Abstract
Chitosan beads carrying various amino acids (a total of 12 kinds) were synthesized through quite simple procedures for selective removal of low density lipoprotein (LDL). Macroporous chitosan beads were prepared by the phase-inversion method, to which the amino acids were then coupled respectively, via either ethyleneglycol diglycidylether (EGDE) or epichlorohydrin (ECH). Among the amino acids used, in vitro tests proved L-Trp to be the best ligand for binding LDL. The adsorbent, which was prepared by coupling L-Trp to the chitosan beads via EGDE, demonstrated satisfactory adsorption performance for selective removal of LDL in human plasma.
Introduction
It is without doubt that low density lipoprotein (LDL) is a very important risk factor in the development and progression of atherosclerosis and its clinical sequelae like coronary heart disease and myocardia infarction (Pekkanen et al., Citation[[1990]]). Various extracorporeal procedures have been developed to achieve therapeutic aim in patients with refractory hyperlipidemia, such as cascade filtration, heparin-induced precipitation and LDL adsorption. Among those selective adsorption which retains much needed plasma components seems to be a better option (Tan, Citation[[1996]]). Although some LDL adsorbents, like cellulose beads carrying dextransulfate and anti-LDL immobilized Sepharose, have been brought into clinical application, considerable effort has been made to develop other LDL binding materials toward good efficacy and biocompatibility, low costs, simple production, easy handling, etc (Borberg et al., Citation[[1988]]; Bosch et al., Citation[[1992]]; Cao et al., Citation[[2002]]; Gamzazade et al., Citation[[1997]]; Kobayashi and Okuyama, Citation[[2002]]; Liu et al., Citation[[2002]]; Sharma et al., Citation[[1993]]; Wang et al., Citation[[2002]]; Yu and He, Citation[[1997]]; Yu et al., Citation[[1997]]). Some researchers have reported LDL adsorbents based on chitosan, a naturally abundant biopolymer with non-toxicity and good biocompatibility (Gamzazade et al., Citation[[1997]]; Liu et al., Citation[[2002]]; Sharma et al., Citation[[1993]]; Yu and He, Citation[[1997]]; Yu et al., Citation[[1997]]). In this article, macroporous chitosan beads with various amino acids as ligands were synthesized through quite simple procedures, and their adsorption properties for lipoproteins were investigated in detail.
Materials and Methods
Materials
Powdered chitosan with a degree of deacetylation 90% and average molecular weight of about 40000 was obtained from Yuhuan Biochemical Co., Ltd. (Zhejiang, China). Ethyleneglycol diglycidylether (EGDE) was purchased from Acros Organics. Epichlorohydrin (ECH) was purchased from Tianjin Chemical Reagents Co., Ltd. (Tianjin, China). All the amino acids to be used were purchased from Aoboxin Biotechnol Co. Ltd. (Beijing, China). All other reagents were of analytical grade and purchased commercially. Plasma taken from the patients suffering from hypercholesterolemia was kindly supplied by Tianjin General Hospital.
Preparation of Chitosan Adsorbents
Phase-inversion technique was employed to form the bare chitosan beads (Kawamura et al., Citation[[1997]]). Briefly, 1 g chitosan was dissolved in 19 mL 3 wt% aqueous solution of acetic acid. This chitosan solution was then sprayed into 8 wt% NaOH aqueous solution, and porous beads were formed due to the fact that chitosan is soluble in acidic solutions, while insoluble in basic ones. The mean diameter of the beads was regulated to about 0.5 mm. The chitosan beads were then washed thoroughly with a large amount of water.
The chitosan beads were activated using two different activator (EGDE and ECH) respectively, to introduce epoxy groups. While using EGDE, 15 mL EGDE and 15 mL 0.6 N NaOH aqueous solution containing 30 mg NaBH4 were mixed with the beads, and the mixture was gently stirred at 25°C for 8 h. On using ECH, the following procedure was applied: 12 mL DMSO and 6 mL ECH were added to the beads, followed by dropping slowly 12 mL 2 N NaOH aqueous solution, then the mixture was heated to 40°C and kept for 4 h. The epoxidized beads were then washed with water to remove the unreacted reagents.
For the coupling of ligands, 10 mmol each of the amino acids was first mixed with 5 mL 2 N NaOH solution to obtain the corresponding salts, which was then dissolved in 20 mL carbonate buffer, pH 10.5. The solution was added to the above epoxidized beads, and the reaction was allowed to proceed under agitation at 65°C for 24 h. Thus modified beads were washed extensively with water, 1 N NaCl, and again with water to remove the remaining carbonates and unbound ligands, then stored in 0.15 M NaCl aqueous solution at 4°C for further use.
Characterization of Adsorbents
Based on the carboxyl groups, the amount of amino acids coupled to the adsorbents was determined using the acid–base titration method described by Qian and Liu (Citation[[1984]]). The water contents of the adsorbents were obtained by determining their swelling in distilled water, and was calculated by the following expression:where w2 and w1 stand for the weights of the adsorbents in wet state (suction-dried after thoroughly swelling) and completely dried state, respectively. Scanning Electron Microscopy (SEM) (S3500 N, HITACHI) was employed to observe the morphology of the adsorbents. The wet beads were freeze-dried following the reported method (Kawamura et al., Citation[[1997]]). Thus dried beads were then coated with gold by an ion spattering apparatus and observed with the SEM.
Determination of Adsorption Capacity
Zero point five grams of suction-dried beads in each case was incubated with 1 mL plasma and stirred for 3 h at 37°C. Total cholesterol (TC), LDL cholesterol (LDL-C), high density lipoprotein (HDL) cholesterol (HDL-C) and triglyceride (TG) contents in the plasma samples before and after adsorption were measured respectively, using a biochemical analyzer (Minilab V, Vital Scientific Co.) and commercial test kits (Zhongsheng High Tech Bioeng Co., Beijing, China). As a measure of adsorption, adsorption percentages for the above constitutes were calculated respectively, according the following formula:where C2 and C1 are the concentrations before and after adsorption, respectively.
Results and Discussion
Adsorbent Characteristics
The chemical structures of the resultant adsorbents are schematically shown in . In contrast to the unactivated chitosan beads, which were soluble in acidic solutions, all the adsorbents were insoluble in 0.5 N aqueous solution of acetic acid for up to 24 h. This indicated that the activating and cross-linking reactions happened simultaneously, therefore the adsorbents provided satisfactory chemical stability. lists the amount of different amino acids coupled to the chitosan beads, and the water contents of the corresponding adsorbents. The amount of bound amino acids varied from 0.50 to 0.74 mmol/g xerogel. The water contents were in the range between 88.6 and 89.5% and very close to that of the bare chitosan beads, demonstrating relatively good hydrophilicity, which is desired for the purpose of blood purification. This can be attributed to the fact that chitosan molecules contain a large amount of −NH2 and −OH groups.
Figure 1. The structures of the modified chitosan adsorbents: P denotes the matrix and NH2R the sodium salt of an amino acid.
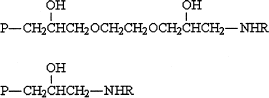
Table 1. The characteristics of adsorbents
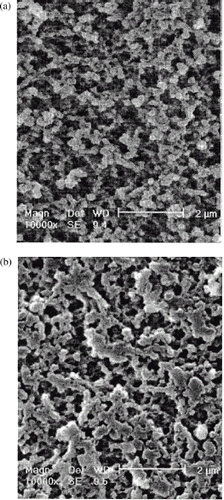
SEM views of the surface and cross-section of a typical adsorbent bead are shown in . Macropores were uniformly formed from the surface to the inside, which seems large enough for LDL particles (about 20–30 nm in diameter) to penetrate the adsorbent.
Effect of Different Ligands on Adsorption Capacity
The most substantial part of LDL adsorbents is the ligands. Based on the characteristics of LDL, the ligands for binding LDL may be chosen according to the biospecific (LDL antibody), electrostatic (dextransulfate and heparin), hydrophobic (cholesterol), and/or hydrogen bond (−NH2 and −OH) interactions (Yu et al., Citation[[2000]]). The adsorption percentages for TC, LDL-C, HDL-C, and TG of various adsorbents are summarized in . As can be seen, the amino acids used as ligands in this study covered about all kinds of amino acids, including acidic, basic and neutral ones. With regard to the adsorption capacities for TC, LDL-C as well as TG, the adsorbents carrying L-Trp, i.e., CS-L-Trp and CS-L-Trp(ECH), provided far larger binding capacities than the others, no matter how the ligands were coupled. Some other researchers also found the relatively good affinity of matrix-bound L-Tip for LDL (Ikonmov et al., Citation[[1992]]), but the mechanism is still open. From , it seems that electrostatic interaction of LDL and the carboxyl groups of the amino acids is not significant, even for the acidic amino acids. Thus, the indole groups in Trp molecules, with appropriate hydrophobicity, probably play an important part in the binding of LDL by hydrophobic interaction. The bare chitosan beads contain a large amount of −OH and −NH2, and for this reason, showed some adsorption of TC and LDL-C probably via the weaker hydrogen-bond interaction. However, the adsorbents carrying the amino acids other than L-Trp did not present better adsorption capacities than the bare chitosan beads.
Table 2. Adsorption percentages for the related plasma constitutes of the various adsorbents
Effect of Spacer on HDL Adsorption
Among the lipoproteins transporting cholesterol in the blood, unlike LDL, which contains a large amount of cholesterol and is known to be causative of atherosclerosis, HDL is involved in reverse cholesterol transport and show antiatherogenic and protective properties (Schimitz and Williamson, Citation[[1991]]). Therefore, any LDL adsorbent is desired to possess sufficient LDL adsorption capacity, with low adsorption of HDL and other needed blood components, that is to say, selective LDL adsorption.
As HDL adsorption is concerned, in contrast to CS-L-Trp(ECH), which was activated by ECH and showed larger HDL adsorption capacity, CS-L-Trp adsorbed less than 10% of the HDL. Other adsorbents prepared via EGDE activating like CS-L-Trp, also adsorbed much less HDL than CS-L-Trp(ECH). Compared with ECH activating, the adsorbents prepared via EGDE activating were attached a much longer hydrophilic spacer arm between the ligand and the matrix, as can be seen from . Therefore, it can be concluded that a long spacer in the structure of a LDL adsorbent can reduce HDL adsorption, thus increasing adsorption selectivity. This was also reported by other researchers (Xu et al., Citation[[2002]]).
Considering both LDL binding capacity and adsorption selectivity, the adsorbent CS-L-Trp prepared in this study demonstrates a much better performance than other chitosan based LDL adsorbents (Gamzazade et al., Citation[[1997]]; Liu et al., Citation[[2002]]; Sharma et al., Citation[[1993]]; Yu and He, Citation[[1997]]; Yu et al., Citation[[1997]]). Furthermore, the preparation route is quite simple.
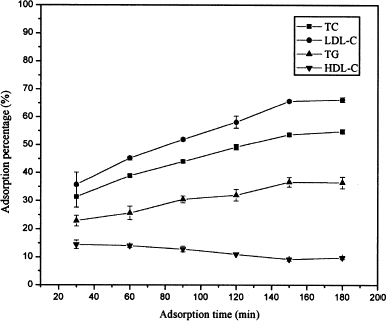
Effect of Adsorption Time on Adsorption Capacity
illustrates the dynamic adsorption behavior of the adsorbent CS-L-Trp. With the increase of adsorption time from 30 to 150 min, the adsorption percentages of TC, LDL-C, and TG increased slowly, while that of HDL-C decreased slowly. However, all the adsorption reached the equilibrium on an elapse of 3 h. The abnormal adsorption of HDL can be explained as follows: unlike LDL, HDL particles (3.5–9 nm in diameter) are much smaller, and their adsorption by the adsorbents is primarily based on their diffusion into the inside of the adsorbents (Yu et al., Citation[[2000]]). Therefore, they could rapidly occupy the adsorptive sites prior to LDL at the initial stages of adsorption. But with the adsorption continuing, they were desorbed gradually by the LDL arriving successively, due to the stronger competitive adsorption until the equilibrium was reached.
Conclusion
Among the various amino acids, L-Trp was proved to a best ligand for binding LDL. A new LDL adsorbent was obtained simply by coupling L-Trp to the macroporous chitosan beads via EGDE. This adsorbent demonstrated satisfactory adsorption capacity and selectivity for LDL removal.
Acknowledgments
This work was supported by The National Key Project of Fundamental Research and Advances (G1999064707).
References
- Borberg H. H., Gaezkowski A., Hombach V. The treatment of familial hypercholesterolemia by means of a specific immunoadsorption. J. Clin. Apheresis. 1988; 4: 59–65, [PUBMED], [INFOTRIEVE]
- Bosch T., Schmidt B., Blumentstein M., Gurland H. J. Lipid apheresis by hemoperfusion: in vitro efficacy and ex vivo biocompatibility of a new low-density lipoprotein adsorber compatible with human whole blood. Artif. Organs 1992; 17(7)640–652
- Cao N., Yu Y., Wang M., Chen C. In vitro study of a novel low-density lipoprotein adsorbent. Artif. Cells, Blood Substitutes, and Immobilization Biotechnol. 2002; 30(1)53–61, [CROSSREF], [CSA]
- Gamzazade A. L., Nasibov S. M., Rogozhin S. V. Study of lipoprotein sorption by some sulfoderivatives of chitosan. Carbohydrate Polymers 1997; 34(2)381–384, [CROSSREF]
- Ikonmov V., Samtleben W., Schmidt B., Blumenstein M., Gurland H. J. Adsorption profile of commercially available adsorbents: an in vitro evaluation. Inter. Artif. Organs 1992; 15(5)312–319
- Kawamura Y., Yoshida H., Asai S., Kurahashi I., Tanibe H. Effects of chitosan concentration and precipitation bath concentration on the material properties of porous cross-linked chitosan beads. Separation Sci. Technol. 1997; 32(12)1959–1974
- Kobayashi A., Okuyama T.. Lipoprotein Adsorbent and Lipoprotein Adsorber Made with the Use of the Same. US Patent 6,337,368, Jan. 8, 2002
- Liu M., Zhao L., Tai L. Adsorption of LDL on the modified chitosan. Chinese J. React. Polymers 2002; 9(2)186–190
- Pekkanen J., Linn S., Heiss G., Suchindran C., Leon A., Rifkind B. Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. New. Engl. J. Med. 1990; 322: 1700–1707, [PUBMED], [INFOTRIEVE]
- Qian T., Liu W. The Application Handbook of Ion Exchangers. Chemical Industry Publishing House, BeijingChina 1984; 57–65
- Schimitz G., Williamson E. High density lipoprotein matabolism, reverse cholesterol transport and membrane protection. Curr. Opin. Lipidol. 1991; 2: 177–189
- Sharma C. P., Chandy T., Kumari T. V., Paul W. Lipoprotein adsorption onto modified chitosan beads: preliminary study. Biomater. Artif. Immobilization Biotechnol. 1993; 21(5)659–664, [CSA]
- Tan N. Development of selective low density lipoprotein (LDL) apheresis system: immobilized polyanion as LDL-specific adsorption for LDL apheresis system. Artif. Organs 1996; 20(8)922–929, [CSA]
- Wang S., Yu Y., Cui T., Cheng Y. Cellulose amphiphilic adsorbent for the removal of low density lipoprotein. Artif. Cells, Blood Substitutes, and Immobilization Biotechnol. 2002; 30(4)285–292, [CROSSREF], [CSA]
- Xu J., Lu L., Li H., He B. Studies on the selective adsorption of low-density lipoprotein by sulfated polyvinyl alcohol beads. Chinese Science Bulletin 2002; 46(23)1958–1961
- Yu X., Wan C., Yue Y. Low-density lipoprotein apheresis. Space Medicine and Medical Eng. 2000; 15(2)144–148
- Yu Y., Gu H., He B. The studies of adsorption properties of sulfonated hydroxyethyl cross-linked chitosan beads for low density lipoprotein in plasma. Acta Polymeric Sinica 1997; 12(5)606–610
- Yu Y., He B. A new low density lipoprotein (LDL) adsorbent. Artif. Cells, Blood Substitutes, and Immobilization Biotechnol. 1997; 25(5)445–450, [CSA]