Abstract
A model system for encapsulation of pancreatic islets which has potential properties for improving biocompatibility and immunosuppression was investigated. In vitro and in vivo studies have shown that phosphorylcholine-containing polymers have high biocompatibility due to low adsorption of proteins and reduced thrombus formation. Encapsulation of islets isolated from rats with a compound membrane composed of phosphorylcholine-containing polymers and cellulose acetate led to rapid insulin production and diffusion across the membrane in response to glucose challenge. The phosphorylcholine-containing polymer had a molecular weight of about 1.3 × 104 Da. The polymer-coated membrane excluded larger molecules such as IgG (molecular weight 150 kDa), thereby acting as a physical immuno-barrier, but allowed smaller molecules such as glucose and insulin to pass through.
Introduction
Transplantation of encapsulated living cells has developed into a promising strategy for the treatment of a wide variety of diseases, for example, hormone deficiencies such as diabetes, and neurodegenerative conditions such as Parkinson's disease. The process of encapsulation physically isolates cells from the outside environment and aims to maintain cellular physiology within a selectively permeable membrane. It has two advantages over the implantation of free cells. Firstly, by mechanically blocking the cells from immune attack, the surrounding synthetic membrane should eliminate the need for immunosuppressive therapy. Secondly, encapsulation offers a solution to the shortage of donors for functional cell transplantations because it allows xenobiotic or allogeneic cells, such as from engineered stem cells to be used.
The materials used for encapsulation of living cells have a dual function. The inner and outer capsule surfaces experience different physiological environments and must therefore be engineered to different specifications to provide a maintenance environment for living cells. To control the synthesis and secretion of a desired cellular product, the encapsulation membrane should be permeable to the nutrients necessary for the survival of the encapsulated cells and to the physiological signals such as glucose or insulin in the case of islet transplantation. To meet the requirement of immuno-isolation and to minimize both the humoral and cellular immune responses of the host, the membrane should restrict direct contact with cells of the immune system, and exclude the penetration of larger molecules such as immunoglobulins and antibodies.
Permeability and immunoprotection can be achieved by an appropriate selection of polymers with a certain pore size. However, capsule biocompatibility is a more complex issue (Rihova, Citation[[2000]]). The direct consequence of a nonbiocompatible membrane is fibrotic overgrowth on the membrane surface, which interferes with the diffusive transport of molecules and the oxygenated blood supply. This fibrous tissue reaction also results in macrophage activation, and/or cytokine and cytotoxic-agent release.
Uludag et al. (Citation[[2000]]) proposed that a fully biocompatible system is one manufactured from membranes which elicit a negligible foreign body reaction. However, the existence of a completely inert biomaterial is doubtful. Implanted capsules inevitably provoke a well-programmed cascade of events associated with the wound-healing process, similar to that observed following the implantation of any biomedical device. The adsorbed proteins or cell layers which form on the external surface of the materials eventually result in a diffusion barrier. Thus the encapsulated cells starve to death due to lack of nutrients, and consequently there is no secretion of desired cellular products from the capsules.
Alginate–polylysine is the most widely utilized material for encapsulation originally described by Lim and Sun (Citation[[1980]]), although other types of alginate based capsules, such as alginate–agarose (Orive et al., Citation[[2003]]), alginate–chitosan (Li et al., Citation[[2002]]), have been investigated. Because of the simple processing techniques and good chemical and physical properties of alginate–polylysine capsules for cell encapsulation, great efforts have been made to produce a better system of alginate–polylysine capsules. In particular, a number of approaches have been studied to tackle the biocompatibility problem. These strategies include using intermediate guluronic acid content in alginate (De Vos et al., Citation[[2003]]), purification of crude alginate (De Vos et al., Citation[[1997]]), shielding the surface charge of alginate–polylysine capsules (Sawhney and Hubbell, Citation[[1992]]) and grafting other charged molecules (Lou et al., Citation[[2000]]).
In this study, we investigate an alternative strategy to improve the biocompatibility of encapsulation, by using a phosphorylcholine-containing polymer. Phosphorylcholine-containing polymers have been widely studied. The applications of this material to biosensors (Chen et al., Citation[[1993]]), dialysis membranes (Campbell et al., Citation[[1993]]), thoracic drain catheters (Hunter and Angelini, Citation[[1993]]), and stents (Zheng et al., Citation[[1999]]) have shown a great potential for biocompatibility. The aim of this study was to construct a compound membrane from a phosphorylcholine-containing polymer and cellulose for the purpose of encapsulation of living islets cells and to evaluate the biocompatibility and permeability of the encapsulation cell strategy.
Experimental
Materials and Membrane Preparation
A copolymer comprising 2-methacryloyloxyethyl phosphorylcholine (MPC) and butyl methacrylate (MPC-BA) with 0.15 mole fraction of MPC was synthesised according to the method described by Ueda et al. (Citation[[1992]]). The molecular weight of the copolymer was measured by gel permeation chromatography in tetrahydrofuran solution using a polystyrene standard. Cellulose acetate (CA) and glucose were purchased from BDH. The buffer used was bicarbonate buffered saline (BBS), which was fortified with 1% bovine serum albumin (BSA) for the protein adsorption experiments. The IgG turbidity assay kit was purchased from Diasorin, and the human IgG from Sigma.
The CA membrane was prepared by casting 2 mL of 2.5% CA solution in tetrahydrofuran in a petri dish. After the solvent had completely evaporated, the membranes were coated with 1 mL of 1% MPC-BA solution in methanol. The thickness of CA was approximately 100 µm and the MPC-BA was approximately 10 µm. The membranes were soaked in BBS solution overnight before use. For the biocompatibility measurement, the MPC-BA membrane was formed on a polyurethane (PU) tubing (Tecoflex, SG-60D, O.D. 0.5 mm), by dipping the tubing into a 1% MPC-BA solution. The solvent was evaporated at room temperature.
Evaluation of Biocompatibility
In vivo experiments were used to evaluate the biocompatibility of the MPC-BA coating. Adult male Wistar rats (350–550 g) were anaesthetized with sodium pentobarbitone (Sagatal). Either noncoated or MPC-BA coated tubing was placed inside a Teflon catheter and inserted into the left common carotid artery. Once in position the tubing was advanced, so that ∼ 3 mm of tubing was exposed to the blood flow. After a period of 120 ± 20 min, the animal was humanely killed, and the tubing was removed by dissection, rinsed in BBS, and immersed in 2.5% glutaraldehyde for 2 h to fix the biological components on the surface of the tubing. It was then rinsed with distilled water, dried under vacuum and examined by scanning electron microscopy (SEM).
In vitro protein adsorption experiments were performed by inserting the MPC-BA coated or noncoated PU tubing in BBS solution with stirring for 50 h, then in bovine serum albumin solution (1%) for 1 h. The tubing was rinsed, fixed, and examined by the method stated as above.
Isolation of Islets
The islets were extracted from the pancreas of adult male Wistar rats by the methods described by Montague and Taylor (Citation[[1968]]) and Howell and Taylor (Citation[[1968]]). In brief, the removed pancreases were trimmed of surplus connective tissue and fat, and were injected with 20 mL of BBS, which expanded and separated the tissue structure. This oedematous tissue was chopped and washed with 15 mL buffer in order to remove any remaining fat, then placed in a conical flask and approximately 3–5 mg/mL of type XI collagenase solution in buffer was added. The mixture was shaken vigorously on a Griffin side arm flask shaker in a water bath at 37°C for 5–8 min, until islets free from exocrine tissue could be detected. At this point, the digestion was terminated by the addition of 10 mL of buffer and the contents of the flask were thoroughly mixed. The tissue was collected by centrifugation at 500 g for 15 s, and the pellet re-suspended in 10 mL of fresh buffer. Islets were then picked by hand under a binocular dissection microscope using a finely drawn out Pasteur pipette. The yield of 150–250 islets were obtained from a single rat pancreas.
Encapsulation of Islets
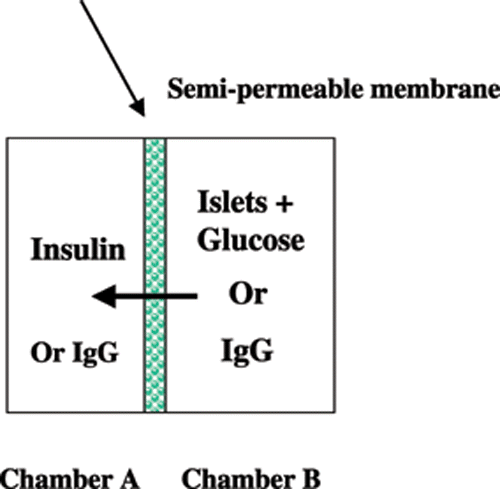
Islet encapsulation was achieved in poly(methylmethacrylate) diffusion chambers, which composed of two complementary countersunk compartments. When a semi-permeable membrane was applied between the two compartments, two chambers were formed, one with a volume of 1.34 cm3 into which BBS was loaded (Chamber A), and another with a volume of 1.66 cm3, into which the islets were loaded (Chamber B). Each chamber had a 1 mm diameter sampling port (). The two chambers were sealed by external clamps.
Sampling and Analysis of Insulin
One hundred islets in BBS were added to Chamber B, and BBS only to Chamber A. The glucose solution (final chamber concentration of 20 mM) was added to Chamber B. During the measurements, the whole apparatus was shaken gently and intermittently to enable even distribution of both islets and secreted insulin. Aliquots (50 µL) from Chamber A were sampled at specific time intervals. The insulin concentration in the samples were determined by a radiolabeling technique using 125Iodine.
Permeability of IgG
The permeability of the compound membrane to IgG was evaluated. Human IgG (1500 mg/L) in distilled water was added to Chamber B and distilled water was added to Chamber A. Fifty microliter aliquots were removed from Chamber A at 1, 3, 5, 7, and 24 h intervals during the diffusion process. The IgG concentration in the aliquots was measured using an IgG turbidity assay, according to the manufacturer's instructions.
Results
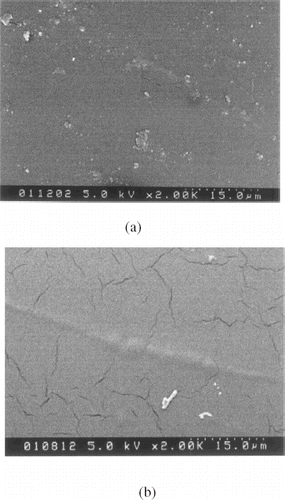
shows the SEM image of the MPC-BA copolymer-coated and noncoated PU tubing following contact with bovine serum albumin for 1 h. The coated tubing exhibited a clearer surface than that of its noncoated counterpart, which was more heavily deposited with protein. The clearer surface demonstrates that the MPC-BA copolymer has an ability to reduce adsorption of proteins.
Figure 2. SEM micrographs of the MPC-BA coated (a) and noncoated (b) PU tubing after circulating in BBS solution for 50 h and bovine serum albumin for 1 h.
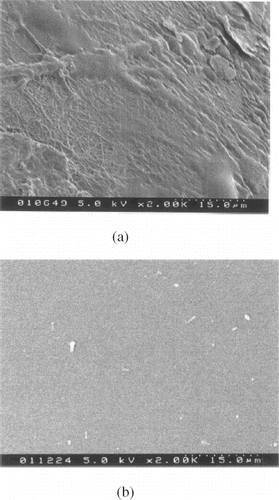
shows the SEM images of the copolymer-coated and noncoated PU tubing after exposure to rat blood circulation for 2 h. It can be seen that the noncoated tubing was covered by fibrin networks which included numerous embedded red cells. These were not detected on the copolymer-coated tubing, indicating that the compound membrane is able to inhibit thrombus formation.
Figure 3. SEM micrographs of the MPC-BA coated (a) and noncoated (b) PU tubing after insertion in rat blood in vivo for 2 h.
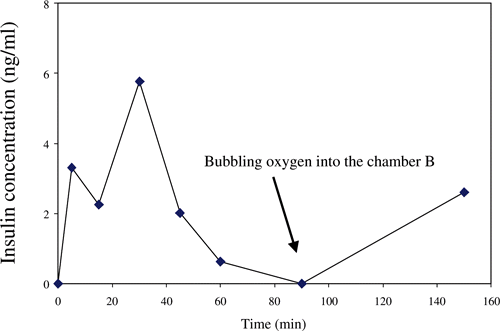
Insulin was detected in Chamber A. This had diffused from Chamber B containing the encapsulated islets. shows a typical plot of the concentration of insulin against diffusion time (n = 4). Insulin was detected after 5 min diffusion. The concentration peaked at 30 min, after which it decreased. When oxygen was bubbled into the chamber as indicated by the arrow in the figure, the insulin concentration increased again.
Figure 4. The plot of insulin concentration against the diffusion time in response to a 20 mM glucose challenge in the rat islets.
Using the immunoturbidometric assay, no IgG was detected in aliquots from Chamber A during the entire 24 h diffusion period, indicating that the copolymer-coated membrane was not permeable to IgG.
Discussion
In recent years, much research has focused on improving the biocompatibility of implanted materials, in particular by physical and chemical modifications of the implant surface (Chu et al., Citation[[2002]]) or by development of bio-hybrid implants (Lee et al., Citation[[2001]]). Capsules comprise a specialized category of implants. Research carried out by our group and other groups (Lewis et al., Citation[[2000]]; Murphy et al., Citation[[1999]]; Zhang et al., Citation[[1996]]) has shown that use of materials which mimic the natural structure of the erythrocyte plasma membrane surface is a feasible approach to enhance implant biocompatibility.
This technique is based on the observation that the erythrocyte plasma membrane is highly biocompatible with other blood components. The most prominent polar head group present in the natural membrane is phosphorylcholine. The majority (88%) of the outer lipid layer of the red cell membrane consists of lecithins and sphingomyelins. These comprise a hydrophilic phosphorylcholine head unit connected to a hydrophobic tail unit of a fatty acid chain and hydrocarbon chain of sphingosine (Chapman, Citation[[1993]]; Stryer, 1981). In this study, a synthetic phosphorylcholine-containing copolymer demonstrated high biocompatibility, indicated by low protein adsorption and no thrombus formation. Various studies of phosphorylcholine-containing copolymers by X-ray photoelectron spectroscope (XPS), differential scanning calorimetry (DSC) and Fourier transform infrared spectroscopy (FT-IR) have shown that the hydrophilic phosphorylcholine groups orient preferentially towards the air surface, and the copolymers undergoes a specific interaction with liposome due to the strong affinity between the lipids (Ishihara and Nakabayashi, Citation[[1991]]; Ishihara et al., Citation[[1990]]; Ruiz et al., Citation[[1998]]; Zhang et al., Citation[[1998]]). The phospholipid layer excludes protein adhesion, and this may be responsible for the high biocompatibility of phosphorylcholine-containing copolymers. Ruiz et al. (Citation[[1998]]) proposed a “flip-flopping” mechanism to explain the biocompatible property of the copolymer, in which the phosphorylcholine group can move freely to and from the surface. Thus, by coating the cellulose membrane with a layer of the copolymer, the protein adsorption pathway was blocked, and the subsequent cascade of events leading to thrombus formation or cell adhesion was inhibited.
In the clinical setting, cellulose membranes have been used extensively in hemodialysis for many years (Hoenich et al., Citation[[1994]]), since they possess robust mechanical properties and the required permeability. However, hemocompatibility would need to be improved if these membranes were to be considered as potential candidates for long-term implantation (Woffindin and Hoenich, Citation[[1988]]). Our study showed that coating cellulose membranes with a biocompatible copolymer did not inhibit diffusion of small molecules such as insulin and glucose. The rapid transfer of insulin across the membrane from Chamber B to Chamber A indicated that the islet cells had responded to the glucose challenge and that they remained bioactive in the presence of the copolymer membrane.
A potential strategy for blocking the passage of immunoglobulins is controlling the molecular weight cut-off of membranes. In this model system, it was found that the coating of phosphorylcholine-containing copolymers did not only act as a biocompatible layer, but also as a physical immuno-barrier. The molecular weight of the copolymer was measured as 1.3 × 104 Da and the coating solution used was as low as 1%. This indicates that with proper molecular weight of the forming-membrane material, the right concentration and processing conditions of solutions which form the membrane, the outmost layer can have right molecular weight cut-off, i.e., blocking IgG and accessing insulin and glucose. Our results were consistent with Ishihara's work (Ishihara et al., Citation[[1990]]).
As stated earlier, the requirements for materials used for encapsulation of living cells are complex. None of the existing polymers has all the required properties, which is a major reason for the delay in introducing clinical applications of cell encapsulation protocols. Circumventing the problem by blend of materials or forming compound membranes or capsules may be a feasible alternative. Lacik et al. (Citation[[1998]]) developed a new capsule by blending five active materials. They found that the capsule size, mechanical strength, membrane thickness, and permeability could be precisely adjusted and quantified. This study established a model system in which cellulose acetate forms the base membrane with tough mechanical properties and phosphorylcholine copolymer provides the properties of biocompatibility and immunosuppression. Our results confirmed that the compound membrane is a good candidate for cell encapsulation because of the rapid diffusion rate of small molecules and impermeability to large molecules.
There may be two approaches which can lead to the development of biocompatible capsules by using phosphorylcholine-containing copolymers discussed in this study: coating cell-containing capsules by phosphorylcholine-containing copolymers or development of a gelation agent which contain phosphorylcholine groups. Synthesis of phosphorylcholine-containing copolymers with proper dissolution properties will make the coating of cell-contained capsules feasible. Ideally, the copolymers should dissolve in less toxic organic solvents to prevent damage to encapsulated cells. Alternatively, incorporating phosphorylcholine groups to polycation polymers as gelation agents for alginate hydrogel system can introduce the biocompatible group into the outermost layer of capsules. With such approaches, the gelation and coating of capsules can be combined in a single step. Such strategies are under investigation in our lab.
Oxygen is a crucial requirement for islet function (Schrezenmeir et al., Citation[[1994]]). Our results showed a high oxygen consummation in the capsules. Although inadequate oxygen supply reduced the production of insulin, overall the compound membrane permeated oxygen well. Once oxygen was bubbled through the chamber, the insulin production resumed. Therefore, enhancement of the supply and distribution of oxygen during encapsulation is an important prerequisite.
Conclusions
A model system with biocompatibility and immunosuppresion properties for islet encapsulation has been studied. Using a cellulose membrane coated with a phosphorylcholine-containing polymer, encapsulation of islets led to rapid insulin delivery in response to glucose challenge. This demonstrates that a compound membrane could be a good candidate for cell encapsulation. On the other hand, the results suggest the possibility of creating biocompatible capsules either physically or covalently bonding phosphorylcholine groups to capsule-forming materials.
References
- Campbell E. J., Hall B., Parmer M. R., Sullivan A. M. Improved hemocompatibility of artificial surfaces by treatment using phosphorylcholine derivatives. Thromb Haemostasis 1993; 69: 935–935
- Chapman D. Biomembranes and new hemocompatible materials. Langmuir 1993; 9: 39–45, [CSA]
- Chen C. Y., Su Y. C., Ishihara K., Nakabayashi N., Tamiya E., Karybe I. Biocompatible needle-type glucose sensor with potential for use in vivo. Electroanalysis 1993; 5: 269–276
- Chu P. K., Chen J. Y., Wang L. P., Huang N. Plasma-surface modification of biomaterials. Mat. Sci. Eng. R. 2002; 36: 143–206, [CROSSREF]
- De Vos P., Dehaan B., Van Schilfgaarde R. Effect of the alginate composition on the biocompatibility of alginate–polylysine microcapsules. Biomaterials 1997; 18: 273–278, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- De Vos P., van Hoogmoed C. G., van Zanten J., Netter S., Strubbe J. H., Busscher H. J. Long-term biocompatibility, chemistry, and function of microencapsulated pancreatic islets. Biomaterials 2003; 24: 305–312, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Hoenich N. A., Woffindin C., Matthews J. N. S., Goldfinch M. E., Turnbull J. Clinical comparison of high-flux cellulose-acetate and synthetic membranes. Nephrol. Dial. Transpl. 1994; 9: 60–66, [CSA]
- Howell S. L., Taylor K. W. Potassium ions and the secretion of insulin by islets of Langerhans incubated in vitro. Biochem. J. 1968; 108: 17–23, [PUBMED], [INFOTRIEVE]
- Hunter S., Angelini G. D. Phosphorylcholine-coated chest tubes improve drainage after open-heart operation. Ann. Thorac. Surg. 1993; 56: 1339–1342, [PUBMED], [INFOTRIEVE], [CSA]
- Ishihara K., Aragaki R., Yamazaki J., Ueda T., Watanabe A., Nakabayashi N. Organized adsorption of phospholipid on the polymer surface with phospholipid polar group and its blood compatibility. Seitai Zairyo. 1990; 8: 231–237, [CSA]
- Ishihara K., Nakabayashi N. Specific interaction between water-soluble phospholipid polymer and liposome. J. Polym. Sci. Polym. Chem. 1991; 29: 831–835
- Ishihara K., Ueda T., Nakabayashi N. Preparation of phospholipid polymers and their properties as polymer hydrogel membranes. Polym. J. 1990; 22: 355–360
- Lacik I., Brissova M., Anilkumar A. V., Powers A. C., Wang T. New capsule with tailored properties for the encapsulation of living cells. J. Biomed. Mater. Res. 1998; 39: 52–60, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Lee W. K., Park K. D., Kim Y. H., Suh H., Park J. C., Lee J. E., Sun K., Baek M. J., Kim H. M., Kim S. H. Improved calcification resistance and biocompatibility of tissue patch grafted with sulfonated PEO or heparin after glutaraldehyde fixation. J. Biomed. Mater. Res. 2001; 58: 27–35, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Lewis A. L., Hughes P. D., Kirkwood L. C., Leppard S. W., Redman R. P., Tolhurst L. A., Stratford P. W. Synthesis and characterisation of phosphorylcholine-based polymers useful for coating blood filtration devices. Biomaterials 2000; 21: 1847–1859, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Li S., Wang X. T., Zhang X. B., Yang R. J., Zhang H. Z., Zhu L. Z., Hou X. P. Studies on alginate–chitosan microcapsules and renal arterial embolization in rabbits. J. Control. Release 2002; 84: 87–98, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Lim F., Sun A. M. Microcapsulated islets as bioartificial endocrine pancreas. Science 1980; 210: 908–910, [PUBMED], [INFOTRIEVE]
- Lou W. H., Qin X. Y., Wu Z. G. Preliminary research on biocompatibility of alginate chitosan-polyethyleneglycol microcapsules. Minerva Biotechnol. 2000; 12: 235–240
- Montague W., Taylor K. W. Pentitols and insulin release by isolated rat islets of Langerhans. Biochem. J. 1968; 109: 333–370, [PUBMED], [INFOTRIEVE]
- Murphy E. F., Lu J. R., Brewer J., Russell J., Penfold J. The reduced adsorption of proteins at the phosphorylcholine incorporated polymer–water interface. Langmuir 1999; 15: 1313–1322, [CROSSREF]
- Orive G., Hernandez R. M., Gascon A. R., Igartua M., Pedraz J. L. Survival of different cell lines in alginate–agarose microcapsules. Eur. J. Pharm. Sci. 2003; 18: 23–30, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Rihova B. Immunocompatibility and biocompatibility of cell delivery systems. Adv. Drug Deliver Rev. 2000; 42: 65–80, [CROSSREF], [CSA]
- Ruiz L., Hilborn J. G., Leonard D., Mathieu H. J. Synthesis, structure, and surface dynamics of phosphorylcholine functional biomimicking polymers. Biomaterials 1998; 19: 987–998, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Sawhney A. S., Hubbell J. A. Poly(ethylene oxide)-graft-poly (1-lysine) copolymers to enhance the biocompatibility of poly (1-lysine)–alginate microcapsule membranes. Biomaterials 1992; 13: 863–870, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Schrezenmeir J., Kirchgessner J., Gero L., Kunz L. A., Beyer J., Muellerklieser W. Effect of microencapsulation on oxygen distribution in islets organs. Transplantation 1994; 57: 1308–1314, [PUBMED], [INFOTRIEVE], [CSA]
- Stryer L. Membrane structure and dynamics. Biochemistry. W. H. Freeman & Company, USA 1995; 11: 263–267
- Ueda T., Oshida H., Kurita K., Ishihara K., Nakabayashi N. Preparation of 2-methacryloyloxyethyl phosphorylcholine copolymers with alkyl methacrylates and their blood compatibility. Polym. J. 1992; 24: 1259–1269
- Uludag H., De Vos P., Tresco P. A. Technology of mammalian cell encapsulation. Adv. Drug Deliver Rev. 2000; 42: 29–64, [CROSSREF], [CSA]
- Woffindin C., Hoenich N. A. Blood-membrane interactions during haemodialysis with cellulose and synthetic membranes. Biomaterials 1988; 9: 53–57, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Zhang S. F., Rolfe P., Wright G., Lian W., Milling A. J., Tanaka S., Ishihara K. Physical and biological properties of compound membranes incorporating a copolymer with a phosphorylcholine head group. Biomaterials 1998; 19: 691–700, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Zhang S. F., Wickramasinghe Y. A. B. D., Rolfe P. Investigation of an optical fibre pH sensor with the membrane based on phospholipid copolymer. Biosens. Bioelectron. 1996; 11: 11–16, [CROSSREF], [CSA]
- Zheng H., Barragan P., Corcos T., Simeoni J. B., Favereau X., Roquebert P. O., Guerin J., Sainsous J. Clinical experience with a new biocompatible phosphorylcholine-coated coronary stent. J. Invasive Cardiol. 1999; 11: 608–614, [PUBMED], [INFOTRIEVE]