Abstract
Hemoglobin vesicles (HbV) are artificial oxygen carriers that encapsulate a concentrated hemoglobin (Hb) solution with a phospholipid bilayer membrane. The oxygen transporting ability of HbV in vivo has been demonstrated by the transfusion of HbV into hemorrhagic shock rodent models. However, the compatibility of HbV with human blood cells must be evaluated. Preincubation of platelets with concentrations of 20% or 40% HbV had no effect on the binding of PAC-1, a monoclonal antibody that detects activation-dependent conformational changes in αIIbβ3 on platelets, or the surface expression of CD62P in whole blood. ADP-induced increases in PAC-1 binding were significantly enhanced by exposing the platelets to concentrations of either 20% or 40% HbV, whereas the ADP-induced increases in CD62P expression were not affected by HbV treatment at either concentration. Preincubation of platelet-rich plasma (PRP) with HbV minimally reduced the spontaneous release of TXB2 and RANTES, but did not significantly affect the formation of TXB2 or the release of RANTES and β-TG in platelets stimulated with ADP. Similarly, preincubation of PRP with HbV minimally reduced the spontaneous release of RANTES but did not significantly affect the formation of TXB2 or the release of RANTES and β-TG in platelets stimulated with collagen, although collagen-induced serotonin release tended to decrease with HbV pretreatment. These data suggest that the exposure of human platelets to high concentrations of HbV (up to 40%) in vitro did not cause platelet activation and did not adversely affect the formation and secretion of prothrombotic substances or proinflammatory substances triggered by platelet agonists, although one of the earliest events in ADP-induced platelet activation was slightly potentiated by HbV pretreatment at the doses tested. Taken together, these results imply that HbV, at concentrations of up to 40%, do not have any aberrant interactions with either unstimulated or agonist-induced platelets.
INTRODUCTION
Vigorous efforts have been made to develop hemoglobin (Hb)-based oxygen carriers (HBOCs) for use as red blood cell substitutes [Citation[1]], and some of these carriers are now in the final stages of clinical trials [Citation[2-4]]. HBOCs offer several potential benefits for red blood cell transfusion applications, including the absence of blood-type antigens and infectious viruses and the ability to be stably stored for long time periods [Citation[5]]. HBOCs can be categorized into two types: acellular modified Hb molecules and cellular liposome-encapsulated Hb, or Hb vesicles (HbV) [Citation[6]]. Acellular modified Hb molecules are composed of intramolecularly cross-linked Hb, recombinant cross-linked Hb, polymerized Hb, or intramolecularly polymer-conjugated Hb. An acellular polymerized bovine Hb has already been used in clinical practice in South Africa.
Cellular HbV have a phospholipid vesicle structure and contain concentrated Hb molecules, similar to actual red blood cells [Citation[7-11]]. Although HbV have not been clinically tested, the oxygen transporting abilities of HbV have been shown to be sufficient using a 40% exchange transfusion with HbV suspended in saline [Citation[8]] and a 90% exchange transfusion with HbV in the presence of albumin as a plasma expander in rats [Citation[7]]. Surface modification of HbV with poly(ethyleneglycol)-phospatidylethanolamine reduced the viscosity by suppressing inter-vesicular aggregation, allowing prompt blood circulation in vivo [Citation[9]]. A sufficient O2 transporting ability, comparable with that of blood, was also established in another model [Citation[11]], and the prompt metabolism of HbV in the reticulo-endothelial system has been demonstrated [Citation[10]].
The biocompatibility of HbV is an important factor for the clinical use of these materials. The administration of HbV could lead to interactions with blood cells, including platelets. Circulating platelets bind to the subendothelial matrix of injured vessels and subsequently become activated, resulting in the release or the expression of components in their intracellular granules and the formation of metabolic products. These products include prothrombotic substances (e.g., adenine nucleotides, thromboxane A2 [TXA2], serotonin, and CD62P) [Citation[12]] and an array of potent proinflammatory chemokines (e.g., RANTES, MIP-1) [Citation[13]]. Prothrombotic substances function as agonists for the recruitment of additional platelets into the evolving thrombus. Chemokines released from the activated platelets trigger the recruitment of leukocytes into the evolving thrombus and play a large role in the initiation and perpetuation of inflammatory responses. Platelet activation is apparently necessary to prevent bleeding in vivo; however, nonphysiological activation leads to pathological thrombosis and the modulation of inflammatory responses. With this in mind, the biocompatibility of HbV and human platelets was evaluated by examining the effect of HbV on CD62P expression and the binding of activation–dependent αIIbβ3 antibody PAC-1 to platelets in the presence or absence of agonists in vitro; these two markers are the most frequently used markers of platelet activation. We also studied the effects of HbV on the secretion of other substances (i.e., serotonin, RANTES, and β-thromboglobulin [β-TG]) and the formation of thromboxane B2 (TXB2), a metabolite of TXA2.
MATERIALS AND METHODS
HbV
HbV suspended in phosphate buffered saline were prepared as previously described [Citation[14]]. The encapsulated carbonylhemoglobin contained pyridoxal 5′-phosphate (PLP) at a molar ratio of [Hb]/[PLP] = 1/2.5 as an allosteric effector and 5 mM of DL-homocysteine. The lipid bilayer was composed of 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine, cholesterol, 1,5-dipalmitoyl-L-glutamate-N-succinic acid, and polyethyleneglycol-1, 2-distearyl-sn-glycero-3-phosphatidylethanolamine-N-[poly (ethylene glycol) (5,000)] at a molar ratio of 5:5:1:0.033. The Hb concentration of the HbV dispersion was adjusted to 10 g/dl. The HbV particle size was nearly 240±60nm in diameter.
Determination of CD62P and PAC-1 Expression by Flow Cytometry
The expression of CD62P and PAC-1 on platelets was measured as described previously, with slight modifications [Citation[15], Citation[16]]. Citrated whole blood was obtained from unselected healthy subjects. Whole blood (520 µl) was incubated with 480 µl of HbV or empty liposomes (at concentrations of 0%, 20%, or 40%) at 37°C for 60 minutes. After incubation, the reaction mixture was diluted to 1/5.4 with Hepes-Tyrode's buffer (KCl, 2 mM; NaCl, 127 mM; NaH2PO4, 0.5 mM; glucose, 5.6 mM; NaHCO3, 12 mM; HEPES, 5 mM; 0.35% BSA; pH 7.3). Eighteen microliters of the diluted reaction mixture was added to 18 µl of a cocktail of FITC-conjugated PAC-1, PE-conjugated anti-CD62P and PerCP-conjugated anti-CD42a. FITC-conjugated anti-mouse IgM, PE-conjugated anti-mouse IgG, and PerCP-conjugated anti-mouse IgG were used as negative controls. All antibodies were purchased from BD bioscience-Pharmingen, San Jose, CA. The reaction mixture was then incubated with 4 µl of ADP (final concentration of 0, 0.05, 0.1, 0.5, 5, or 10 µM) for 20 minutes at room temperature in the dark. After incubation, the platelet suspension was fixed with 500 µl of paraformaldehyde (final concentration, 1%) and washed once with PBS. Finally, the platelets were resuspended in 500 µL of PBS. The samples were analyzed by flow cytometry (LSR, Becton-Dickinson, San Jose, CA). Fluorescence data from 10,000 platelet events were collected in logarithmic mode. The platelet population was identified by the number of CD42a-positive events.
Assay of Mediator Release
The platelet mediator release assay was carried out as described by Santos et al. [Citation[17]], with slight modifications. Platelet-rich plasma (PRP) was obtained from citrated venous blood of unselected healthy subjects by centrifugation (140 g, 15 minutes, 22°C), and 600 µl of PRP (final concentration, 1.7 × 108/ml) was incubated with 400 µl of HbV (0%, 20%, or 40%) at 37°C for 60 minutes. After incubation, the mixture was divided into two 480 µl aliquots. For the collagen-induced platelet release reaction, the mixture was activated with 20 µl of collagen (final concentration, 1 µg/ml) (NYCOMED ARZNEIMITTEL BMBH, Germany) or buffer at 37°C for 5 minutes. For the ADP-induced platelet release reaction, the mixture was activated with 20 µl of ADP (final concentration, 2 µM) (SIGMA) or PBS at room temperature for 20 minutes. After incubation, the tube was centrifuged at 10,000 g for 1 minute. The cell-free supernatant was then transferred to another tube and centrifuged at 10,000 g for 30 minutes. The cell-free supernatant was stored at − 20○C until the measurement of platelet release. Commercially available enzyme-linked immunosorbent assays (ELISAs) were used to measure the levels of RANTES (R&D Systems, Minneapolis, MN), serotonin (ICN Biomedicals Inc., Costa Mesa, CA) and TXB2 (Cayman Chemical Company, Ann Arbor, MI) in duplicate experiments, according to the manufacturers' recommendations. Enzyme immunoassays were used to measure the levels of β-TG (Asserachrom β-TG, Roche Diagnostics, Tokyo, Japan).
Statistical Analysis
A two-way repeated measures ANOVA with Bonferroni correction was used for multiple comparisons of mediator levels and surface marker levels among different concentrations of HbV. A p value < 0.05 was considered to indicate a significant difference.
RESULTS
Effect of HbV on the Binding of PAC-1 and the Expression of CD62P on Resting and ADP-stimulated Platelets In Vitro in Whole Blood
First, the effect of HbV on the binding of PAC-1 to platelets and the surface expression of CD62P on platelets with or without ADP stimulation was examined in a whole blood environment in vitro. Without ADP stimulation, PAC-1 binding to platelets was discernible. Preincubation of whole blood with 20% or 40% HbV alone did not cause a significant difference in PAC-1 binding to the platelets. Stimulation of platelets with varying concentrations of ADP caused a gradual increase in the percentage of PAC-1 positive cells (). Preincubation of whole blood with 20% or 40% HbV resulted in a slight, but significant, enhancement in the percentage of PAC-1 positive cells, compared to the results of comparable experiments without HbV, at ADP concentrations ranging from 0.05 µM to 5 µM ().
Figure 1 Effect of HbV on platelet surface activation markers. (A) PAC-1 binding to platelets and (B) CD62P expression on platelets. Whole blood was incubated with HbV at concentrations of 0% (square), 20% (triangle), or 40% (circle). Whole blood was then stimulated with or without various concentrations of ADP, as described in the Materials and Methods section. Values are the means±SE of 4 experiments. *p < 0.05, **p < 0.01, compared with control (0% HbV).
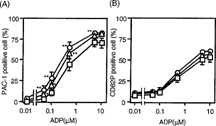
Unstimulated platelets showed only a slight expression of CD62P, regardless of HbV treatment (). The treatment of platelets with varying concentrations of ADP also led to gradual increases in the percentage of CD62P-positive cells, but preincubation of whole blood with 20% or 40% HbV did not affect the ADP-induced increase in the percentage of CD62P-positive cells ().
Effect of HbV on Secretion of Platelet-derived Mediators in Resting and ADP-stimulated Platelets In Vitro
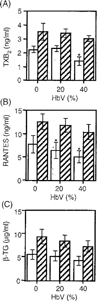
Next, the effect of HbV on the release of mediators from platelets stimulated with or without a submaximal dose of ADP, a weak platelet agonist, was examined. Without ADP stimulation, a slight, but significant, reduction in the spontaneous release of TXB2 from platelets pretreated with 40% HbV was observed (). Similarly, the levels of spontaneous release of RANTES from platelets pretreated with both 20% and 40% HbV were slightly, but significantly, reduced in comparison with those from platelets that were not pretreated with HbV (0% HbV). The treatment of PRP with ADP caused a significant increase in the levels of each mediator in the releasates. Pretreatment of PRP with either 20% or 40% HbV did not affect the ADP-induced release of each mediator, although a slight reduction was observed in each case ().
Figure 2 Effect of HbV on ADP-induced platelet mediator release. ADP-induced release of (A) TXB2, (B) RANTES, and (C) β-TG from human platelets. PRP was incubated with concentrations of 0%, 20%, or 40% HbV and then stimulated with (hatched columns) or without (open columns) ADP, as described in the Materials and Methods section. Values are the means±SE of 5 (A) and 6 (B, C) experiments using blood from different donors. *p < 0.05, compared with control (0% HbV).
Effect of HbV on Secretion of Platelet-derived Mediators in Resting and Collagen-stimulated Platelets In Vitro
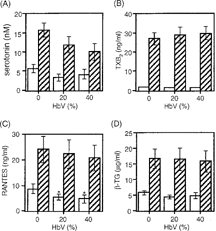
The effect of HbV on mediator release was further examined using platelets stimulated with or without collagen, a strong platelet agonist. Without collagen stimulation, the levels of serotonin, TXB2, and β-TG were not affected in the cell-free releasates from PRP after pretreatment with either 20% or 40% HbV, although the RANTES levels were slightly, but significantly, reduced ( p < 0.05) (). Collagen stimulation of the PRP caused a marked increase in the levels of each mediator but pretreatment with 20% or 40% HbV did not affect the collagen-induced release of TXB2, RANTES, or β-TG. The levels of serotonin in the collagen-stimulated PRP tended to decrease in an HbV-dose dependent manner.
Figure 3 Effect of HbV on collagen-induced platelet mediator release. Collagen-induced release of (A) serotonin, (B) TXB2, (C) RANTES, and (D) β-TG from platelets. PRP was incubated with concentrations of 0%, 20%, or 40% HbV and then stimulated with (hatched columns) or without (open columns) collagen, as described in the Materials and Methods section. Values are the means±SE of 5 experiments using blood from different donors. *p < 0.05, compared with control (0% HbV).
DISCUSSION
In this study, the effect of HbV on the expression of platelet activation markers in the presence or absence of platelet agonists was evaluated in vitro. Integrin αIIbβ3 mediates platelet adhesion and aggregation and plays a crucial role in thrombosis and hemostasis [Citation[18]]. αIIbβ3 is expressed in a low affinity state on resting platelets. On platelet activation, αIIbβ3 shifts to a high affinity conformation that efficiently binds its ligands, including fibrinogen and von Willebrand factor. Thus, such activation is a prerequisite for fibrinogen binding to platelets, which culminates in platelet aggregation. The high affinity conformation of αIIbβ3 on human platelets can be detected by the monoclonal antibody PAC-1 [Citation[15], Citation[16], Citation[19]]. Because low doses of ADP cause an increase in PAC-1 binding within a short time period, this phenomenon is regarded as one of the earliest events in platelet activation, and PAC-1 has been shown to be a highly sensitive and specific marker of platelet activation [Citation[15], Citation[16]].
Activated platelets secrete a number of prothrombotic substances, like TXA2, serotonin, and CD62P that act synergistically to form thrombi. TXA2 is synthesized via the cyclooxygenase-mediated arachidonic metabolic pathway [Citation[20]] and is a potent platelet agonist that induces a rapid positive feedback loop, thereby amplifying the activation signals and enabling robust platelet recruitment at the site of vascular injury [Citation[21]]. Serotonin is a bioactive amine that localizes in dense granules of resting platelets and is secreted upon platelet activation. Serotonin also has a prothrombotic effect on platelets [Citation[12]]. Interactions between platelets via CD62P stabilize the initial αIIbβ3–fibrinogen interactions, thereby promoting the formation of large, stable platelet aggregates [Citation[22]]. In addition, CD62P is the major surface receptor for neutrophils and monocytes on activated platelets, mediating leukocyte adhesion. Thus, platelet CD62P is involved in the recruitment of both platelets and leukocytes into an evolving thrombus [Citation[22-25]].
Recent studies have extended platelet function to include the modulation of local inflammatory events through the release of chemokines and cytokines [Citation[13]]. RANTES and β-TG [Citation[13]] are stored in α-granules in platelets and are released from platelets on activation. RANTES has diverse inflammatory effects, such as histamine release from basophils and the exocytosis of eosinophil cationic protein. Furthermore, RANTES is a powerful chemoattractant for T cells, basophils, and eosinophils [Citation[13], Citation[26]]. β-TG, a platelet-derived CXC chemokine, is also released into the blood at micromolar concentrations and plays an important role in the recruitment of neutrophils to sites of tissue injury [Citation[27]]. Consequently, the aberrant release of serotonin, TXA2, RANTES, or β-TG in response to the inappropriate activation of platelets could result in pathological thrombosis, inflammatory reactions, or allergic responses.
The present study demonstrated that the exposure of blood samples to HbV at concentrations of up to 40% did not cause platelet activation, as measured by various markers, although the levels of RANTES and TXB2 were significantly, but marginally, reduced. In terms of the effect of HbV on platelet activation triggered by submaximal concentrations of agonists, the enhancement of PAC-1 binding to platelets, one of the earliest markers of activation, was observed after the exposure of blood samples to HbV at concentrations of up to 40%, suggesting that HbV might have an enhancing effect on agonist-induced platelet aggregation. Other than the slight enhancing effect of HbV on PAC-1 binding in the presence of agonist stimulation, however, none of the other parameters were significantly affected. Rather, the levels of TXB2, RANTES, and β-TG tended to be reduced by ADP stimulation, while the level of serotonin tended to be reduced by collagen stimulation. Thus, the lack of coordinated potentiation in the levels of prothrombotic substances (i.e., TXB2 and serotonin) and stabilizing molecules involved in the initial αIIbβ3–fibrinogen interactions (i.e., CD62P) in response to the presence of an agonist suggests that the enhancing effect of HbV on platelet reactivity to agonists, if present, is not likely to lead to the deleterious formation of thrombi. In addition, the absence of adverse effects on the secretion of RANTES and β-TG suggest that HbV is unlikely to trigger the initiation and/or aberrant perpetuation of inflammatory and allergic reactions.
The present results are of value for estimating the biocompatibility of HbV and human platelets. Further research is warranted to investigate whether the administration of HbV has any effect on platelet activation and platelet functions in vivo.
Acknowledgments
This work was supported in part by Health and Labor Sciences Research Grants (Research on Pharmaceutical and Medical Safety), Ministry of Health, Labor and Welfare, Japan.
References
- Chang, T.M.S. (2000). Is there a need for blood substitutes in the new millennium and what should we expect in the way of safety and efficacy ? Art. Cells, Blood Subs., and Immob. Biotech. 28: v–xi.
- Johnson, J.L., Moore, E.E., Offner, P.J., Haenel, J.B., Hides, G.A., Tamura, D.Y. (1998). Resuscitation of the injured patient with polymerized stroma-free hemoglobin does not produce systemic or pulmonary hypertension. Am. J. Surg. 176: 612–617. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Mullon, J., Giacoppe, G., Clagett, C., McCune, D., Dillard, T. (2000). Transfusions of polymerized bovine hemoglobin in a patient with severe autoimmune hemolytic anemia. N. Engl. J. Med. 342: 1638–1643. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Carmichael, F.J., Ali, A.C., Campbell, J.A., Langlois, S.F., Biro, G.P., Willan, A.R., Pierce, C.H., Greenburg, A.G. (2000). A phase I study of oxidized raffinose cross-linked human hemoglobin. Crit. Care Med. 28: 2283–2292. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sakai, H., Tomiyama, K.I., Sou, K., Takeoka, S., Tsuchida, E. (2000). Poly(ethylene glycol)-conjugation and deoxygenation enable long-term preservation of hemoglobin-vesicles as oxygen carriers in a liquid state. Bioconjug. Chem. 11: 425–432. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Tsuchida, E., Takeoka, S. (1995). Stabilized hemoglobin vesicles, in Artificial Red Cells: Materials, Performances and Clinical Study as Blood Substitutes, E. Tsuchida, Ed., John Wiley and Sons: Chichester, pp. 35–64.
- Izumi, Y., Sakai, H., Kose, T., Hamada, K., Takeoka, S., Yoshizu, A., Horinouchi, H., Kato, R., Nishide, H., Tsuchida, E., Kobayashi, K. (1997). Evaluation of the capabilities of a hemoglobin vesicle as an artificial oxygen carrier in a rat exchange transfusion model. ASAIO J. 43: 289–297. [PUBMED], [INFOTRIEVE], [CSA]
- Izumi, Y., Sakai, H., Hamada, K., Takeoka, S., Yamahata, T., Kato, R., Nishide, H., Tsuchida, E., Kobayashi, K. (1996). Physiologic responses to exchange transfusion with hemoglobin vesicles as an artificial oxygen carrier in anesthetized rats: changes in mean arterial pressure and renal cortical tissue oxygen tension. Crit. Care Med. 24: 1869–1873. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sakai, H., Takeoka, S., Park, S.I., Kose, T., Nishide, H., Izumi, Y., Yoshizu, A., Kobayashi, K., Tsuchida, E. (1997). Surface modification of hemoglobin vesicles with poly(ethylene glycol) and effects on aggregation, viscosity, and blood flow during 90% exchange transfusion in anesthetized rats. Bioconjug. Chem. 8: 23–30. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sakai, H., Horinouchi, H., Tomiyama, K., Ikeda, E., Takeoka, S., Kobayashi, K., Tsuchida, E. (2001). Hemoglobin-vesicles as oxygen carriers: influence on phagocytic activity and histopathological changes in reticuloendothelial system. Am. J. Pathol. 159: 1079–1088. [PUBMED], [INFOTRIEVE]
- Sakai, H., Takeoka, S., Wettstein, R., Tsai, A.G., Intaglietta, M., Tsuchida, E. (2002). Systemic and microvascular responses to hemorrhagic shock and resuscitation with Hb vesicles. Am. J. Physiol. Heart Circ. Physiol. 283: H1191–1199. [PUBMED], [INFOTRIEVE], [CSA]
- Rand, M.L., Leung, R., Packham, M.A. (2003). Platelet function assays. Transfus Apheresis Sci. 28: 307–317. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Klinger, M.H. (1997). Platelets and inflammation. Anat. Embryol. (Berl). 196: 1–11. [CSA], [CROSSREF]
- Sou, K., Naito, Y., Endo, T., Takeoka, S., Tsuchida, E. (2003). Effective encapsulation of proteins into size-controlled phospholipid vesicles using freeze-thawing and extrusion. Biotechnol. Prog. 19: 1547–1552. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Hagberg, I.A., Lyberg, T. (2000). Blood platelet activation evaluated by flow cytometry: optimised methods for clinical studies. Platelets 11: 137–150. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kohmura, C., Hayashi, Y., Ikeda, H. (1999). Detection of activated platelets by flow cytometry. Rinsho Byori 47: 447–452. [PUBMED], [INFOTRIEVE], [CSA]
- Santos, M.T., Valles, J., Marcus, A.J., Safier, L.B., Broekman, M.J., Islam, N., Ullman, H.L., Eiroa, A.M., Aznar, J. (1991). Enhancement of platelet reactivity and modulation of eicosanoid production by intact erythrocytes. A new approach to platelet activation and recruitment. J. Clin. Invest. 87: 571–580. [PUBMED], [INFOTRIEVE]
- Hynes, R.O. (1992). Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69: 11–25. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Shattil, S.J., Hoxie, J.A., Cunningham, M., Brass, L.F. (1985). Changes in the platelet membrane glycoprotein IIb: IIIa complex during platelet activation. J. Biol. Chem. 260: 11107–11114. [PUBMED], [INFOTRIEVE]
- Samuelsson, B., Goldyne, M., Granstrom, E., Hamberg, M., Hammarstrom, S., Malmsten, C. (1978). Prostaglandins and thromboxanes. Annu. Rev. Biochem. 47: 997–1029. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Ruggeri, Z.M. (2002). Platelets in atherothrombosis. Nat. Med. 8: 1227–1234. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Merten, M., Thiagarajan, P. (2000). P-selectin expression on platelets determines size and stability of platelet aggregates. Circulation 102: 1931–1936. [PUBMED], [INFOTRIEVE]
- Stenberg, P.E., McEver, R.P., Shuman, M.A., Jacques, Y.V., Bainton, D.F. (1985). A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J. Cell. Biol. 101: 880–886. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Palabrica, T., Lobb, R., Furie, B.C., Aronovitz, M., Benjamin, C., Hsu, Y.M., Sajer, S.A., Furie, B. (1992). Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature 359: 848–851. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Schmidtke, D.W., Diamond, S.L. (2000). Direct observation of membrane tethers formed during neutrophil attachment to platelets or P-selectin under physiological flow. J. Cell. Biol. 149: 719–730. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Baggiolini, M., Dahinden, C.A. (1994). CC chemokines in allergic inflammation. Immunol. Today 15: 127–133. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Brandt, E., Ludwig, A., Petersen, F., Flad, H.D. (2000). Platelet-derived CXC chemokines: old players in new games. Immunol. Rev. 177: 204–216. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]