)387–396
- Heinemann L., Schmelzeisen‐‐Redekeron G. Non invasive continuous glucose monitoring in type 1 diabetic patients with optical glucose sensors. Diabetologia 1998; 41(7)848–854
- Hoffman A., Ziv E. Pharmacokinetic consideraration of new insulin formulations and route of administration. Clin. Pharmacokinet. 1997; 33(4)285–301
- Kawamori R., Yamasaki Y., Murata T., Morishima T., Yagi T., Goriya Y., Shichiri M., Abe H. Validation of self‐‐adaptative control algorithm for blood glucose concentration in artificial beta cell. Automedica 1980; 3: 183–189
- Marvin J. S., Hellinga H. W. Engineering biosensors by introducing fluorescent allosteric signal transducers: construction of a novel glucose sensor. J. Am. Chem. Soc. 1998; 120: 7–11
- Nomura M., Shichiri M., Kawamori R., Yamasaki Y., Iwama N., Abe H. A mathematical insulin‐‐secretion model and its validation in isolated rat pancreatic islets perfusion. Comput. Biomed. Res. 1984; 17: 570–579
- Pickup J. Sensitive glucose sensing in diabetes. Lancet 2000; 355: 426–427
- Pickup J., McCartney L., Rolinski O., Birch D. In vivo glucose sensing for diabetes management: progress towards non‐‐invasive monitoring. BMJ 1999; 319(7220)1289–1295
- Schaupp, Ellmerer M., Brunner G. A., Wutte A., Sendlhofer G., Trajanoski Z., Skrabal F., Pieber T. R., Wach P. Direct access to interstitial fluid in adipose tissue in humans by use of open‐‐flow microperfusion. Am. J. Physiol., Endocrinol Metabol. Gastrointest. Physiol. 1999; 276: E401–E408
- Shichiri M., Kawamori R. Optimised algorithms for closet‐‐loop glycemic control system. Computer System for Insulin Adjustment, J. Beyer, M. Albisser, J. Scherezenmeir, L. Lehmann. Diabetes Mellitus, Panscientia‐‐Verlag, Switzerland 1985; 171–183
- Shichiri M., Kawamori R., Yamasaki Y., Hakui N., Abe H. Wearable artificial endocrine pancreas with needle‐‐type glucose sensor. Lancet 1982; 2: 1129–1131
- Shichiri M., Kawamori R., Yamazaki Y. Needle‐‐type glucose sensor. Methods Enzymol. 1988; 137: 326–334
- Tolosa L., Szmacinski H., Rao G., Lakowicz J. R. Lifetime‐‐based sensing of glucose using energy transfer with a long‐‐lifetime donor. Anal. Biochem. 1997; 250: 102–108
- Trajanoski Z., Brunner G. A., Schaupp L., Ellmerer M., Wach P., Pieber T. R., Kotanko P., Skrabal F. Open‐‐flow microperfusion of subcutaneous adipose tissue for on‐‐line continuous ex vivo measurement of glucose concentration. Diabetes Care 1997; 20: 1114–1121
- UK Prospective Diabetes Study Group. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes: UKPDS 33. Lancet 1998; 352: 837–853