Abstract
The unique behavior of perfluorocarbons (PFCs), including their high oxygen dissolving capacity, hydrophobic and lipophobic character, and extreme inertness, derive directly, in a predictable manner, from the electronic structure and spatial requirements of the fluorine atom. Their low water solubility is key to the prolonged in vivo persistence of the now commercially available injectable microbubbles that serve as contrast agents for diagnostic ultrasound imaging. OxygentTM, a stable, small-sized emulsion of a slightly lipophilic, rapidly excreted PFC, perfluorooctyl bromide (perflubron), has been engineered. Significant oxygen delivery has been established in animal models and through Phase II and III human clinical trials. However, an inappropriate testing protocol and the lack of funding led to temporary suspension of the trials.
UNIQUE, BUT NOT “MYSTERIOUS”
Last year, when we met in Stockholm, a distinguished colleague of ours, highly competent in hemoglobin matters, told me that fluorocarbons (or perfluorocarbons, PFCs) were for him “a total mystery” (his words). However, when questioned about what he had read about PFCs, he admitted frankly that although he was intrigued by them, he had never had time to really read any paper about PFCs.
This is one reason why I choose, in this presentation, to return to basics about PFCs and PFC emulsions, in case there still were a few colleagues who hadn't yet had a chance to learn about PFCs and with the hope of solving some of the perceived mysteries. The topic is also timely because of Alliance Pharmaceutical Corp. (San Diego, CA) having voluntarily interrupted a clinical trial of its emulsion, OxygentTM, in a cardio pulmonary bypass surgery setting involving augmented acute normovolemic hemodilution.
My message is “There are no mysteries, just specific molecules with their rather unique attributes and performances. These attributes derive directly, can be understood and can be predicted from the specific electronic structure and spatial requirement of the constituent atoms, especially the fluorine atom.”
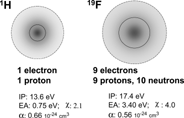
Back to basics implies back to chemistry. There should be little wonder that replacing all the hydrogen atoms by fluorines in an organic molecule should bring about some substantial changes in behavior.[Citation[1-4]] Fluorine has 9 electrons (and 9 protons and 10 neutrons) as compared to only one electron (and one proton) for hydrogen. These 9 electrons are packed in—proportionally—less space, hence in a more compact way (). In other words, fluorine has a much denser electron cloud. Fluorine also has a higher ionization potential than hydrogen (just after the inert gases He and Ne), a considerably larger electron affinity, the highest electronegativity of all atoms, and a lower polarizability than hydrogen, second only to Ne.
Figure 1 The hydrogen and fluorine atoms compared (IP: ionization potential; EA: electron affinity; χ: electronegativity; α: polarizability; see Riess, Tetrahedron 58: 4113 (2002)).
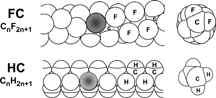
As a result, perfluoroalkyl chains (F-chains) are structurally quite different from standard alkyl chains (). The fluorine atom, being more space demanding (CF3 is only marginally smaller than C(CH3)3), forces the C—C skeleton to adopt a helical arrangement rather than the usual planar zig-zag configuration found in hydrocarbon chains (H-chains). F-chains are also bulkier than H-chains (cross section of 30 vs. 20 Å, respectively). The larger trans/gauche interchange energy barrier (4.6 vs. 2.0 kJ mol−1, respectively) makes them more rigid (it takes more energy to twist them) and allows for fewer kinks. Finally, the larger, electronically more dense fluorine atoms cover and protect the C—C backbone much more effectively than hydrogen atoms do.
Figure 2 A schematic, comparative view showing the structural differences between fluorocarbon and hydrocarbon chains, with cross-sections on the right.
Fluorocarbons: Among the Most Stable, Most Inert Chemicals Known to Man
Why are PFCs more stable (in the thermodynamic sense) and chemically more inert (in the kinetic sense) than their hydrocarbon (HC) counterparts? A better match between carbon and fluorine orbitals as compared to that between carbon and hydrogen leads to the strongest single bond found in molecular compounds (e.g. 530 kJ mol−1 for C—F in C2F6 vs. 439 kJ mol−1 for C—H in CH4). Moreover, the extreme electron attracting character of fluorine enhances the C—C bond energy in the skeleton by “shrinking” the orbitals of the carbons (e.g. 413 kJ mol−1 in CF3—CF3 vs. 376 kJ mol−1 in CH3—CH3).
From the reactivity standpoint, there simply exist no low energy molecular orbitals accessible for binding O2, CO, NO. The fluorine atoms shield the C—C skeleton sterically. Additionally, the dense electronic sheath repels approaching reagent, i.e. exercises some sort of a “Scotchguard” effect at the molecular level.
The following few examples illustrate the difference in reactivity between F-alkylated and alkylated compounds. While HCs (think of octane) are highly flammable, PFCs (e.g. F-octane) are not, and could even serve as fire extinguishers! Typical n-CnF2n+2 compounds resist heating to 400°C; n-CnF2n+1Br withstands 300°C for 24 h; cooking with 5 N H2SO4 at 105°C for 10 days; exposure to 300 nm UV (ICH test), etc. Polytetrafluoroethylene (PTFE, well-known under the brand name Teflon®) is one of the most inert organic materials known. Expanded PTFE (Gore-Tex®) is used in body implant devices and allows natural tissues to grow in its pores. Fluorinated surfactants can resist highly aggressive media, including strong acids, alkalies and oxidants, even at high temperatures, e.g. can withstand contact with 98% sulfuric acid containing 10 g/L chromic acid for 28 days at 90°C; no non-fluorinated surfactant is known that resists such harsh conditions. Which H-surfactant could possibly have a hydrophobic moiety that is also lipophobic? Finally, PFCs are not metabolized; since Mother Nature did not exploit the PFC route, she did not develop the enzymes that would have been needed to recycle them. Pure PFCs have no effects on cell cultures either, other than the benefits that result from their capacity to provide O2 or CO2. One can drink PFCs by the liter without side effects other than wet pants.
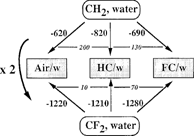
relates to the difference in behavior of F-chains and H-chains at interfaces. It compares the difference in the contribution to the free energy of adsorption of CF2 and CH2 segments from water to air/water or HC/water or PFC/water interfaces. It shows that these free energy differences are roughly twice as large for CF2 as compared to CH2. This reflects the higher interfacial activity of CF2s (lower surface tension) and their higher affinity for (and alikeness to) gases as compared to CH2s. For example, the surface tension of F-n-octane is 13.6 mN m−1 vs. 21.1 mN m−1 for n-octane (as compared to 72.8 mN m−1 for water).
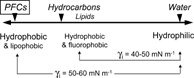
Highly Hydrophobic and Lipophobic, as Well
PFCs are the most hydrophobic organic substances ever invented. They are considerably more hydrophobic than HC oils. Increased hydrophobicity is primarily a matter of low polarizability and, for a given PFC molecular structure, of increased surface area exposed to the surrounding medium, as compared to the “parent” HC. On a polarity scale (where water would be on the high polarity side, ), PFCs are located further out than HCs with respect to water: The PFC/water interfacial tension (which opposes the dispersion of PFCs in water) can reach 60 mN m−1. The mixing of PFCs and HCs is also highly non-ideal, resulting for PFCs in the unique attribute of being not only hydrophobic but lipophobic (or oleophobic) as well.
Figure 4 The relative positions of fluorocarbons, hydrocarbons and water on a polarity scale: fluorocarbons are more hydrophobic than olive oil, and lipophobic as well.
The well-known “hydrophobic effect” is the basis for the self-association of lipids into bilayer membranes, such as cell membranes. Being both extremely hydrophobic and substantially lipophobic as well, PFCs and F-chains tend to keep to themselves and do not tend to mix with either aqueous phases or lipids.
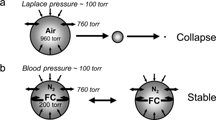
The extreme hydrophobicity of PFCs translates into very low water solubility (typically one order of magnitude lower than HCs). These low water solubilities, lower than those of any volatile HC, combined with high volatility (that also results from low intermolecular forces), are the basis for the stabilization of injectable dispersions of micron-size PFC-containing gas bubbles that serve as in vivo reflectors for contrast ultrasound imaging. Gas bubbles, small enough to pass the capillary beds, are indeed ideal sound wave scatterers. However, plain air bubbles, when injected in the circulation, dissolve within seconds under the combined effect of blood pressure and surface tension pressure (). Rapid microbubble dissolution could be prevented by introducing a volatile PFC inside the bubbles. The water-soluble gases, i.e. O2, N2 and CO2, equilibrate then with the gases present in the plasma, while the very poorly water-soluble PFC stays in the bubbles and compensates for blood pressure and Laplace pressure ().[Citation[6-7]]
Figure 5 Due to extremely low water solubility, volatile fluorocarbons stabilize injectable gas microbubbles used as contrast agents for ultrasound imaging (see Schutt et al., Angew. Chem. Int. Ed. 42: 3218 (2003)).
Several such perfluorochemical-based contrast agents, namely Optison® (Amersham Health Corp.), SonoVue® (Bracco), Definity® (Bristol Myers Squib) and Imagent® (Alliance Pharmaceutical Corp.), have been licensed by the FDA in the United States or EMEA in Europe in recent years and are now commercially available.
The same stabilization phenomenon is likely to play a key role in stabilizing the O2 microbubbles that are being investigated as O2 carriers by Lundgren and Tyssebotn.
Oxygen Dissolving and Delivery Capacity—Gas-Like Liquids
Why PFCs dissolve gases better than any other liquid is one of the most frequently asked questions. Remember that there exists no possibility for PFCs to bind gases chemically; PFCs dissolve them, as water does, or HCs. The exceptionally high gas-dissolving capacity of PFCs derives from fluorine's extremely low polarizability: low polarizability translates into low van der Waals interactions between PFC molecules, as van der Waals interactions depend directly on fluctuations in polarity of the electronic cloud. Since van der Waals interactions are the only intermolecular forces that keep together non-polar molecules, the intermolecular forces in PFCs are very feeble, in sharp contrast with their strong intramolecular bonds. Consequently, liquid PFCs behave like nearly ideal, gas-like fluids. They easily dissolve other substances of similarly low cohesivity, namely gases, including O2, CO2, N2, NO, etc. Birds of a feather flock together. The low cohesivity of PFCs is also reflected by their low boiling points and high volatility relative to their molecular weight (MW). If one compares the Hildebrandt parameters (which express the cohesive energy density of fluids, hence their aptitude for mutual solubility) of oxygen, typical PFCs and HCs, and water:one sees that PFCs are very much like O2, more so than HCs, not to speak of water, which, with its highly structured three-dimensional network of hydrogen bonds, lies at the opposite end of the scale.
In other words, it takes less energy to make a hole in a less cohesive material, such as a PFC and host a guest of similar cohesiveness, such as O2 ().
Figure 6 It takes less energy to make a hole in a less cohesive material, e.g. a fluorocarbon (left) and host a guest molecule of similarly low cohesiveness (a gas) than in a more cohesive material, e.g. a hydrocarbon (right).
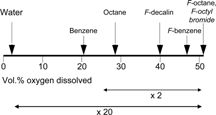
On an O2 solubility scale (), water is at one end of the scale while the least structured of fluids, the gas-like PFCs, are at the other; HCs lie in-between. There is roughly a factor 20 in terms of volume percent O2 solubility between PFCs and water, and a factor 2 between PFCs and HCs.
Figure 7 An oxygen solubility scale. Water, a highly cohesive medium due to extensive hydrogen bonding, is an exceptionally poor O2 solvent.
reminds us that, with PFCs, one has a physical gas dissolution phenomenon that follows Henry's law, i.e. solubility is directly proportional to the gas's partial pressure, and not a localized chemical binding situation as in hemoglobin.
Figure 8 Oxygen solubility in fluorocarbons follows Henry's law, i.e. is directly proportional to the gas' partial pressure, as expected in the absence of chemical bonding, while hemoglobin binds O2 through a strong covalent (coordination) bond to its iron atoms, with consequent saturation at pO2 exceeding that of O2 in the earth's atmosphere. Oxygen extraction from a PFC emulsion can reach 90% of O2 content (see Riess, Chem. Rev. 101: 2797 (2001).
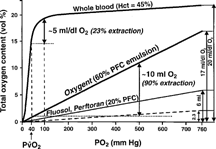
Consequently, O2 can be rapidly and extensively extracted from PFCs when needed. also reminds us that one should use high inspired O2 fractions in order to maximize the benefits of PFC administration to the patient.
Superior O2 solubilities would not suffice to make PFCs good candidate in vivo O2 carriers; it is the combination of O2/CO2 solubilities and of exceptional biological inertness that creates the potential. The fact that PFCs do not tend to mix with either water or lipids certainly contributes to their biological inertness.
In summary, the properties and behavior of PFCs (and of perfluoroalkylated (F-alkylated) compounds) are in essence of the same nature as those of regular organic (HC-derived) compounds. However, the exceptionally strong intramolecular binding and uniquely low intermolecular cohesiveness of liquid PFCs related to the low polarizability of fluorine result in properties that can become substantially different (exceeding the usual range) from those of H-analogs and, in practice, unique (but in no way mysterious). Many F-compounds can reach a level of effectiveness in their performances that cannot be attained by HC compounds, leading to technological feats that just cannot be achieved with non-fluorinated materials. Compared to HCs, PFCs are typically much more inert, have higher densities, compressibilities, fluidity, spreading coefficients and gas-dissolving capacities, and lower refraction indexes, surface tensions, dielectric constants and water solubilities, and magnetic susceptibilities comparable to that of water. Moreover, F-compounds offer unique combinations of properties that can make them irreplaceable and constitute the basis for further potential biomedical applications.
SELECTING A PFC FOR IN VIVO OXYGEN TRANSPORT
Using a PFC as a carrier for in vivo O2 delivery assumes that a number of issues be resolved. Where chemistry is concerned, the principal difficulties are to decide which PFC[Citation[1-4]] to use and to develop a stable injectable emulsion of that PFC. It has been determined that rapid excretion of the PFC requires a touch of lipid solubility, meaning that the PFC should not be too heavy in terms of MW. On the other hand, emulsion stability requires low water solubility, hence that the PFC not be too light. A good candidate PFC should thus have relatively high lipid solubility and the lowest possible water solubility, two conditions that are difficult to satisfy simultaneously. Vapor pressure, which also depends on MW, is an important parameter; too light a PFC can favor retention of air in the alveoli, resulting in increased pulmonary residual volume. In order to avoid this phenomenon, the vapor pressure of the PFC phase should not exceed about 10 torr.
There are, a priori, many PFCs to choose from since, in principle, almost any molecular structure can be synthesized. displays some of the PFCs that have been investigated as candidate O2 carriers. Actually, there are very few candidate PFCs acceptable for parenteral use, i.e. PFCs that optimally combine rapid excretion with the capability of producing stable emulsions. displays some characteristics of PFC emulsions that are directly related to the PFC that constitutes its dispersed phase.
Table 1 Characteristics of PFC emulsions related to the dispersed PFC(s)
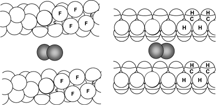
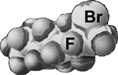
reminds us that organ retention of PFCs is primarily an exponential function of MW, with cyclization, branching and the presence of heteroatoms within their structure having little effect on excretion rate other than through their effect on MW. There are, however, a few interesting exceptions to this rule, i.e. PFCs that are excreted more rapidly than would be predicted on the sole basis of their MW. This is the case of F-octyl bromide (PFOB, perflubron). F-octyl bromide is slightly more lipophilic than a standard PFC of the same MW, due to its well-exposed, polarizable terminal bromine atom (), which facilitates carriage of PFC molecules by circulating lipoproteins.
Figure 10 Organ retention of true fluorocarbons is essentially an exponential function of molecular weight. Fluorocarbons containing lipophilic elements (squares), such as a terminal bromine atom, are excreted more rapidly than what would be predicted on the sole basis of their molecular weight.
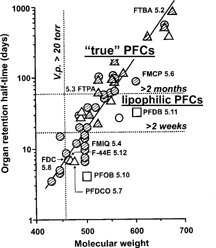
Figure 11 Molecular model of F-octyl bromide showing the lipophilic bromine atom protruding at the end of the linear chain.
This lesser lipophobicity of F-octyl bromide as compared to F-decalin is, for example, reflected by an 8 times larger solubility in olive oil and by a ca. 40°C lower critical solution temperature in hexane (). also illustrates the fact that all PFCs are not equivalent.
Table 2 Some physical properties of F-octyl bromide (PFOB) and F-decalin (FDC) compared
At this point, F-octyl bromide offers the best combination of rapid excretion, ability to form stable emulsions with phospholipids and easy manufacture. Additionally, it has among the largest O2 and CO2 solubilities relative to its MW.
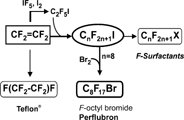
F-octyl bromide has the advantage of being a totally synthetic material. Industrial access to perflubron is achieved via a simple bromination of a key intermediate, F-octyl iodide, on the route to commercial fluorosurfactants (). Teflon also proceeds from the same manufacturing line. The telomerization process used for synthesizing F-octyl iodide from tetrafluoroethylene, CF2CF2, ensures that perflubron can be produced on a multi-ton scale in better than 99.9% purity.
ENGINEERING A STABLE INJECTABLE EMULSION
The second key issue for chemists is the development of a stable, small sized, narrowly dispersed, sterile, injectable emulsion.[Citation[1-4], Citation[8]] PFC emulsions share several key features with the fat emulsions (e.g. Intralipid®) commonly used for parenteral nutrition, including use of the same emulsifier, egg yolk phospholipids, and the same production process through high-shear homogenization and terminal heat sterilization ().
Figure 13 Producing a fluorocarbon emulsion requires use of a pharmaceutically accepted surfactant and energy in order to overcome the PFC/water interfacial tension. Terminal heat sterilization is achieved in a rotary sterilizer. The surfactant is identical and the process similar to those used to produce injectable fat emulsions.
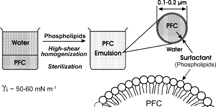
Emulsifying PFCs is difficult a priori, because of the high interfacial tension, γi, of 50–60 mN m−1 that opposes the dispersion of PFCs in water. The target PFC droplets are in the 0.1–0.2 μm range and are typically covered by a monomolecular film of the phospholipids. Practical PFC emulsions need to remain stable for several years without significant changes in particle sizes and particle size distribution. Frozen storage, thawing and reconstitution are clearly impractical and not acceptable.
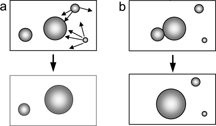
Over time, submicronic PFC droplets grow, not through droplet coalescence, but as a result of molecular diffusion (also known as Ostwald ripening). In this process, individual PFC molecules leave the smaller droplets, where the chemical potential is higher, to join larger droplets, where curvature and, consequently, chemical potential is smaller (). Molecular diffusion is characterized by a linear increase of the average droplet volume over time and by a time-invariant droplet size distribution function.
Figure 14 Particle size growth over time in fluorocarbon emulsions is due to molecular diffusion (a) rather than to droplet coalescence (b) The thin arrows represent individual molecules leaving the smaller droplets to join larger ones.
Droplet growth by molecular diffusion follows the Lifshitz-Slezov equation:which says that the average droplet volume r3 in a given emulsion increases over time t proportionally to the water/PFC interfacial tension γi and to the solubility and diffusibility, C and D, of the PFC in the aqueous phase. What can one do about slowing down molecular diffusion? By chance, phospholipids (the emulsifier used in Intralipid and other pharmaceuticals, including liposome preparations) are particularly apt at reducing γi. Additionally, the solubility of the PFC phase in water diminishes rapidly when a “heavier” (higher MW) PFC is added. The longer organ retention of higher MW PFCs can be mitigated by using a somewhat lipophilic PFC. This is the case when F-decyl bromide is selected as the heavier PFC. shows that the droplet growth in an F-octyl bromide emulsion can be very effectively reduced by adding just a small percentage of its higher homologue, F-decyl bromide. summarizes the properties of PFC emulsions that are related to their being a dispersion of droplets.
Figure 15 Droplet growth by molecular diffusion in an F-octyl bromide emulsion can be effectively repressed by addition of a small amount of a heavier PFC; effect on organ retention can be limited by using F-decyl bromide, which is slightly lipophilic and benefits from faster excretion than non-lipophilic PFCs of similar molecular weight (see Weers et al., Artif. Cells, Blood Subst., Immob. Biotech. 22: 1175 (1994)).
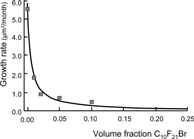
Table 3 PFC emulsion characteristics related to their particulate nature
Manufacturing of practical, small-sized, narrowly dispersed emulsions requires a good deal of know-how. Extensive formulation and process optimization led to OxygentTM AF0144, a 60% w/v concentrated PFC emulsion, that is heat sterilized, has an average droplet size of 0.16 µm after terminal heat sterilization, and a viscosity around 4 cP, i.e. slightly above that of water. Its pH and osmolarity were adjusted to 7.1 and 304 mOsm, respectively. It is stable for 2 years at 5–10°C and is ready for use.
PROSPECTS
This short overview will, hopefully, have solved some of the perceived mysteries about PFCs and their emulsions, at least from the standpoint of their chemistry.
Where do we presently stand in terms of using PFCs as O2 carriers? There is no doubt that PFCs dissolve, transport and deliver O2 in vivo.[Citation3, Citation5,Citation7,Citation8] A randomized, multicenter, European, Phase III clinical evaluation of Oxygent in general surgery patients has established the ability of the emulsion to significantly reduce and avoid red blood cell transfusion. The trial was conducted using an augmented acute normovolemic hemodilution with PFC emulsion protocol. In the protocol-defined target population (330 subjects with blood loss ≥ 20 mL/kg body weight) significantly greater avoidance of any red blood cell transfusion, as compared to controls, was maintained through day 21 or day of hospital discharge (P < 0.05). There was also a significant reduction in the number of units of blood transfused (P < 0.001). From the clinical data collected, the hemoglobin equivalency, in terms of added O2-delivering capacity, of a 1 g PFC/kg body weight dose was estimated to be around 1.5 g hemoglobin.
F-octyl bromide (perflubron) still stands out as the best candidate PFC for intravascular O2 delivery. Oxygent is a well-designed, stable emulsion, although by now, even more stable and smaller-sized emulsions have become feasible. A further objective is to prolong intravascular persistence. There is little doubt that improved products are feasible.
On the other hand, the voluntary suspension of a cardiopulmonary bypass surgery trial with Oxygent because of side effects most likely due to an inadequate clinical protocol that resulted in overly aggressive autologous blood harvesting in the treatment group prior to bypass, is a serious setback in the development of PFC-based O2-carriers.
Further efforts will undoubtedly improve our understanding of their in vivo behavior, teach us more about how to perform clinical trials and, more generally, about how to use the O2 delivering capacity of PFC emulsions optimally. Therefore, we need more research, open dialog, time, and resources.
REFERENCES
- Krafft, M.P., Riess, J.G., Weers, J.G. (1998). The design and engineering of oxygen-delivering fluorocarbon emulsions, in Submicronic Emulsions in Drug Targeting and Delivery, S. Benita, Ed., Harwood Academic Publ.: Amsterdam, pp. 235–333.
- Riess, J.G., Krafft, M.P. (1999). Fluorocarbons and fluorosurfactants for in vivo oxygen transport (blood substitutes), imaging and drug delivery. Mat. Res. Soc. Bull. 24: 42–48.
- Riess, J.G. (2001). Injectable oxygen carriers (blood substitutes)–Raison d'être, chemistry, and some physiology – Chem. Rev. 101: 2797–2920. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Riess, J.G. (2002). Fluorous micro- and nanophases with a biomedical perspective. Tetrahedron 58: 4113–4131. [CROSSREF]
- Spahn, D.R., Waschke, K.F., Standl, T., Motsch, J., Van Huynegem, L., Welte, M., Gombotz, H., Coriat, P., Verkh, L., Faithfull, S., Keipert, P. (2002). Use of perflubron emulsion to decrease allogeneic blood transfusion in high-blood-loss non-cardiac surgery: Results of a European Phase 3 study. Anesthesiology 97: 1338–1349. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Schutt, E.S., Klein, D.H., Mattrey, R.M., Riess, J.G. (2003). Injectable microbubbles as contrast agents for diagnostic ultrasound imaging: The key role of perfluorochemicals. Angew. Chem. Int. Ed. 42: 3218–3235. [CSA], [CROSSREF]
- Riess, J.G. Biomedical applications of fluorous materials, in Handbook of Fluorous Chemistry, J.A. Gladysz, D.P. Curran, I. Horváth, Eds., Wiley-VCH, pp. 521–573.
- Krafft, M.P., Chittofrati, A., Riess, J.G. (2003). Emulsions and microemulsions with a fluorocarbon phase. Curr. Opin. Colloid Interf. Sci. 8: 251–258. [CROSSREF]