Abstract
A study on liquid state bioconversion of sewage treatment plant (STP) sludge was assisted to evaluate the performance of batch fermenter compared to shake flask in a laboratory. Bioconversion of STP sludge was highly influenced by the mixed fungal culture of Penicillium corylophilum and Aspergillus niger after 4 days of treatment. The results showed that about 24.9 g kg−1 dry sludge cake (DSC) was produced with enrichment of fungal biomass protein in fermenter while 20.1 g kg−1 in shake flask after 4 days of fungal treatment. The effective biodegradation of STP sludge was recorded in both fermenter and shake flask experiment compared to control (uninnoculated sample). The results presented in this study revealed that the overall performance of fermenter in terms of sludge cake (biosolids) accumulation and biodegradation of STP sludge was higher than the shake flask.
Introduction
Sewage sludge is an unavoidable by-product of wastewater treatment facilities. Recently, with the invention towards increasingly efficient wastewater treatment coupled with rapid industrial growth has created an over-proportional increase of sludge. About 3.2 million cubic meters of sewage sludge is produced annually by Indah Water Konsortium (IWK) throughout Malaysia. By the year 2005, it is expected to increase the sludge volume to about 4.3 million cubic meters annually and its management cost is estimated to USD27 million (Kadir and Velayutham, Citation[[1999]]). The handling and disposal of huge quantity of sludge produced from wastewater treatment facilities has become one of the main environmental problems and has been considered the most troublesome phase of sewage sludge treatment.
The biodegradability of wastewater sludge can be improved by using thermal energy (Baier, Citation[[1997]]; Li and Noik, Citation[[1992]]) enzymes (Knapp and Howell, Citation[[1978]]), ozonation (Yasui and Shibata, Citation[[1994]]), acidification (Woodard and Wukasch, Citation[[1994]]), alkalin addition (Lin et al., Citation[[1989]]; Mukherjee and Levine, Citation[[1992]]), high pressure (Dollerer and Willder, Citation[[1993]]), mechanical disintegration (Baier and Schmidheiny, Citation[[1997]]; Kopp et al., Citation[[1997]]) and ultrasound (Tiehm et al., Citation[[1997]]). Most of these solutions are: not suitable for toxic pollutants (existing biological process); only for low concentration (adsorption); very expensive for dilute organic compounds (oxidation); low cut-off molecular weight is needed for the removal of soluble compounds (membrane process). Among these processes, the employment of microbial treatment of sewage treatment plant (STP) sludge using liquid state bioconversion (LSB) process is being considered as a new solution for the biodegradation, bioseparation, and biosolids accumulation of soluble and insoluble pollutant which exhibits the benefit of nonhazardous, safe/scientific, and environmental friendly for all living beings (Alam, Citation[[2002]]; Alam et al., Citation[[2001]], Citation[[2002]]).
In LSB process, filamentous fungi isolated from its relevant sources (wastewater, sewage sludge, sludge cake) can immobilize/entrap on waste particles of STP sludge (<1.5 w/w of TSS) with the formation of pellets/flocs and as a result, the treated sludge will clarify. Since the fungus entraps the solid particles of sludge, the settling and dewatering characteristics may be enhanced considerably (Alam et al., Citation[[2002]]). The fungal growth and its secondary metabolite i.e., enzymes influence the reduction of soluble and insoluble materials present in waste sludge and enhance the bioconversion process significantly (Alam et al., Citation[[2002]]; Friedrich et al., Citation[[1983]]; Friedrich et al., Citation[[1987]]; Jin et al., Citation[[1999]]). Therefore, this study was undertaken to evaluate the performance of batch fermenter compared to shake flake in optimized process conditions in terms of biosolids accumulation and biodegradation of STP sludge.
Materials and Methods
Fungal Strains
A mixed culture of Penicillium corylophilum WWZP1003 (IMI385277) and Aspergillus niger SCahmA103 (IMI385267) obtained from a series of experiments of isolation/identification (Fakhru’l-Razi et al., Citation[[2002]]), screening (Alam et al., Citation[[2003a]]), and optimization of mixed culture (Alam et al., Citation[[2003b]]) was used. The strains of mixed culture used were isolated from relevant sources (wastewater and sludge cake) and optimized on the basis of potential performance for the treatment of domestic wastewater sludge using bioconversion process. The cultures were maintained on 3.9% w/v of Potato Dextrose Agar (PDA, Oxoid, UK) slants and subsequently subcultured once in a month and stored at 4°C.
Sewage Treatment Plant Sludge
The sewage treatment plant (STP) sludge of 0.75–1% w/w of TSS (pH 6.6) was collected from secondary clarifier in a sewage treatment plant, Kuala Lumpur, Malaysia. The required percent of sludge was prepared with dilution or drying to exactly 1% w/w of TSS. The STP sludge supplemented by 2% w/w of wheat flour (WF, optimized carbon source) as co-substrate was used for fungal growth throughout the study.
Experimental Procedures for Bioconversion Process
Two different experiments such as bioconversion of STP sludge in shake flask and fermenter were conducted throughout the study. The optimized carbon source as co-substrate and process conditions were operated in both treatments. Optimum conditions were cheap carbon source (co-substrate) of wheat flour, temperature of 33°C, initial pH of 5.5, inoculum size of 2% v/w, and co-substrate (WF) concentration of 2% w/w for shake flask experiment (Alam et al., Citation[[2003c]]). In fermenter experiment, above-mentioned conditions were followed including optimal agitation of 150 rpm and aeration of 0.5 vvm (volume per volume of substrate per minute) to evaluate its performance (Alam and Fakhru'l-Razi, Citation[[2002]]).
Bioconversion in Shake Flask
An experiment was carried out in a 500 mL Erlenmeyer flask containing 100 gm of STP sludge medium containing 2% w/w of wheat flour. The samples were autoclaved at 121°C for 30 min and inoculated with mixed culture of 2% v/w of inoculum (1% v/w of inoculum for each fungus). It was incubated in a rotary shaker at 150 rpm for 6 days (sampling was carried at everyday). The spore suspension of inoculum was used in the experiment. The concentration of mixed inoculum of P. corylophilum and A. niger used were 1.25 × 104 and 2 × 104 spores mL−1 respectively.
Bioconversion in Batch Fermenter
A 6-liter capacity BIOSTAT®CT compact laboratory-scale fermenter (B. Braun Biotech International) with a 3-liter working volume was used throughout. The fermenter was a vertical glass cylinder with the height/diameter ratio of 2.2:1. Fermenter and all its parts were sterilized to be used along with the substrate at 1.5 barg and 121°C for 45 min. Filtered air was supplied into the substrate in the fermenter just below the impeller by an air compressor. The initial pH of the substrate was adjusted to 5.5 but it was not controlled thereafter. Temperature, agitation, and aeration rate were maintained automatically by microprocesser controller in fermenter. The treatment was started with 3-kg of STP sludge containing 2% w/w of co-substrate. It was inoculated with 2% (v/w) of spore suspension of mixed inoculum (1:1 ratio of individual) of P. corylophilum and A. niger for 6 days. The sampling, inoculum preparation and its strength (spores/mL) were followed as similar as shake flask experiment.
Sample Analysis
The biomass containing dry sludge cake was collected by filtering the fermentation broth (treated sludge) and dried at 105°C with 24 and 48 h for shake flask and fermenter experiment respectively. The supernatant was used for analysis after centrifuging the fermented broth at 3000 rpm for 30 min. The TS, TSS, and COD were measured following the standard methods (APHA, Citation[[1989]]). The optical density (OD) of supernatant at 660 nm was measured against distilled water in an UV spectrophotometer (UVIKON 933) (Alam and Fakhru'l-Razi, Citation[[2002]]). pH was measured by the pH meter (Hanna Instruments, Italy) according to the manual instructions. Soluble protein was determined according to Lowry method (Lowry et al., Citation[[1951]]). Total dissolved solid (TDS) was determined by using EC (electrical conductivity)/TDS meter (Hanna Instruments, HI9835) according to manual instructions of Hanna Instruments. Reducing sugar was measured according to the method of DNS (Miller, Citation[[1959]]). The experiment was conducted by using factorial completely randomized design (CRD). The data were the average of three replicates.
Results and Discussion
This study was assisted to evaluate the performance of a batch fermenter compared to shake flask for the biological treatment of STP sludge by using LSB process. The optimized process conditions such as co-substrate concentration, initial pH, temperature, and inoculum size were used in both of fermenter experiment (FE) and shake flask experiment (SFE). The FE was additionally maintained with optimum agitation and aeration rates. The recorded results were performed to evaluate bioconversion of STP sludge in terms of dry sludge cake production and its protein enrichment, reduction of effluent discharge level such as TSS, TDS, turbidity, COD, soluble protein, substrate concentration (reducing sugar), and pH values.
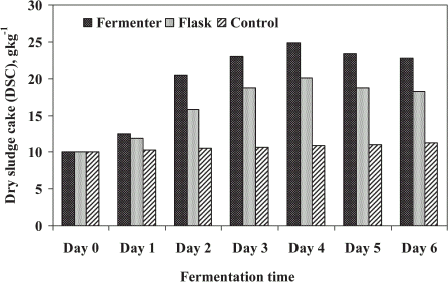
The fungal strains grown in STP sludge resulted in increase of the solid content which was expressed by dry sludge cake (DSC) production. The DSC production in treated sludge is shown in . In both experiments, DSC produced by fungi was maximum after 4 days of treatment and the growth was declined thereafter. Highest production of DSC recorded was 24.87 g kg−1 in FE while 20.05 g kg−1 was produced in SFE (). On the basis of DSC production, the performance of fermenter was higher than the shake flask experiment by about 20%. It may be achieved with optimum process conditions used in FE where SFE was not optimized with all conditions (except agitation and aeration). The production rate was significantly influenced by both experiments compared to uninnoculated experiment (control). The highest increased of solids observed was 129 and 85% for FE and SFE, respectively. DSC production has been studied by several authors in different wastes material such as olive oil mill effluent, apple distillery wastes, domestic wastewater sludge with different fungal strains (Alam, 2001, Citation[[2002]]; Friedrich et al., Citation[[1983]], Citation[[1986]]; Jin et al., Citation[[1999]]).
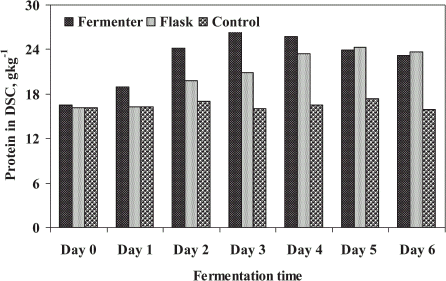
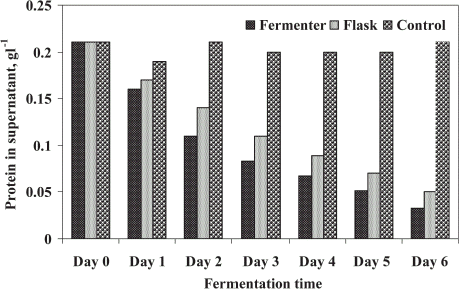
The enriched protein in DSC was determined to evaluate DSC quality as nutrient value of treated biosolids which can be converted into compost in future study. Protein was enriched with fungal mycelia during their growth in STP sludge. Enriched protein in DSC and untreated sludge (control) are illustrated in . The results indicated the enrichment of protein by 56 and 42% in DSC for FE and SFE, respectively after 4 days of fungal treatment. The production trend of protein was similar as followed by DSC production, as it was determined from DSC. The highest value of protein was accounted after 4 days of microbial treatment for FE (25.78 g kg−1) and 5 days for SFE (24.28 g kg−1), respectively, and no marked difference was observed thereafter in both cases. Nigam (Citation[[1994]]) has studied the enrichment of protein in sugar industry by-products. Enrichment of protein in sludge cake produced from domestic wastewater sludge is supported by Alam et al. (Citation[[2002]]).
Figure 2. Enrichment of protein in dry sludge cake (DSC) by microbial treatment of STP sludge in a batch fermenter as well as the shake flask.
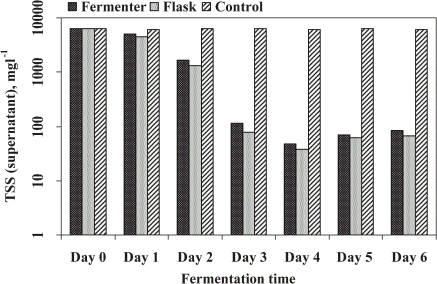
Total suspended solids (TSS) in supernatant was determined in both experiments (FE and SFE) is shown in . The TSS removal was highly influenced by the fungal treatment of sludge after 3 days of treatment. The results recorded had no significant difference in between both experiments after microbial treatment of STP sludge. The removal rate was maximum until 4 days of fermentation after that the value was slightly increased up to final days of treatment (6 days). The reduction of TSS in supernatant was slightly higher (99%) in SFE than the FE (98.8%) compared to control (uninnoculated). This may be due to higher entrapment/immobilization in SFE as compared to FE, which involved higher mechanical agitation caused in damage fungal mycelia. Jin et al. (Citation[[1999]]) has removed 95% of TSS in starch processing wastewater by fungal treatment. Alam et al. (Citation[[2002]]) has observed the removal of TSS in domestic wastewater sludge which was highly affected by the fungal strains.
Figure 3. Total suspended solids (TSS) in supernatant of treated sludge recorded in fermenter and shake flask.
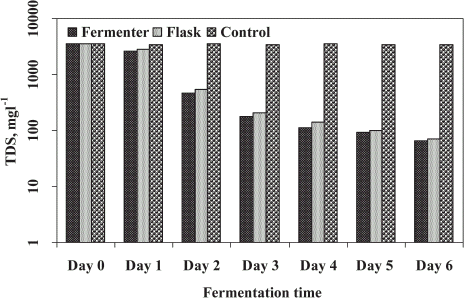
The total dissolved solids (TDS) in fungal treated sludge is shown in . The fungi utilized the dissolved solids as substrate during their proliferation and as a result the TDS decreased effectively in treated supernatant of sludge. The reduction of TDS by fungal treatment was highly affected after 3 days of treatment. The decreasing rate was observed up to 6 days of treatment in both the experiments (FE and SFE). No significant reduction of TDS was recorded after 3 days of fermentation. The results showed that removal percent of TDS for FE (98.1%) and SFE (97.9%) was almost same compared to untreated sludge after 6 days of treatment. Recently, Alam and Fakhru'l-Razi (Citation[[2002]]) have studied that the fungi reduced the dissolved solid by 96% after 3 days of treatment in a batch fermenter with optimum agitation and aeration conditions.
Figure 4. Total dissolved solids (TDS) in STP sludge to evaluate the fermenter and shake flask performance.
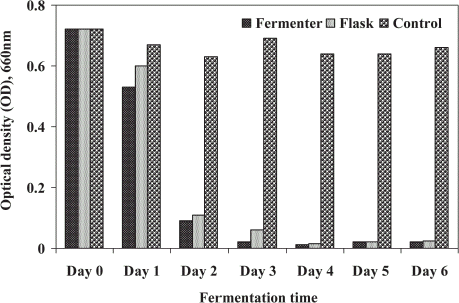
The optical density (OD, 660 nm) of supernatant against distilled water was determined and considered as turbidity of treated sludge (Alam and Fakhru'l-Razi, Citation[[2002]]). The effect of microbial treatment on the reduction of turbidity in STP sludge is shown in . The microorganisms utilized the soluble and insoluble substances in waste sludge resulting in effective removal of turbidity. The highest removal of turbidity observed after 4 days of treatment in both cases. The higher decreasing trend was recorded for first four days of treatment and it was increased slightly until 6 days of fermentation. The removal rate was higher (97.1%) in FE than the SFE (91.3%) for first 3 days of operation thereafter no marked difference was perceived up to 6 days of treatment. The similar study has been done in optimization of bioconversion process for biological treatment of domestic wastewater sludge (Alam et al., Citation[[2002]]; Alam and Fakhru’l-Razi, Citation[[2002]]).
Figure 5. Optical density (turbidity) in microbially treated sludge using fermenter and shake flask.
The reduction of soluble protein (SP) in supernatant of STP sludge is an important parameter to evaluate the treatment performance as nutrient removal in wastewater treatment plant. The removal of SP with the treatment of fungal inoculum was observed in . The microbial strains consumed SP as nutrient and energy sources and as a result, reduction process of SP was stimulated. Highest removal of SP was recorded after 6 days of treatment in both cases. The results indicated that the maximum removal of SP was higher in FE (84.3%) than the SFE (76.2%) compared to untreated sludge (control). The efficiency in FE was about 8% higher than the SFE in terms of SP reduction after treatment. This observation agreed with the study of Alam et al. (Citation[[2002]]).
Figure 6. Reduction of soluble protein by fungal mixed culture of A. niger and P. corylophilum in batch fermenter and shake flask.
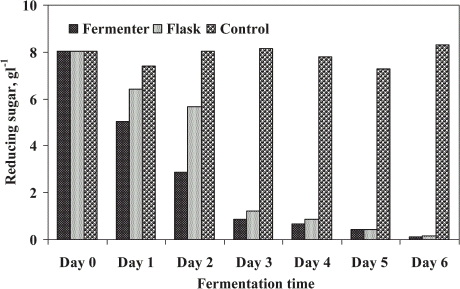
Reducing sugar (RS) was the parameter for determining the consumption of substrate by microfungi. The mixed culture of fungi influenced the reduction rate of RS by consuming the substrate as food and it was also reduced by fungal secondary metabolites. The removal of RS is shown in . The highest removal was perceived up to final days of treatment for both cases. The higher RS reduction rate was accounted for first 3 days of fermentation and after that no marked difference was observed for FE and SFE. The maximum substrate utilization was 98.7% by the FE and 98% of RS was utilized by SFE after final treatment. The observed results showed about 1% higher removal rate in FE than the SFE. Alam et al. (Citation[[2002]]) achieved the similar results during the bioconversion process of wastewater sludge in shake flask experiment. In a batch fermenter, RS was utilized by fungi to assess optimum agitation and aeration which was recorded by Alam and Fakhru'l-Razi (Citation[[2002]]).
Figure 7. Substrate utilization (reducing sugar) by the mixed microbial treatment of STP sludge in a fermenter compared to shake flask.
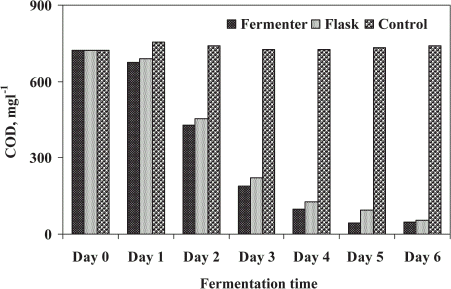
The STP sludge contained higher level of chemical oxygen demand (COD) caused serious problems to dispose into the environment without any treatment. The effect of microfungi on removal of COD in sludge is shown in . The removal of COD of treated sludge was exhibited by using fungal mixed culture of A. niger and P. corylophilum. The maximum removal of COD (94.1%) was observed in FE after 5 days of fermentation. For SFE, highest reduction rate (92.5%) was recorded after 6 days of treatment. The increased removal percent of COD was about 2% higher in FE than the SFE. This may due to higher growth rate of filamentous fungi in FE than the SFE. The filamentous fungi utilized the soluble and insoluble organic matters in sludge and enhanced the removal of COD in treated sludge. The increasing rate of reduction of COD in different wastes such as domestic wastewater sludge, olive oil mill waste, apple distillery waste, kraft bleaching effluents and pulp and paper mill effluent have been studied using different fungal inoculum (Friedrich et al., Citation[[1983]]; Hamdi et al., Citation[[1991]]; Marwaha et al., Citation[[1998]]; Nagarathnamma et al., Citation[[1999]]; Robles et al., Citation[[2000]]).
Figure 8. Chemical oxygen demand (COD) removal by microbial treatment of STP sludge in fermenter and shake flask.
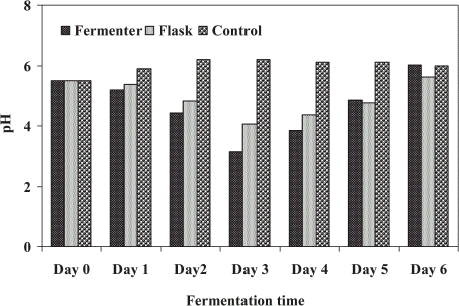
The pH values were recorded in both treatments (FE and SFE) during the bioconversion process (). The values of pH were highly affected by the microbial treatment of sludge. Initially pH was decreased for first 3 days from its initial value and it was increased up to final treatment of sludge. In both experiments, acidic sludge (pH < 6.2) was produced due to the secretion of organic acids by acting microbes. The final pH recorded was 6.02 and 5.63 for FE and SFE, respectively. Several authors have reported the production of acidic pH by fungi during treatment in different wastes (Alam et al., Citation[[2001]]; Miller, Citation[[1959]]; Friedrich et al., Citation[[1986]]).
Conclusion
The study on fungal treatment of STP sludge in shake flask and fermenter was conducted to evaluate their performance in LSB process. In both experiments, bioconversion process was effectively influenced by the mixed microbial treatment compared to uninnoculated treatment (control). The results in this study indicated that the bioconversion efficiency was higher in fermenter than the shake flask in terms of biosolids accumulation and biodegradation of STP sludge. About 0.2–20% higher efficiency was recorded in fermenter due to significant controlled conditions as compared to shake flask that would be positive approach for scaling up treatment process in future applications.
Acknowledgments
The authors would like to convey their heartfelt gratitude to the Department of Chemical and Environmental Engineering, Universiti Putra Malaysia (UPM) for their kind support and IWK for their research grant and for providing the sludge sample during this study.
References
- Alam M. Z. Microbial treatment of domestic wastewater treatment plant sludge by liquid state bioconversion process. Faculty of Engineering, Universiti Putra Malaysia. 2002, Ph.D. thesis
- Alam M. Z., Fakhru'l-Razi A. Effect of agitation and aeration on bioconversion of domestic wastewater sludge in a batch fermenter. J. Environ. Sci. Health 2002; A37: 1087–1097
- Alam M. Z., Fakhru'l-Razi A., Molla A. H., Roychoudhury P. K. Treatment of wastewater sludge by liquid state bioconversion process. J. Environ. Sci. Health 2001; A36: 1237–1243
- Alam M. Z., Fakhru'l-Razi A., Abd-Aziz S., Idris A. Bioconversion of domestic wastewater sludge by immobilized mixed culture of Penicillium corylophilum WWZP1003 and Aspergillus niger SCahmA103. Art. Cells, Blood Subs., and Immob. Biotechnol. 2002; 30: 307–318, [CROSSREF]
- Alam M. Z., Fakhru'l-Razi A., Molla A. H. Evaluation of fungal potentiality for bioconversion of domestic wastewater sludge. J. Environ. Sci. 2003a; 16: 132–137
- Alam M. Z., Fakhru'l-Razi A., Molla A. H. Optimization of compatible mixed cultures for liquid state bioconversion of municipal wastewater sludge. Water, Air Soil Poll. 2003b; 149: 113–126, [CROSSREF]
- Alam M. Z., Fakhru'l-Razi A., Molla A. H. Optimization of liquid state bioconversion process for microbial treatment of domestic wastewater sludge. J. Environ. Eng. Sci. 2003c; 2(4)299–306, [CROSSREF]
- APHA. Standard methods for the examination of water and wastewater, 17th ed. American Public Health Association, Washington, DC 1989
- Baier U. Thermal inactivation of plant seeds in sewage sludge. Water Sci. Technol. 1997; 36(11)197–202, [CROSSREF]
- Baier U., Schmidheiny P. Enhanced anaerobic degradation of mechanically disintegrated sludge. Water Sci. Technol. 1997; 36(11)137–143, [CROSSREF]
- Dollerer J., Willder P. A. High pressure treatment of organic wastes. Water Sci. Technol. 1993; 28(1)243–248
- Fakhru'l-Razi A., Alam M. Z., Idris A., Abd-Aziz S., Molla A. H. Filamentous fungi in Indah Water Konsortium (IWK) sewage treatment plant for biological treatment of domestic wastewater sludge. J. Environ. Sci. Health 2002; A37: 309–320
- Friedrich J., Cimerman A., Perdih A. The use of Aspergillus niger for bioconversion of apple distillery waste. Eur. J. Appl. Microbiol. Biotechnol. 1983; 17: 243–247, [CROSSREF]
- Friedrich J., Cimerman A., Perdih A. Comparison of different cellulolytic fungi for bioconversion of apple distillery waste. Appl. Microbiol. Biotechnol. 1986; 24: 432–434, [CROSSREF]
- Friedrich J., Cimerman A., Perdih A. Mixed culture of Aspergillus awamori and Trichoderma reesei for bioconversion of apple distillery waste. Appl. Microbiol. Biotechnol. 1987; 26: 299–303, [CROSSREF]
- Hamdi M., Hamed H. B., Ellouz R. Optimisation of olive mill waste-waters by Aspergillus niger. Appl. Microbial. Technol. 1991; 36: 285–288
- Jin B., van Leeuwen J., Yu Q., Patel B. Screening and selection of microfungi for microbial biomass protein production and water reclamation from starch processing wastewater. J. Chem. Technol. Biotechnol. 1999; 74: 106–110, [CROSSREF]
- Kadir M. D. A., Velayutham S. The management of municipal wastewater sludge in Malaysia. Symp on sludge management. Universiti Technologi Malaysia. Aug. 18–19, 1999
- Knapp J. S., Howell J. A. Treatment of primary sewage sludge with enzymes. Biotechnol. Bioeng. 1978; 20: 1221–1234
- Kopp J., Muller J., Dichtl N., Schwedes J. Anaerobic digestion and dewatering characteristics of mechanically excess sludge. Water Sci. Technol. 1997; 36(11)129–136, [CROSSREF]
- Li Y. Y., Noik T. Upgrading of anaerobic digestion of waste activated sludge by thermal pre-treatment. Water Sci. Technol. 1992; 26(3–4)857–866
- Lin J. G., Rajan R. V., Ray B. T. Low-level chemical pretreatment for enhanced sludge solubilization. J. Water Pollut. Control Fed. 1989; 61: 1678–1683
- Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951; 193: 265–275, [PUBMED], [INFOTRIEVE]
- Marwaha S. S., Grover R., Prakash C., Kennedy J. F. Continuous biobleaching of black liquor from the pulp and paper industry using an immobilized cell system. J. Chem. Technol. Biotechnol. 1998; 73: 292–296, [CROSSREF]
- Miller G. L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959; 31: 426–428
- Mukherjee S. R., Levine A. D. Chemical solubilization of particulate organics as a pretreatment approach. Water Sci. Technol. 1992; 26(9–11)2289–2292
- Nagarathnamma R., Bajpai P., Bajpai P. K. Studies on decolorization, degradation, and detoxification of chlorinated lignin compounds in kraft bleaching effluents by Ceriporiopsis subvermispora. Process Biochem. 1999; 34: 939–948, [CROSSREF]
- Nigam P. Process selection for protein-enrichment: fermentation of the sugar industry by products molasses and sugar beet pulp. Process Biochem. 1994; 29: 337–342, [CROSSREF]
- Robles A., Lucas R., Alvarez de Cienfuegos G., Galvez A. Biomass production and detoxification of wastewaters from the olive oil industry by strains of Penicillium isolated from wastewater disposal ponds. Bioresour. Technol. 2000; 74: 217–221, [CROSSREF]
- Tiehm A., Nickel K., Neis U. The use of ultrasound to accelerate the anaerobic of sewage sludge. Water Sci. Technol. 1997; 36(11)121–128, [CROSSREF]
- Woodard S. E., Wukasch R. F. A hydrolysis/thickening/filtration process for the treatment of waste activated sludge. Water Sci. Technol. 1994; 30(3)29–38
- Yasui H., Shibata M. An innovative approach to reduce excess sludge production in the activated sludge process. Water Sci. Technol. 1994; 30(9)11–20