Abstract
Porcine pancreatic lipase and Candida cylindracea lipase were immobilized on Celite and Amberlite IRA-938. Activities and stabilities of immobilized lipases were investigated. The immobilized lipase derivatives on Celite exhibited grater residual activity and more resistance to thermal inactivation than their immobilized counterpart on Amberlite IRA-938. The apparent optimum temperatures of the immobilized lipases were 7–10°C higher than that of the free enzymes. The native lipase and lipases immobilized on Celite showed same behaviors of pH dependence. But the pH optimum values for lipases immobilized on Amberlite IRA-938 were shifted to the acidic region relative to that of free enzymes. The stabilities of free and immobilized lipases were also investigated.
Introduction
Lipases (triacylglycerol ester hydrolases EC (3.1.1.3)) catalyze both, the hydrolysis of triglycerides and other carboxylic acid esters in aqueous solution (Mettson and Volpenheim, [Citation1969]) and their synthesis in hydrophobic organic solvents with limited water content (Doumèche et al., [Citation2002]; Zaks and Klibanov, [Citation1985]). In recent years lipases have become of great interest to the chemical and pharmaceutical industries owing to their usefulness in both hydrolysis and synthesis reactions. Lipases are often used because they are inexpensive and easy to handle. Other reasons for the enormous interest with regard to the use of lipases as biocatalysts include: (i) they are stable in organic solvent, (ii) do not require cofactors, (iii) possess broad substrate specificity, (iv) exhibit a high enantioselectivity (Jaeger and Reetz, [Citation1998]). However, due to economical consideration their industrial applications require their immobilization and thus re-usability. Immobilized enzymes have a wide range of potential practical applications. They display a number of advantages over the use of soluble enzymes, e.g., the possibility of recovery and reuse, simplicity in operation, and improved stability. Many techniques for the immobilization of lipases have been reported, using synthetic or natural polymers, inorganic materials, controlled pore glasses, silica, and zeolite (Bagi et al., [Citation1997]; Dimitriu et al., [Citation2003]; Dosanjh and Kaur, [Citation2002]; Ivanov and Scheider, [Citation1997]; Kilinç et al., [Citation2002]; Meteo et al., [Citation2000]; Moreno and Sinisterra, [Citation1994]; Sağiroğlu and Telefoncu, [Citation1993]).
The aim of the present work was to produce various immobilized forms of porcine pancreatic lipase (PPL) and Candida cylindracea lipase (CCL) with advantageous catalytic properties and stabilities. Lipases were immobilized on anion-exchange resin (Amberlite IRA-938) and Celite. Influence of several parameters on the activity of the immobilized lipase preparations has been studied.
Materials And Methods
Materials
Porcine pancreatic lipase (PPL), Candida cylindracea lipase (CCL), and bovine serum albumin were obtained from Sigma Chemical Co. (St.Louis, MO). Amberlite IRA-938 was provided by Röhm and Haas, Italy S.P.A. (Milan, Italy). Celite 545 was purchased from Aldrich Chemical Co. (Milwaukee, WI). All other chemicals were analytical reagent grade and were obtained from E. Merck (Darmstadt, Germany). Olive oil was purchased in a local market.
Immobilization of Lipases on Celite
Lipases from porcine pancreas and Candida cylindracea were immobilized by adsorption on Celite using 0.1 M phosphate buffer (pH 7.0) as an immobilization medium. Five hundred milligrams of PPL and CCL were dissolved separately in 15 mL phosphate buffer, filtered off, and diluted with bidistilled water up to 50 mL. The protein concentration and lipolytic activity in filtrates were determined. Two point five grams of Celite was added to both enzyme solutions. The mixture was shaken for 30 min at room temperature and dried under reduced pressure on a rotevapor at 40°C. Immobilized lipase preparates were stored as a dry material at 4°C.
Immobilization of Lipases on Amberlite IRA-938
Lipases from porcine pancreas and Candida cylindracea were immobilized on Amberlite IRA-938 by a physical adsorption. Five hundred milligrams of crude PPL and CCL were dissolved separately in 15 mL phosphate buffer (pH 7.0), filtered off, and diluted as described above. The protein concentration and enzymatic activity in filtrates were determined. Two point five grams of Amberlite IRA-938 was added to both enzyme solutions. The mixtures were shaken for 1 h at room temperature. The resin beads were separated by filtration through a glass filter. They were then washed by resuspending and filtering three times with 20 mL bidistilled water. The beads were lyophilized and stored at 4°C.
Protein Determination
Determination of protein concentration in adsorption filtrates was performed according to the method of Lowry et al. ([Citation1951]) by using bovine serum albumin as the standard. The amount of bound protein was determined indirectly from the difference between the amount of protein introduced into the coupling reaction mixture and the amount of protein present in the filtrate.
Determination of Lipolytic activity
Lipolytic activities of free and immobilized enzyme derivatives were measured by a titrimetric assay with 0.05 M NaOH using emulsified olive oil as the substrate. The substrate emulsion was prepared in a blender by mixing 10 mL of olive oil with 20 mL of 10% gum arabic solution. The reaction mixture, consisting of 5 mL of the substrate emulsion, 4 mL of phosphate buffer (pH 7.0), and either free (1 mL, 0.1 mg/mL) or immobilized lipase (100–200 mg) was incubated for 30 min at 37°C. After this time interval, the reaction was stopped by the addition of 10 mL acetone/ethanol solution (1:1). The liberated fatty acids were titrated with 0.05 M NaOH solution in presence of phenolphthalein as an indicator (Hoppe and Theimer, [Citation1996]). One unit (U) of enzyme activity was defined as the amount of enzyme liberated 1 µmol of free fatty acids per minute under the assay conditions.
Stability Tests
The pH stabilities of free and immobilized lipases were compared in the 0.1 M phosphate buffer between pH 6.0 and 8.0. Free and immobilized forms of PPL and CCL were incubated in phosphate buffer for 30 min at 4°C and then the remaining activity was assayed at the standard assay conditions.
For the thermal stability study, the free and immobilized forms of PPL and CCL were incubated at various temperatures (25–55°C) for 1 h and residual activity was measured at standard assay conditions.
The operational stabilities of the free and immobilized lipases were performed with hydrolysis of olive oil at 37°C and optimum pH of the free and immobilized enzyme preparations in a continuous stirred tank reactor, by measuring the activity of samples taken at regular time intervals. The relationship between operation time and the decrease in enzyme activity was determined. The reaction vessel containing 2 g olive oil in 100 mL of bidistilled water and about 100 U of different forms of lipases (free and immobilized forms of PPL and CCL). After appropriate incubation periods aliquots were withdrawn and the amount of free fatty acids were determined by titration with 0.01 M sodium hydroxide.
Results And Discussion
Porcine pancreatic lipase (PPL) and Candida cylindracea lipase (CCL) were immobilized on Celite and Amberlite IRA-938. The results of immobilizations are summarized in .
Table 1. Immobilization of porcine pancreatic lipase (PPL) and Candida cylindracea lipase (CCL)
Amberlite IRA-938 is a macroreticular, strongly basic, anion exchange resin with a pore size distribution between 25,000 and 230,000Å. Its porosity and anion exchange capacity are 0.972 cc pore/g dry resin and 3.8 meq/g dry resin, respectively. The binding capacities for both lipases immobilized on Amberlite IRA-938 were greater than that of immobilized on Celite. But activity yields of PPL and CCL immobilized on Celite higher than that of immobilized on Amberlite IRA-938.
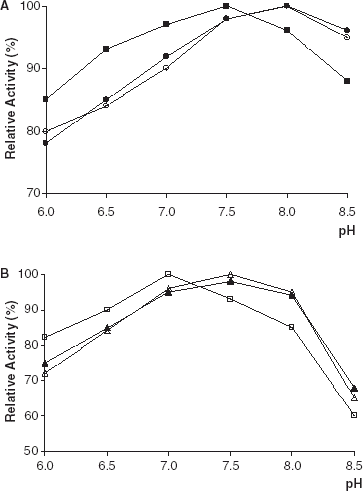
The lipolytic activities of free and immobilized PPL and CCL were determined at different pH values. The optimum pH values were determined from the graph of pH plotted against the percentage of relative activity (). The pH optima of PPL and CCL immobilized on Amberlite IRA-938 were shifted slightly to the acidic region relative to that of free enzymes. The reason for this shift is the polycationic character of Amberlite IRA-938 (Telefoncu, [Citation1983]; Shaw et al., [Citation1990]).
Figure 1. Effect of pH on lipolytic activity of free and immobilized PPL and CCL. (a) (○): free PPL, (•): PPL immobilized on Celite, (▪): PPL immobilized on Amberlite IRA-938. (b) (▵): free CCL, (▴): CCL immobilized on Celite, (□): CCL immobilized on Amberlite IRA-938.
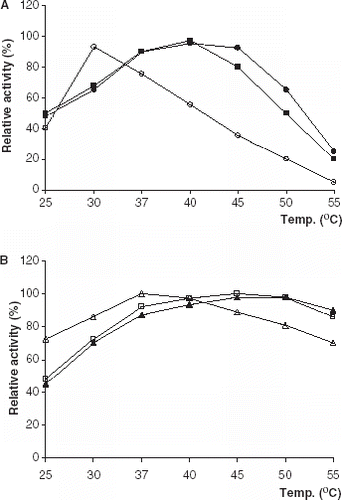
The temperature dependence of the lipolytic activity was investigated in 0.1 M phosphate buffer at the optimum pH values in the temperature range 25–55°C (). The optimum temperatures for all of immobilized lipase derivatives were higher than that of free lipases. The similar results has been reported (Bagi et al., [Citation1997]; Kilinç et al., [Citation2002]; Moreno and Sinisterra, [Citation1994]).
Figure 2. Effect of temperature on lipolytic activity of free and immobilized PPL and CCL. (a) (○): free PPL, (•): PPL immobilized on Celite, (▪): PPL immobilized on Amberlite IRA-938. (b) (▵): free CCL, (▴): CCL immobilized on Celite, (□): CCL immobilized on Amberlite IRA-938.
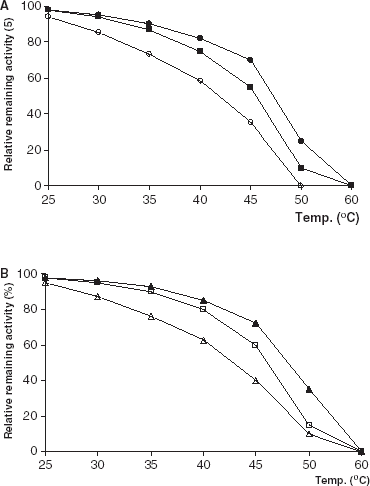
The effect of incubation temperature on free and immobilized enzyme stabilities were studied in the temperature range 30–60°C at pH 7.0 in 0.1 M phosphate buffer. The thermal stabilities for all forms of immobilized lipases were better than that of free lipases (). In general, thermal stability of an immobilized enzyme increases relative to soluble form (Fagain and Kennedy, [Citation1991]; Illanes, [Citation1999]; Tombs, [Citation1985]).
Figure 3. Thermal stability of free and immobilized PPL and CCL. (a) (○): free PPL, (•): PPL immobilized on Celite, (□): PPL immobilized on Amberlite IRA-938. (b) (▵): free CCL, (▴): CCL immobilized on Celite, (□): CCL immobilized on Amberlite IRA-938.
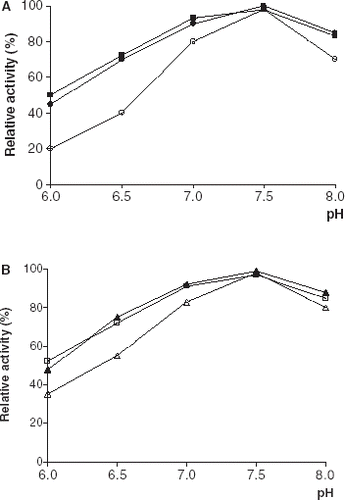
The effect of incubation pH on free and immobilized lipase stabilities were compared in 0.1 M phosphate buffer in the pH range 6.0–8.0 for 30 min at 4°C (). As can be seen from the immobilized enzyme derivatives were more stable than their free counterpart at all pH values. A similar observation has been reported by Guo et al. ([Citation2003]).
Figure 4. pH stabilities of free and immobilized PPL and CCL. (a) (○): free PPL, (•): PPL immobilized on Celite, (▪): PPL immobilized on Amberlite IRA-938. (b) (▵): free CCL, (▴): CCL immobilized on Celite, (□): CCL immobilized on Amberlite IRA-938.
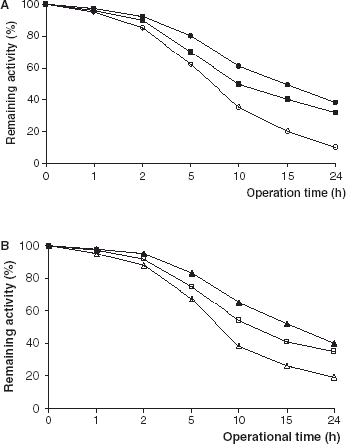
The hydrolysis of olive oil catalyzed by immobilized lipase derivatives was carried out continuously for 24 h (). For the industrial or preparative uses of immobilized enzymes, characterization of their operational stabilities is very important. Protein stabilization has been achieved by several methods, including immobilization and crosslinking (Sharma and Gupta, [Citation2001]; Sundaram and Venkatesh, [Citation1999]; Wu et al., [Citation2001]). As can be seen from PPL and CCL immobilized on Celite lost their initial activities more quickly than that of immobilized on Amberlite IRA-938.
Figure 5. Operational stabilities of free and immobilized PPL and CCL. (a) (○): free PPL, (•): PPL immobilized on Celite, (▪): PPL immobilized on Amberlite IRA-938. (b) (▵): free CCL, (▴): CCL immobilized on Celite, (□): CCL immobilized on Amberlite IRA-938.
In conclusion, Celite and Amberlite IRA-938 are good carriers for the immobilization of porcine pancreatic lipase and Candida cylindracea lipase and the immobilization procedure is very easy to carry out. The lipases immobilized on both supports showed good catalytic properties and stabilities.
References
- Bagi K., Simon L. M., Szajani B. Immobilization and characterization of porcine pancreas lipase. Enzyme and Microbial Technology 1997; 20: 531–535
- Dosanjh N. S., Kaur J. Immobilization, stability and esterification studies of a lipase from a Bacillus sp. Biotechnol. Appl. Biochem. 2002; 36: 7–12
- Doumèche B., Heinemann M., Büchs J., Hartmeier W., Ansorge-Schumacher M. B. Enzymatic catalysis in gel-stabilized two-phase systems: improvement of the solvent phase. J. Mol. Cat. B: Enzymatic 2002; 18: 19–27
- Dumitriu E., Secundo F., Patarin J., Fechete I. Preparation and properties of lipase immobilized on MCM-36 support. J. Mol. Cat. B: Enzymatic 2003; 22: 119–133
- Fagain C. O., Kennedy R. O. Functionally-stabilized proteins. Biotechnol. Adv. 1991; 9: 351–409
- Guo Z., Bai S., Sun Y. Preparation and characterization of immobilized lipase on magnetic hydrophobic microspheres. Enzyme Microb. Technol. 2003; 32: 776–782
- Hoppe A., Theimer R. R. Titrimetric test for lipase activity using stabilized triolein emulsions. Phytochemistry 1996; 42: 973–978
- Illanes A. Stability of biocatalysts. Electron. J. Biotechnol. 1999; 2: 7–8
- Ivanov A., Scheider M. P. Methods for the immobilization of lipases and their use for ester synthesis. J. Mol. Cat. B: Enzymatic. 1997; 3(6)303–309
- Jaeger K. E., Reetz M. T. Microbial lipases form versatile tools in biotechnology. Trends Biotechnol. 1998; 16: 396–403
- Kilinç A., Önal S., Telefoncu A. Chemical attachment of porcine pancreatic lipase to crosslinked poly(vinyl alcohol) by means of adipoyldichloride. Process Biochemistry 2002; 38: 641–647
- Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951; 193: 265
- Meteo C., Albian O., Lafuente R., Gusian J.M. Reversible enzyme immobilization via a very strong and non-distorting adsorption on supports-polyethylene imine composite. Biotechnol. Bioeng. 2000; 68(1)98–105
- Mettson F. H., Volpenheim R. A. Relative rates of hydrolysis by rat pancreatic lipase of esters of C2-C18 fatty acids with C1-C18 primary n-alcohols. J. Lipid Res. 1969; 10: 271–276
- Moreno J. M., Sinisterra J. V. Immobilization of lipase from Candida cylindracea on inorganic supports. J. Mol. Cat. 1994; 93(3)357–369
- Sağiroğlu A., Telefoncu A. Synthesis of stereospecific esters and resolution of racemic alchols. Indian J. Chem. 1993; 32B: 85–87
- Sharma S., Gupta M.N. Alginate as a macroaffinity ligand and an additive for enhanced activity and thermostability of lipases. Biotechnol. Appl. Biochem. 2001; 33: 161–165
- Shaw J.F., Chang R.C., Wang F.F., Wang Y.J. Lipolytic activities of lipase immobilized on six support materials. Biotechnol. Bioeng. 1990; 35: 132–137
- Sundaram P., Venkatesh R. Retardation of thermal and urea induced inactivation of alpha-chymotrypsin by modification with carbohydrate polymers. Protein Eng. 1999; 11: 699–705
- Telefoncu A. Preparation and properties of trypsin chemically attached to EEDQ activated styrene methacrylic acid copolymers. Biotechnol. Bioeng. 1983; 25: 703–724
- Tombs M.P. Stability of enzymes. J. Appl. Biochem. 1985; 7: 3–24
- Wu J.C., Zhang G.F., He Z.M. Enhanced activity of Candida rugosa lipase modified by polyethylene glycol derivatives. Biotechnol. Lett. 2001; 23(3)211–214
- Zaks A., Klibanov A. Enzyme-catalyzed processes inorganic solvents. Proc. Natl. Acad. Sci. USA 1985; 82: 3192–3196