Abstract
We have previously reported the experimental use of genetically engineered Escherichia coli with microencapsulation to lower nitrogenous waste. Concern has surfaced, nonetheless, about safety of genetically engineered product. The purpose of this study is to explore the alternative use of probiotics in removal of plasma urea. After repeated cycles of exposure of Lactobacillus delbrueckii in urea‐rich medium under anaerobic environment, the organisms were demonstrated to lower plasma urea concentration in vitro. Suspension of Lactobacillus in uremic plasma reduced the urea nitrogen levels from 51.5 ± 5.2 mg/dL to 44.3 ± 3.9 mg/dL (P = 0.02) after 24 hours. With microencapsulation of Lactobacillus (inside semipermeable alginate‐polylysine‐alginate polymeric membrane), further lowering of urea nitrogen levels was achieved (35.4 ± 0.8 mg/dL, P = 0.03) at 24 hours. These preliminary data show that expression of certain enzymes could be induced in Lactobacillus delbrueckii and thus capable of lowering plasma urea. Further studies and molecular analysis would be indicated to explore and refine the techniques.
Introduction
We have previously demonstrated the use of nonpathogenic strain Escherichia coli DH5 (after transfected with the Klebsiella aerogenes urease gene) to remove waste metabolites in the body (Chang, [Citation1997]; Prakash and Chang, [Citation1996]). With the technique of microencapsulation (Chang, [Citation1964]; Chang and Prakash, [Citation2001]), these genetically engineered cells lowered urea levels significantly in vitro and efficiently in vivo by oral administration in rat model of renal failure.
Given the financial restraints with dialysis therapy (Kher, [Citation2002]; Li and Chow, [Citation2001]), such novel supplementary renal replacement therapy would therefore be of great value and potential. However, safety issues in genetic engineering biotechnology have yet been subject to considerable controversy, with particular focus on the degree of uncertainty associated with environmental and consumer risk (Kappeli and Auberson, [Citation1997]). In this article, for the first time, we therefore describe a new approach of using Lactobacillus for lowering urea. Experiments were designed to evaluate if Lactobacillus would express enzymes capable of lowering plasma urea in vitro. The purpose is twofold; first to explore the possibility of probiotic removal of urea and thereby circumventing administration of genetically engineered cells; second, we tested the feasibility of enhancing urea removal by microencapsulation of the bacterial cells.
Materials and Methods
Cultures of Lactobacilli delbrueckii subsp. lactis ATCC 4797 were grown at 30°C in MRS Broth (HiMedia Laboratories, Bombay, India), which contained 1.0% proteose peptone, 1.0% beef extract, 0.5% yeast extract, 2.0% dextrose, 0.02% ammonium citrate, 0.01% MgSO4 and 0.005% MnSO4. The pH of the medium was 6.5. To induce urea adaptation, the medium was further modified by addition of urea, after which the urea concentration was approximately 50–60 mg/dL. Urea concentration was determined quantitatively using a colorimetric assay (Sigma Diagnostic Urea Nitrogen, Sigma Diagnostics, Inc., St. Louis, United States), and expressed as urea nitrogen concentration. Plasma ammonia concentrations were determined enzymatically (Sigma Diagnostics Ammonia, Sigma Diagnostics, Inc., St. Louis, United States). Inoculum of Lactobacillus was made in 1.5 mL of urea‐enriched MRS medium in a test tube and incubated by shaking the mixture overnight at 30°C. Inoculum (0.1 mL) was then transferred to 1.5 mL of similar medium for incubation.
All experiments were carried out in anaerobic condition, unless otherwise specified, which was generated by reducing the oxygen level to below 1% (AnaeroGen sachet AN35, Oxoid Ltd., Hampshire, United Kingdom) in a sealed jar (AnaeroJar AG25, Oxoid Ltd., Hampshire, United Kingdom).
After 20 cycles of suspension in urea‐rich medium, log phase bacteria were harvested by centrifugation. Bacteria were then washed with sterile cold water to remove media components. The ability of Lactobacillus to remove plasma urea was thereafter tested in vitro. Briefly, the cells were weighed and then suspended in non‐heparinized plasma from male uremic Wistar rats, with urea nitrogen concentration approximately 50 mg/dL. Urea concentration was monitored and compared with baseline and control experiment in which no Lactobacilli were added.
The second part of the study further explored the use of microencapsulated Lactobacilli for the removal of urea in vitro. This is based upon the premise that use of semipermeable polymer membrane would immobilize and protect Lactobacilli inside the microcapsules on one hand, and allow diffusion of urea for their nitrogen source on the other hand. The details of bacteria cell microencapsulation procedure had been described elsewhere (Chang and Prakash, [Citation2001]). Briefly, Lactobacillus cells (after induction) were suspended in autoclaved sodium alginate solution at room temperature, and then pressed through a 23‐gauge needle using a syringe pump. Compressed air was used to shear the droplets coming from the tip of the 23‐gauge needle. The droplets were allowed to gel for 15 minutes in gently stirred calcium chloride (1.4%) solution. After gel formation in the calcium chloride, alginate beads were coated with poly‐L‐lysine (0.05% in HEPES buffered saline, pH 7.20) for 10 minutes. The beads were then washed with HEPES and coated with an alginate solution (0.2%) for 10 minutes. The alginate‐poly‐L‐lysing‐alginate microcapsules were then washed in 3% citrate bath to liquefy the gel in the microcapsules. The microcapsules containing Lactobacilli were then washed and suspended in uremic plasma to study their capacity of lowering urea level, as compared with equivalent amount of non‐microencapsulated Lactobacilli.
Statistical analyses including Student's t test for paired data were used where appropriate. A two‐tailed P value of less than 0.05 is defined as statistically significant.
Results
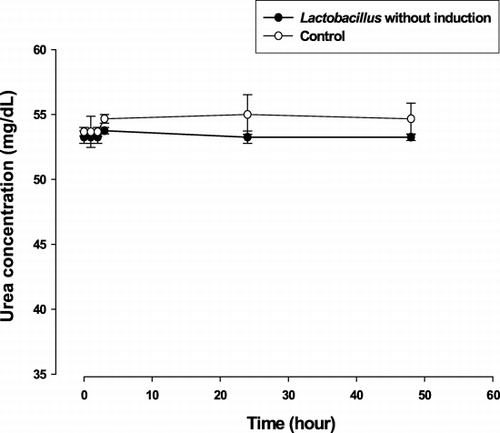
Before metabolic induction of Lactobacillus delbrueckii in a high urea environment, their ability to lower urea was tested in non‐heparinized plasma from uremic rats. As shown in , no significant change in plasma urea nitrogen concentration was detected throughout 48 hours of incubation. Likewise, no significant change in urea nitrogen concentration was noted in the control experiment, as carried out under identical condition in uremic plasma except omission of Lactobacillus.
Figure 1. The mean (± SE) plasma urea nitrogen level at specified time of in vitro experiments: No significant change in plasma urea after incubation with Lactobacillus without preceding induction.
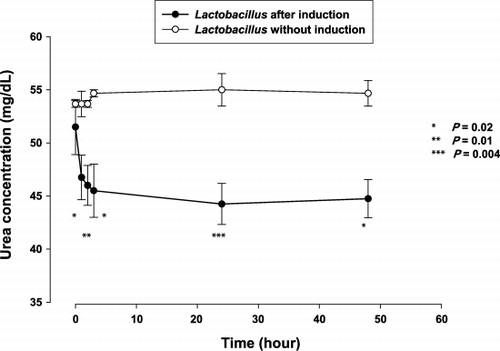
After metabolic induction based on 20 cycles of incubation in urea‐rich medium, they were further tested for ability to remove lower in vitro. illustrates the trend of plasma urea nitrogen concentration after incubation with Lactobacillus prepared as above. At 3 hours, the plasma urea nitrogen concentration was 46.0 ± 3.7 mg/dL, as compared to baseline value of 51.5 ± 5.2 mg/dL (P = 0.15). By 24 hours, the bacteria were able to lower plasma urea nitrogen concentration from 51.5 ± 5.2 mg/dL to 44.3 ± 3.9 mg/dL (P = 0.02). The plasma urea nitrogen concentration was also significantly lower as compared to that incubated with Lactobacillus without metabolic induction (), by the independent samples t test. There was no elevation of plasma ammonia concentration.
Figure 2. The mean (± SE) plasma urea nitrogen level at specified time of in vitro experiments: Removal of plasma urea by Lactobacillus after induction as shown by significantly lower concentration as compared to baseline after 24 and 48 hours of incubation.
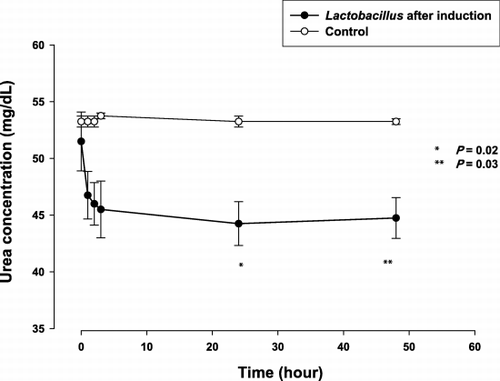
Figure 3. The mean (± SE) plasma urea nitrogen level at specified time of in vitro experiments: Significantly lower plasma urea with Lactobacillus after induction, compared using the independent samples t test.
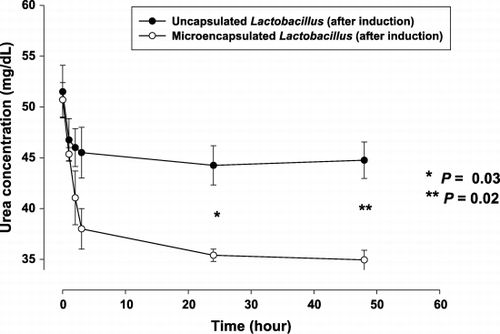
Bacteria cells of Lactobacilli were microencapsulated after 20 induction cycles (). Addition of the microencapsulated bacteria lowered the plasma urea nitrogen from 50.7 ± 2.4 mg/dL at baseline to 35.4 ± 0.8 mg/dL at 24 hours. This decrease was greater than the values achieved by the same amount of bacteria without encapsulation (P = 0.03). The trend of plasma urea concentration was shown in . Final urea nitrogen concentration at 48 hours was 35.0 ± 1.3 mg/dL, as compared to 44.8 ± 3.6 mg/dL in the experiments using Lactobacilli without microencapsulation (P = 0.02).
Discussion and Conclusion
Our results have shown that repeated exposure of Lactobacillus to urea‐enriched environment could upregulate their ability to lower urea in vitro. Our study further demonstrated that microencapsulated bacteria are much more effective for the removal of urea in vitro. There is little reason to believe that the results should differ qualitatively in vivo, given the foreseen benefit of protecting bacterial cells from the uremic moieties of the body system (Kailasapathy, [Citation2002]; Orive et al., [Citation2003]). On the basis of our experimental results, it was estimated that administration of a biomass of 1 g microencapsulated Lactobacillus would remove 105 mg of urea nitrogen. This is about one‐tenth of that achieved by the genetically engineered E. coli DH5 cells. Thus, the fall in plasma urea nitrogen concentration might not be as effective as the genetically engineered bacteria in order to achieve clinical benefit. However, being the bacteria similar to that in yoghurts, the larger amounts needed would more than compensate the need to use genetically engineered microorganisms with their inherent problems.
One of the prerequisites for probiotic use in removal of metabolic wastes should be ability of the microorganisms to use that metabolite as substrate. Whereas bacterial urease is only synthesized constitutively in certain organisms (Kailasapathy, [Citation2002]; Mobley et al., [Citation1995]), this enzyme is by and large thought to be inducible (Mobley et al., [Citation1995]). Expression of urease had been induced by growing several species of Proteus in the presence of urea; whether this applies to Lactobacillus has not been substantiated (Collins and D'Orazio, [Citation1993]; Magaña‐Plaza and Ruiz‐Herrera, [Citation1967]; Mobley et al., [Citation1995]). The ability of Lactobacillus in removing urea in vitro was speculated to result from an increased urease enzyme activity although it was not specifically determined in our experiment. Interestingly enough, the fecal urease activity of uremic patients had been previously demonstrated to increase in proportion to their blood urea concentration, an indicator of body‐urea pool size (Brown et al., [Citation1971]; Rosenstein et al., [Citation1980]). Increased bacterial (including Lactobacilli) urease in the colon was thus postulated to confer benefit to the uremic host instead of the bacteria. Potential beneficial role of the latter is further supported by other investigators who have shown previously that oral administration of Lactobacillus acidophilus probiotics effectively reduced the levels of uremic toxins in dialysis patients (Brown et al., [Citation1971]). In our case, the conversion of urea did not result in an increase in ammonia levels. Most likely the metabolic induction involves an increase not just in urease activity but also an increase in the metabolic cycle that converts the nitrogen source from urea into another product.
Taken together, current evidence supports the premise that Lactobacillus enzyme system could be incrementally induced to remove urea in vitro. Further studies are needed to refine the experimental conditions for optimal enzyme induction and hence potential therapeutic efficacy in vivo.
References
- Brown C. L., Hill M. J., Richards P. Bacterial ureases in uraemic men. Lancet 1971; 2: 406–408
- Chang T. M.S. Semipermeable microcapsules. Science 1964; 146: 524–525
- Chang T. M.S. Live E. coli cells to treat uremia: replies to letters to the editor. Nat. Med. 1997; 3: 3–4
- Chang T. M., Prakash S. Procedures for microencapsulation of enzymes, cells and genetically engineered microorganisms. Mol. Biotechnol. 2001; 17: 249–260
- Collins C. M., D'Orazio S. E. Bacterial ureases: structure, regulation of expression and role in pathogenesis. Mol. Microbiol. 1993; 9: 907–913
- Hida M., Aiba Y., Sawamura S., Suzuki N., Satoh T., Koga Y. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin®, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 1996; 74: 349–355
- Kailasapathy K. Microencapsulation of probiotic bacteria: technology and potential applications. Curr. Issues Intest. Microbiol. 2002; 3: 39–48
- Kappeli O., Auberson L. The science and intricacy of environmental safety evaluations. Trends Biotechnol. 1997; 15: 342–349
- Kher V. End‐stage renal disease in developing countries. Kidney Int. 2002; 62: 350–362
- Li P. K., Chow K. M. The cost barrier of peritoneal dialysis in the developing world—an Asian perspective. Perit. Dial. Int. 2001; 21(Suppl. 3)S307–S313
- Magaña‐Plaza I., Ruiz‐Herrera J. Mechanisms of regulation of urease biosynthesis in Proteus rettgeri. J. Bacteriol. 1967; 93: 1294–1301
- Mobley H. L., Island M. D., Hausinger R. P. Molecular biology of microbial ureases. Microbiol. Rev. 1995; 59: 451–480
- Orive G., Hernández R. M., Gascón A. R., Calafiore R., Chang T. M., De Vos P., Hortelano G., Hunkeler D., Lacík I., Shapiro A. M., Pedraz J. L. Cell encapsulation: promise and progress. Nat. Med. 2003; 9: 104–107
- Prakash S., Chang T. M.S. Microencapsulated genetically engineered live E. coli DH5 cells administered orally to maintain normal plasma urea level in uremic rats. Nat. Med. 1996; 2: 883–887
- Rosenstein I., Hamilton‐Miller J. M., Brumfitt W. The effect of acetohydroxamic acid on the induction of bacterial ureases. Invest. Urol. 1980; 18: 112–114