Abstract
The FEV1 is widely used by physicians in the diagnosis, staging, treatment, monitoring and establishing prognosis for patients with COPD. The MCID is the smallest difference which patients perceive as beneficial and which would mandate a change in patient management. A precise MCID for FEV1 has not been established.
In attempt to establish a MCID for predose or trough FEV1, several limitations need to be addressed. There are issues such as reproducibility, repeatability, acceptability, variability, placebo effect, and equipment effects. Patient factors, such as baseline level of FEV1, albuterol reversibility, diurnal variation, influence the results.
Nonetheless, using anchoring techniques, a change in pre dose FEV1 of about 100 mL can be perceived by patients, correlates with fewer relapses following exacerbations and is in the range usually achieved with bronchodilators approved for COPD.
In the future, consistent reporting of spirometric variables, such as a predose FEV1 and other outcomes, can be incorporated into a more quantitative effort to establish the MCID. Also distributional/statistical methods may be useful in determining the MCID FEV1.
Introduction
The objectives of COPD management according to the Global Obstructive Lung Desease (GOLD) Guidelines and American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines are to prevent disease progression, relieve symptoms, improve exercise tolerance, improve health status, prevent and treat exacerbations, prevent and treat complications, reduce mortality, and minimize side effects from treatment Citation[1&2]. Improving lung function, including the FEV1, is not mentioned. However, the FEV1 is used widely by physicians in the diagnosis, staging, treatment, monitoring, and determining prognosis for their patients with COPD. The association between lung function and minimal clinically important difference (MCID) will be discussed.
Jaeschke defined the MCID as “the smallest difference in score in the domain of interest which patients perceive as beneficial and which would mandate, in the absence of troublesome side effects and excessive costs, a change in the patient's management” Citation[[3]]. Other definitions include “the smallest difference in a score that is considered to be worthwhile or important” and “the minimum absolute risk reduction for which patients would take a treatment given their understanding of the risk without that treatment” Citation[[4]]. Thus, there are varied constructs with the MCID label, some weighing change against potential risk, others weighing the impact on treatment decision making, while still others weighing impact on the magnitude of change alone.
Change in FEV1 can be practically useful only if it exceeds the bounds of measurement error. One methodological technique is the Bland and Altman bounds of agreement wherein the noise is defined by taking the mean change score for the group ± 2 standard deviations of the change Citation[[4]]. Anything within the upper and lower boundaries would be indistinguishable from the background noise or measurement error. Change would have to be greater than the boundaries for it to be interpreted as a change at all. Another distributionalapproach for identifying meaningful intra-individual change is developing criteria based on the Standard Error of the Measurement (SEM) developed by Wyrwich Citation[[5]].Theoretically, the MCID for FEV1 should exceed the noise level, which can be substantial.
Furthermore, the MCID may be dependent on baseline lung status. For example, a change of 100 mL may be more meaningful to a patient with an FEV1 of 1.0 L/s rather than to one with an FEV1 of 2.5 L/s. Moreover, the variability and accuracy of spirometry may differ with these two examples. Also, predose reversibility to albuterol (eq. 12% increase in FEV1 and 200 mL in volume) may be a factor since those more reversible respond more to interventions such as Long Acting β Agonists (LABA), Anticholinergic (AC) and Inhaled Corticosteroids (ICS). The entry criteria differ between US and European (less than 9% reversibility) COPD studies, and the resulting change in FEV1with bronchodilator is usually less in European studies ().
Table 1. Other Factors Influencing MCID for FEV1.
Since major decisions about pharmacotherapy of COPD are based on the FEV1 (e.g., regulatory approval, guideline algorithms, staging schemes, as well as decisions about individual patients), the MCID for FEV1 will be explored and recommendations made for further study.
Background
Spirometry is the most important measurement historically in clinical trials of therapeutic agents used in COPD and is essential for making the diagnosis and staging the severity of COPD. Maximal expiratory flow and the FEV1 are routinely used in clinical practice. A relationship exists between maximum expiratory flow, intrathoracic pressure, and lung volume. The FEV1 is determined by the lung elastic recoil pressure, upstream frictional pressure loss, the cross-sectional area, and transmural pressure at the choke point. FEV1 and Forced Vital Capacity (FVC) are expressed in both absolute values (liters) and as a percent of predicted value for the individual based on height, gender, age and ethnicity. The FEV1 has been used to measure bronchodilator response and can be reported as: as a percent of baselineFEV1; as a percent of the patients predicted FEV1; in absolute units of volume; and as a percent of the highest FEV1 ever recorded by the individual. Airflow obstruction is defined in the ATS/ERS and GOLD Guidelines as an FEV1 less than 80% predicted and an FEV1/FVC ratio > 70% Citation[1&2]. For optimal performance of spirometry, standardized technique must be employed, and the patient must exhale down to a plateau and exhalation should usually exceed 6 seconds. The American Thoracic Society has issued Guidelines for the standardization of spirometry Citation[[6]]. The spirometer must be capable of measuring volumes of at least 8 liters with an accuracy of at least ± 3% or ± 0.050 L with flows between zero and 14 L/s.
Benefits
The FEV1 is relatively quick and simple to measure at any level of patient impairment. It is fairly reproducible and provides an objective measure with well-established normal ranges for age, sex, height and race. Serial measures allow monitoring of therapy and documenting disease progression. The FEV1 is effort dependent but less so than the FVC and is less variable. The variance in a given individual with repeated measures is reasonably low.
Despite its shortcomings, it has been used as the parameter followed in longitudinal studies of the effects of smoking cessation and therapeutic interventions, examples include the long-term (3-year) evaluation of the role of inhaled steroids on decline in lung function Citation[[7]], combination therapy of an ICS plus LABA on FEV1and survival Citation[[8]], clinical trials of tiotropium bromide on preserving lung function Citation[[9]], anti-oxidants(n-acetyl cysteine) on exacerbations Citation[[10]], and alpha 1-antitrypsin therapy effects on decline in lung function (e.g. 66 mL/s with augmentation therapy verses 93 mL/yr in those untreated in patients with FEV1 of 35–50% predicted) and survival Citation[[11]]. On average patients without COPD have a decline in FEV1 of 25 cc while those with COPD often lose 50–60 mL/y. The decline in FEV1 also been measured in the National Institute of Health (NIH) sponsored Lung healthstudies where the rate of decline over 5 years falls from 62 mL/y to 32 mL/y in those that continue to smoke verses those who stop Citation[[12]].
The FEV1 is also used in the staging of COPD (). Both the GOLD Guidelines and the ATS/ERS stage the severity of COPD on the basis of spirometry Citation[1&2] since it is readily available, inexpensive, and standardized.
Table 2. Classification of Severity.
Nonetheless, there is some degree of variability between each maneuver on the same day, diurnal changes, and differences from day to day. Moreover, the maneuver does not correspond well to normal daily activities, does not detect small airway disease, and does not correlate all that well with symptoms, survival, quality of life, and economic outcomes (). Also, spirometric data from community sites tends to differ from academic institutions. Therefore, despite the importance of trying to define a precise MCID for FEV1 to guide decision making, serious issues abound.
Table 3. Considerations in Determining the MCID for FEV1.
While the FEV1 is widely reported and does provide useful information, it does not correlate well with some important clinical outcomes. For example, the composite BODE Index may be a better indicator of survival in COPD Citation[[13]]. This index includes FEV1, Basal Metabolic Index (BMI), a Dyspnea scale, and the six minute walk distance, providing more functional data Citation[[14]]. In addition, the FEV1 parameter has been shown by Nishimura et al. to be a poorer predictor of 5-year survival in patients with COPD than dyspnea Citation[[14]]. Using the ATS Disease Severity Staging based on FEV1, there was only a slight difference between stage 1, 2 and 3 on survival over 60 months of follow up. However, using the 5-point Medical Research Council Scale of dyspnea, grades 2, 3, 4 and 5 were rapidly distinguishable on survival over 30 months Citation[[14]]. When reversibility to albuterol is used, the FEV1 correlates a little better with survival Citation[[15]]. Again, this raises the issue of how important is it to define a MCID for FEV1?
Which FEV1?
The FEV1 is reported as the trough, predose or pre-bronchodilator value as a primary outcome for more recent clinical trials of long-acting bronchodilators or anti-inflammatory agents in COPD because this reflects the efficacy over 12 to 24 hours Citation[16-19]. Also, this value reflects the morning lung function when patients awaken, usually a nadir when symptoms are more noticeable. Some studies report the FEV1 post-bronchodilation with albuterol to minimize variability. Depending on the duration of effect of an agent, such as a bronchodilator, the FEV1 can be reported as a mean, average, peak, 2 hour postdose value, or as area under the FEV1 curve. When testing bronchodilator response, time of onset and duration of effect are also noted. No systematic correlation with other outcomes such as mortality or quality of life has been established for any of these parameters nor has a MCID been established for each of these parameters.
Moreover, there are inconsistencies between trials. For example, the predose or trough FEV1 can be reported as the mean of 2 measures taken at 1 h and a second at 5 min predose Citation[16&17], immediately predose Citation[18&19] or 12 hours post dose. Some report last observation carried forward for patients discontinued, while for those completing studies, mean trough over visits is reported, while others report end of study values (). As for bronchodilator response, some trials report 2 h postdose FEV1 Citation[19&20] while other trials reports the average FEV1 for the first 3 h Citation[21&22], while still others report the peak effect Citation[[23]].
Table 4. Predose (Trough) FEV1.
The area under the curve for FEV1 (FEV1 AUC12) is determined by serial hourly measurements of spirometry over a duration of time such as 6, 12, or 24 h. The clinical trials program with formoterol used FEV1 AUC12 as the primary efficacy variable. The between patient SD was assumed to be 400 mL. A sample size was required to demonstrate a clinically meaningful difference of 120 mL between treatment groups based on “previous studies by the sponsor” Citation[[24]]. Since recent clinical trials consistently report predose or trough FEV1 as a primary outcome upon which the study is powered, this review will primarily focus on this parameter when available as well as available post bronchodilator FEV1 parameters such as peak FEV1, mean FEV1, or FEV1AUC.
Historical Benchmarks
The so called “outcomes with established thresholds for clinically meaningful changes” in clinical trials of pharmaceutical interventions in COPD have historically included improvement in FEV1 of 150 mL, six-minute walk of 30–50 m, a change in the Chronic Respiratory Disease Questionnaire (CRDQ) of 0.5 units per question, St. George's Respiratory Questionnaire (SGRQ) change of 4 unit, and an increase in Transitional Dyspnea Index (TDI) of 1 unit. Many of these outcomes are not established in evidence and are currently being reviewed.
There is no definitive consensus supporting a 150 mL increase as a MCID, and this number appears to be based on the natural variability, reproducibility, and repeatability of FEV1. Since correlation with survival is poor, the important question is what level of improvement in FEV1 correlates in a meaningful way with improvement in patient's symptoms, particularly dyspnea, and reduced frequency of exacerbations. The ATS and GOLD authors consider a 12% increase in baseline value of FEV1 and 200 mL/s absolute increase involume to be a “significant” bronchodilator response Citation[1&2]. The European Respiratory Society recommends an FEV increment of 9% of predicted FEV1 for the individual which can be 250–300 mL/s for bronchodilator responsiveness Citation[[25]]. These levels are frequently attained in the first few hours following bronchodilators and are basically peak effects which then taper off. However, the primary outcome for newer long-acting approved agents given once or twice daily for COPD is the trough effect, which is typically far less than these levels. The correlations with dyspnea and exacerbations between the various FEV1 parameters including trough will be analyzed.
Limitations
Acceptability and Reproducibility Criteria
In 1995 the American Thoracic Society published spirometry recommendations which also included acceptability and reproducibility criteria Citation[[6]]. Acceptability criteria are: satisfactory start of test; minimum FVC exhalation time of 6 s; and end of test criteria (a plateau with in the volume-time curve). As a goal, the largest FVC and second largest FVC must not vary by more than 200 mL. Similarly the largest FEV1 and second largest FEV1 must not vary by 200 mL/s. This represents a shift for in 1987 the ATS published an update to their 1979 recommendation, which recommended reproducibility criterion for the FVC and FEV1 of 5% or 100 ml, whichever is greater Citation[[26]]. The ATS also recommended that 3 acceptable curves be obtained.
The reproducibility criterion is to be used only as a guide as to whether more than 3 FVC maneuvers are needed. Reproducibility criteria were not to be used for excluding subjects from reports or for excluding subjects from a study. One potential pitfall is that children and individuals of short stature may require different standards. However, Hankinson & Bang, found when a reproducibility criterion of 200 mL were used there was no significant differences between the number of reproducibility criterion and failures for 14 different height groups used Citation[[27]]. Therefore, the minimum reproducibility criterion recommends that the largest FVC and FEV1 and the second largest FVC and FEV1 be within 10% or 200 mL, but most should be well below this threshold. Also the acceptability criteria must be applied before the reproducibity criteria. Therefore, this degree of variability must be considered in a discussion of MCID for FEV1.
Validity of Spirometric Testing in a General Practice Population in Patients with COPD and Other Pitfalls
There are a number of potential limitations due to other technical issues (). Schermer et al., recently investigated the validity of spirometric tests performed in a general practice Citation[[28]]. He repeated within subject comparisons of spirometric tests with a “gold standard” performed in a central reference pulmonary function laboratory for 388 subjects with COPD from 61 general practices. Within subject differences in the FVC and FEV1 between the standard, reference laboratory and the general practice were measured. The quality marker was the FEV1 reproducibility test of < 5% or < 200 mL. The mean Δ FEV1 was 0.069 L (95% CL 0.054 to 0.084) and ΔFVC was 0.081 L (95% CL 0.053 to 0.109). The authors conclude that relevant spirometric indices measured by trained general practice staff were marginally but statistically significantly higher than those measured in reference pulmonary function laboratories.
Table 5. Additional Considerations in MCID for FEV1.
Another potential pitfall producing spurious results appears to be caused by inaccurate zeroing of the flow sensor or by condensation, mucus deposition, or unstable calibration of various flow type spirometers. These errors elevated some FVC measurement to 144 to 204% of baseline Citation[[29]]. A third consideration is the effect of an inspiratory maneuver preceding forced expiration. A rapid inspiration or a slow inspiration with a 4–6 s pause can alter the subsequent FEV1by as much as 8% Citation[[30]]. This lack of interchangeability adds another element of complexity when trying to determine an MCID for FEV1. A fourth consideration is the effect of gas compression on expiratory flow in those with COPD Citation[[31]]. In a typical clinical pulmonary function laboratory, flow is measured by a flowmeter at the mouth level and FEV1 is obtained by integration of flow. However this volume can differ significantly from that measured with body plethysmography in patients with COPD leading to an error.
Differences Due to Selection of Spirometric Measurements in Determining FEV1
Forced expiratory spirometry is a well-standardized test used frequently in clinical practice in population studies. Among the several parameters derived from forced expiratory spirometry, forced expirated volume in one second (FEV1) and the forced vital capacity (FVC) are the most commonly reported because of good reproducibility, ease of measurement, and correlation although imperfect with disease state, morbidity, mortality and functional status. In the United States, the standards for measurement of the FEV1 and FVC have been recommended by several organizations. In addition to specifications for test performance and equipment, all of these groups recommend selection of the FEV1 and FVC as the largest value taken from technically acceptable maneuvers,even though they might be from different maneuvers. The British Medical Research Council recommends discarding the first two “trial” maneuvers and reporting the mean value of the next three acceptable maneuvers Citation[[32]].
Some recommend using the mean of the best three of five acceptable maneuvers while others suggest that the best value for the FVC is the mean of the last two efforts from six trials. Freeman suggests reporting the mean of the second and the third effort if they agree within 10% Citation[[33]]. The American Thoracic Society standards are widely adopted but have not been vigorously evaluated Citation[[6]]. The rationale for using the highest level of FEV1 and FVC from different maneuvers is based on the notion that the FEV1 is an effort-dependent test, the largest value representing the greatest effort. Thus, among a set of values during an individual testing session, it is expected that one could not exceed this maximal effort. Therefore, there is not universal agreement on which maneuver to report.
Limitations Due To The Placebo Effect
The magnitude and direction of the placebo effect on lung function in COPD clinical trials is not well established. Joyce et al., performed a meta-analysis in asthma drug therapy trials, and the absolute increase in FEV1 for placebo groups was 110 mL which was a 4.8% mean increase Citation[[34]]. The Peak Expiratory Flow rate (PEF) changes were in the opposite direction to the FEV1, an absolute decrease of 2.24 L/min or a decrease of 4.21%. Noteworthy was the fact that mean increases in FEV1 exceeded 10% in 5 of 33 placebo groups and 13 of 33 active treatment groups. Therefore, in clinical trials, there is a small but measurable placebo effect, and a few have changes that might be interpreted as “clinically significant.” The placebo impact must also be considered in COPD trials where FEV1 may increase or decrease at least short-term.
Limitations Due to Repeatability of Spirometry
Enright et al., determined the limits for repeatability of FEV1, FVC and PEF during spirometry test sessions in 18,000 consecutive patients, aged 20 to 90 years Citation[[35]]. Ninety percent of the patients were able to reproduce FEV1 within 120 mL (6.1%), FVC within 150 ml (5.3%), and peak flows within 0.80 L (12%). They concluded that the ability of patients to meet or exceed spirometry repeatability goals does not depend on patient characteristics. The current American Thoracic Society repeatability goal of 200 mL for FEV1 and FVC may be too lenient.
Limitation Due to Short Term Variability in FEV1 and Bronchodilator Responsiveness in Patients with COPD
Tweeddale et al., measured short term variability in FEV1 and responsiveness to inhaled bronchodilators in 150 patients with obstructive ventilatory defects Citation[[36]]. The range of initial FEV1 was 0.5–4.7 L, and the natural variability over a 20 min period when expressed in absolute terms was different insignificantly from that found in normal subjects. The increase in the FEV1 and vital capacity required to exclude natural variability with 95% confidence in these patients was 160 mL and 330 mL respectively.
Investigators from the Lung Health Study examined the spirometry test sessions from two visits of 5,885 individual with mild to moderate COPD Citation[[37]]. Sessions were 17 days apart. They compared different selection methods for the FEV1 and FVC. The coefficient of variation ranged from 4.1 to 4.9% for FEV1 and from 3.5 to 5.7% for FVC. The average absolute difference between the two test sessions ranged from 110 to 123 mL for FEV1and from 149 to 200 mL for FVC. The mean of the three highest values and the largest single value from all maneuvers provided the least short-term variability for both FEV1 and FVC.
The Dutch CNSLD study group studied variability of bronchodilator response and the effects of inhaled corticosteroids in obstructive airways disease Citation[[38]]. Two hundred and seventy-four patients from age 18 to 60 with both asthma and COPD were enrolled. The FEV1 was measured before and 20 min after a 1000 µg of terbutaline and 40 min after additional 80 mcg of ipratropium bromide. Bronchodilator tests, however, were quite variable with standard deviations of 186 mL or 11% of initial value. The patients were followed up to 21 months. There was a significant reduction in bronchodilator responsiveness of 117 mL after three months of treatment with β2 agonists plus a corticosteroid but not after bronchodilators alone. Bronchodilator response at the start of the study was a poor predictor of improvement in FEV1 with bronchodilator and corticosteroid treatment. They concluded that bronchodilator response decreased substantially with inhaled corticosteroid therapy but within subject variability is considerable in chronic obstructive pulmonary disease. This group also found that the Δ FEV1 percent predicted appeared to be the most useful method of expressing bronchodilator responses both for clinical and research proposes. Therefore, reversibility of airway obstruction in response to a bronchodilator is a continuous variable and not a dichotomous trait. Any cut off level for a so called “positive bronchodilator response” is therefore arbitrary. Therefore, a sharp cut off to define the FEV1 that is a MCID might also be arbitrary with a continuous variable.
Other Consideration for Determining MCID
Spirometric Reference Values for the Six Second Maneuver
The National Lung Health Education Program for COPD screening proposed a shorter FVC maneuver (forced expiratory volume at 6 s exhalation) to reduce variability. A minimum exhalation time of 6 s is recommended whenthere is a plateau in the volume-time curve display. Using the third National Health and Nutrition Survey raw volume-time curves, Hankinson et al., calculated the reference values from the 6 s FVC maneuver Citation[[39]]. Swanney analyzed the FEV1/FEV6 and FEV1/FVC results of 502 patients with airway obstruction and found the reproducibility of FEV6 superior to FVC and less physically demanding for patients Citation[[40]]. Similarly, Enright evaluated data from the Lung Health Study and also concluded that FEV1/FEV6 is an independent predictor of decline in lung function and may be used to detect smokers at risk for COPD Citation[[41]]. New reference values for the FEV6 and FEF25–75 may need to be incorporated into future discussion about MCID in spirometry.
Baseline Lung Function
Patients with different levels of severity of COPD may respond in different ways to bronchodilators. For example those with mild or moderate disease usually have a larger increase in volume of FEV1 while those with Stage 4 disease may increase the percent FEV1. Therefore, the ATS guidelines for bronchodilator responsiveness includes both a volume change of 200 mL and a percent change of 12%. However, those with severe disease and hyperinflation may respond more to albuterol, not by increased FEV1 but via a change in Inspiratory Capacity (IC) or a decrease in Residual Volume (RV) or Thoracic Gas Volume (TGV). These two observations can impact the discussion of MCID FEV1.
Since COPD is a progressive disease, the baseline lung function declines over a year time, and the slope of decline can be more rapid in those with an FEV1 of 35–50% verses those with higher or lower levels. This was noted in the NIH registry of patients with Alpha I antitryspin deficiency. Furthermore, those patients withdrawing from a clinical trial (ISOLDE) were most evident when the baseline FEV1 was below 50% of predicted, and dropouts had a more rapid decline in FEV1, health status, and more exacerbations. Therefore, the efforts of dropouts can influence FEV1 MCID.
Inspiratory Capacity MCID
The inspiratory capacity may correlate better with exercise performance than the FEV1. O'Donnell et al., studied the change in inspiratory capacity verses the percentage change in exercise endurance time Citation[[42]]. The correlation with IC was r = 0.303, whereas with FEV1 the correlation was only r = 0.141. Furthermore, in patients with decreased baseline inspiratory capacity, Di Marco noted a much greater increase in IC verse FEV1 following bronchodilation with β2-agonists Citation[[43]]. Usual changes in IC are around 100 mL. Therefore, parameters other than the FEV1 must be addressed when evaluating a clinically meaningful response to a drug.
Response of Lung Volumes to Inhaled Albuterol
Many patients with COPD do respond to albuterol, but this may not be detected by an FEV1 change assessed the response of lung volumes to albuterol and the relationships of change in lung volume to spirometric improvements in patients with severe hyperinflation Citation[[44]]. Patients, who had undergone pulmonary function tests (PFT's) from 1982–1998 and had a total lung capacity (TLC) of greater than 115% and a FEV1/FVC of less than 85% were evaluated. Among those with severe hyperinflation verses those with moderate hyperinflation, the lung volume measurements were better than the FEV1 in determining bronchodilator responsiveness. However, the inspiratory capacity performed best in those with severe hyperinflation. They conclude that the FEV1 improved following albuterol inhalation in 33% of severe hyperinflated and 26% of moderate hyperinflated patients. If the inspiratory capacity, the FVC and RV are measured, then 76% of severe hyperinflated and 62% of moderate hyperinflated patients improve following bronchodilator. Therefore, measure of lung volumes improves the sensitivity of detecting those improving with bronchodilators and, more importantly, reduced dyspnea parallels reduced air-trapping better than improved air-flow. Usual changes in RV and FRC are about 200–300 mL.
Therefore, the MCID in FEV1 for a bronchodilator response determined exclusively by FEV1 may not be as meaningful to those with moderate to severe hyperinflation, especially during activity as the changes in IC or lung volumes.
The MCID Lung Function: Anchor-Based Approaches
Perceptions of Patients
Since FEV1 correlates poorly with survival, the relationship to the clinically meaningful outcomes of dyspnea and exacerbations are evaluated (). Redelmeier, studied the relationship between spirometry and dyspnea in patients with COPD Citation[[45]]. A cross-sectional study of 120 patients in a pulmonary rehabilitation program was performed with an evaluation of 832 contrasts. The mean FEV1 was 35%, and a subjective comparison was undertaken in an attempt to determine when a difference in FEV1 is sufficiently large to be associated with noticeable difference in dyspnea. A subjective comparison rating of breathing ability based on questions about whether or not your breathing was “much worse than someone else's, somewhat worse, a little bit worse, about the same, a little bit better, somewhat better and much better.” These responses were correlated with the frequency of the difference in FEV1 with a range of 10%. The results of this study show that the FEV1 moderately correlated withpatients rating of dyspnea (r = 0.29), and the FEV1 minimally correlated with patients rating of overall health (r = 0.10). The FEV1 needed to differ by 4% of the predicted FEV1 for the average patient to start rating his/her dyspnea “a little better or a little worse” relative to others. This calculates out to 112 mL. Therefore, the authors concluded that a 4% change or 112 mL is the smallest difference in FEV1 that is noticeable to patients. This may help clinicians interpret the effectiveness in treatment. This is one calculation of MCID anchored to patient's perception.
Table 6. MCID FEV1 Anchors.
In contrast, the MCID in asthma is reported to be quite a bit larger. Using an entirely different technique, Santanello, evaluating a large database in a comparison of the effectiveness of oral montelukast vs. beclomethasone (an inhaled corticosteroid), determined that patients could perceive a change in FEV1 of 230 mL or 10%, a Peak flow of 18.79L, − 0.81 puffs of albuterol and − 0.31 in symptom scores Citation[[46]].
Exacerbation Data
Exacerbations are potentially serious events in the COPD patient's life and often lead to death or hospitalization with prolonged deterioration in health status and are very costly. Niewoehner looked at the relationship of FEV1 to acute exacerbation of COPD relapse rate Citation[[47]]. Two hundred sixty-one subjects were treated with prednisone or placebo for 14 days following discharge from the hospital for an Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD). Steroid treated patients had a more rapid rate of improvement in FEV1 and a maximal difference of 120 mL was noted on day 1 and persisted for 3 days. Sixty-four had treatment failure. The FEV1 change from baseline over the first several days is predictive of failure. All subjects increased 100 mL in FEV1. There were a few individuals with larger changes such as 700 cc. A 100 mL increase in FEV1 was significant at baseline and at day 2. At entry, the FEV1 was 767 mL. By day 14, it was 1054 mL. Using a Cox Proportional Hazard Model, the relative hazard of failure correlates with a FEV1 at entry 100 mL and a change of a delta FEV1 of 100 mL. Therefore, a difference in 100 mL was associated with a lower relapse rate.
Singh et al., reviewed 8 studies of corticosteroid therapy for patients with AECOPD and concluded that short courses of systemic corticosteroids improve spirometric outcomes and clinical outcomes Citation[[48]]. In another study, the rate of change in FEV1 was 90 mL/day with Oral Corticosteroid (OCS) verses 30 mL/day with placebo. The OCS group had fewer drop outs, shorter hospital stay, and better control of symptoms, but these effects were of short duration and the benefit did not last beyond hospitalization Citation[[49]]. Aaron studied 147 patients in the Emergency Department. Ten days of oral prednisone increased the FEV1 34% + 42% or 0.3 L/s verses 15% + 31% or 0.16 L with placebo. This correlation with fewer relapses (RR 0.63; 95% CL 0.40L to 1.01) Citation[[50]]. In other trials, a correlation with improvement in FEV1 and exacerbations following oral corticosteroid use was not possible.
MCID Anchored to Decline in Lung Function—The Lung Health Study
The classic smoking cessation study of Fletcher and Peto correlated the relationship between FEV1 and age demonstrated the relationship with current smoking and smoking cessation Citation[[51]]. Data such as these have lead to the estimate that smokers lose 50 mL/year while non-smokers lose 25 mL in the FEV1. If a bronchodilator or inhaled corticosteroid increases the FEV1 predose by 100 mL, does this offset the typical 2 year decline in lung function in a smoker? The answer is not yet known.
The NIH Lung Health study was the landmark study that confirmed the benefits of smoking cessation on lung function. The purpose of the Lung Health Study was to determined if smoking intervention and inhaled bronchodilator use can slow the rate of decline in FEV1 in smokers with mild COPD. Five thousand eight hundred eighty-seven smokers were randomized to smoking intervention plus bronchodilator, smoking intervention plus placebo, or usual care (UC) and or no intervention were followed for 5 years. Participants in the two smoking intervention groups showed significantly smaller declines in FEV1 than those in the UC group. Most of this difference occurred during the first year. Bronchodilator therapy with ipratropium bromide increased FEV1but had no benefit on the slope of the decline in FEV1 over time Citation[[52]]. Similarly, the 4 longitudinal studies with inhaled corticosteroids increased FEV1 modestly over baseline but did not change the slope of the decline Citation[[53]]. Only prospective studies over many years will determine if these changes in FEV1 equate with any survival or other benefit.
Quitters have an improvement in post-bronchodilator FEV1 of 47 mL or 2% in the first year. Subsequently, the mean annual decline in sustained quitters is 31 mL ± 48, which is half the 62 mL ± 55 (mean ± SD) in continuing smokers. Over the five year period, the mean decrement in FEV1 was 77 mL in quitters and 296 mL in continuing smokers. The results suggest that quitters have a decline similar to healthy never smokers. However, the standard deviation of the annual rate of decline in FEV1 in the Lung Health Study was 48 mL/y in quitters and 55 mL/y in continuing smokers. This indicates that even over 5 years of follow-up, confidence in a value for an individual is low, and there is plenty of room for individuals to show continuing accelerating decline Citation[[52]]. This study was performed in mild to moderately impaired individuals, and data on decline in lung function in more severely affected patients is necessary. None the less long term data shows that smoking cessation is associated with decline in lung cancer, and all cause mortality. Among British Doctors born around 1920, prolonged cigarette smoking from early adult life tripled age specific mortality rates, but cessation at age 50 halved the hazard, and cessation at age 30 avoided most of it Citation[[54]]. What are the implications on survival and outcomes of a change in decline of FEV1 from 61 to 32 mL/year?
Minimal Clinical Important Difference: Correlation of FEV1 with Other Outcomes, from Clinical Trials
Role of Short-Acting Beta Agonist in COPD
Sestini reviewed 13 studies of regular short-acting beta agonists in COPD Citation[[55]] (). The increase in FEV1 was 0.14l WMD (Weighted Mean Difference) post bronchodilator. The increase in am Peak flow was 29.2 WMD, and the pm Peak flow was 36.8 WMD. They concluded that short-acting bronchodilators via MDI on a regular basis improved lung function and breathlessness but not exercise performance. They also concluded there was no evidence of long-term side effects, and it should still be considered first line therapy. Therefore, short acting beta agonists increase post-bd FEV1 by 140 mls, which has modest clinical correlation with improving dyspnea. This change is below the 200 mL in FEV1 postdose, which is considered historically to be a positive response. Short acting beta agonists as well as anticholinergics have a 4–6 h duration of action; Therefore, they have no measureable effect on trough FEV1 when compared to longer acting agents Citation[[18]].
Table 7. MCID FEV1—Clinical Trials Anchors.
Long-Acting Beta Agonists in COPD
Considerable new data are emerging on the effects of the LABAs, formoterol, and salmeterol, on lung function. The increase in trough FEV1 with salmeterol monotherapy was: 78 mL, 91 mL, and 107 mL in the 3 trials when compared to the combination of fluticasone/salmeterol Citation[[19]]Citation[[20]]Citation[[56]]. The postdose FEV1 at 2 hours was 200 mL and 233 mL. Sin et al., reviewed 9 placebo controlled trials of LABAs in moderate to severe COPD, and therapy lowered exacerbations by 21% (95% CI 10–31%) Citation[[57]]. Therefore, predose values around 100 mL are anchored to improvements in exacerbations, a meaningful outcome.
In contrast, Appleton looked at 33 studies of which only 4 met the Randomized Control Trial criteria Citation[[58]]. Salmeterol 50 mcg increased FEV1 0.10l WMD; Salmeterol 100 mcg increased FEV1 0.12 L. Salmeterol, in some studies, improved the Quality of Life (QOL), but not SF 36. They concluded the long-acting beta agonists produced only small increases in FEV1,significant improvement in QOL, and reduced breathlessness, but the effects are modest. Nonetheless, these clinical trials data lead to approval by the FDA as well as international regulatory bodies of salmeterol for bronchospasm due to COPD.
In two one-year trials, the long acting β2 agonist formoterol non-significantly increased FEV1 on an average by 82 mL (95% confidence limits, < 26 to 190 ml per year) when compared to placebo Citation[[57]]. This agent is also FDA approved for bronchospasm in COPD. Analyses such as these allow anchoring of the FEV1 improvement and suggest that a predose improvement of 100mls might be related to a reduction in exacerbations but more data are needed. In addition, each of these long-acting agents improved postdose FEV1 by over 200 mL and this may be equally as meaningful as the trough change.
Long-Acting Anticholinergics
Long-acting anticholinergics in contrast have a powerful effect on FEV1. In the two trials that had one year follow up, the trough FEV1 increased by 121 mL, (95% confidence, 102 to 141 ml per year) when compared with placebo or ipratropium bromide Citation[[57]]. These studies support a beneficial effect on exacerbation reduction and a benefit in health status relative to placebo. In two trials that compared long-acting anticholinergics with long-acting beta-agonist over six months, tiotropium bromide, the long-acting anticholinergics had a more favorable effect on trough FEV1, 37 ml (95% confidence limit 12 to 61 ml) greater than salmeterol (), but no consistent data supported superiority over the LABAs in reducing exacerbations or improving health status Citation[[17]]Citation[[22]]. Therefore, the larger change in predose FEV1 in the placebo controlled and ipratropium comparisons, correlate better with other outcomes than the small changes in FEV1 seen in the salmeterol comparisons.
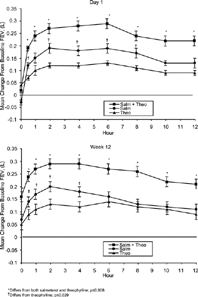
Figure 1. Response of FEV1 over 12 hours to tiotropium, salmeterol and placebo. Reprinted from Ref. Citation[[17]].
![Figure 1. Response of FEV1 over 12 hours to tiotropium, salmeterol and placebo. Reprinted from Ref. Citation[[17]].](/cms/asset/0e72e77e-d7e7-4c6c-a7f7-4de3459ad9f6/icop_a_53377_uf0001_b.gif)
In specific comparisons, tiotropium improved the trough FEV1and FVC above baseline compared with ipratropium (trough FEV1 was 120 mL vs. − 30 mL, trough FVC was 320 mL vs. 110 mL) Citation[[21]]. More patients receiving tiotropium achieved meaningful differences in TDI focal scores reflecting less dyspnea. Most patient's receiving tiotropium achieved clinically meaningful improvement of 4 units in HRQOL scores, and fewer patients receiving tiotropium experienced exacerbations (36% vs. 46%). Little difference between tiotropium and ipratropium was noted on the first day of therapy. In fact, ipratropium has a faster onset of action, but rapidly declines so even on day one tiotropium significantly improves FEV1 and FVC at hours 3, 4, 5 and 6 Citation[[59]].
Tiotropium has clinical effects on static and dynamic lung volumes. In a placebo controlled study of 187 patients for 42 days, Tiotropium increased FEV1 by 120 mL ± 30; FVC 250 mL ± 60; IC 100 mL ± 50; while reducing RV360 mL ± 90; and FRC by 300 mL ± 80 Citation[[60]]. The expansion of inspiratory capacity enabled improvements in both exertional dyspnea and exercise endurance. Since improvements in hyperinflation correlate well with dyspnea and exercise performance, calculation of a MCID based on IC or RV may be possible in the future as more studies are reported.
Inhaled Corticosteroids in COPD
The precise role of inhaled corticosteroids (ICS) in COPD remains contentious. The increment in FEV1 are small and more apparent in those with a more asthmatic phenotype. Highland and others have shown that regular use of ICS do not have much of an effect on the rate of decline in FEV1 Citation[[53]]. Sutherland showed a slightly greater improvement following regular use of inhaled steroids Citation[[7]]. All of these values are quite low when compared to the effects of smoking cessation. In contrast, Sin showed that ICS had an overall effect on all-cause mortality and the relative risk ranged from 0.79 to 0.89 Citation[[59]].
ICS have a modest effect on FEV1. In the first six months of therapy, they increased baseline trough FEV1 on an average by 45 mL, (95% confidence limit, 22 to 69 mL) in the six largest trials Citation[[57]]. After the initial six months, however, decline was unaffected by ICS by 5 mL/y, (95% confidence limits, minus 1 to 11 mL/y) relative to placebo in four trials.
As an example, the annual rate of decline in FEV1 was 50 mL per for fluticasone vs. 59 mL/y for placebo in the ISOLDE Trial Citation[[61]]. Patients given an initial dose of oral corticosteroids had an increase of FEV1 of slightly below 65 mL. While inhaled steroids had little effect on the rate of decline, the mean postbronchodilator FEV1 remained approximately 100 mL higher at least through the first year of treatment but then declined.
Combination Therapy of a ICS and LABA
For regulatory approval, combination therapy must be superior to the individual components in efficacy, but there should not be any additional safety issues. Effects on trough FEV1 or pre-bronchodilator FEV1 with ICS/LABA combination have been reported in many recent studies. Calverley et al., evaluated the combination verses the individual components over one year's study Citation[[56]]. The predose change in FEV1with the combination was 133 mL (95% CI:105 to161), 73 mL (95% CI;46 to 101) with salmeterol alone, and 95 mL (95%CI;67 to 122) with fluticasone. This predose value compares favorably with other studies of this combination Citation[19&20]. Similarly, the two hour postdose FEV1shows a significant improvement with the combination over the individual components.
Sin Analyzed 3 studies and found an ICS and LABA was effective in improving trough FEV1 compared to: placebo; 101 mL/y, (95% confidence limit 76 to 126 mL/y): Long-acting β-agonist: 34 mL/y, (95% confidence limit, 11 to 57 mL/y): and inhaled corticosteroids: 50 mL/y, (95% confidence limit interval 26 to 74 mL/y) Citation[[57]]. While clearly the difference with placebo is above a hypothetical MCID of 100 mL, is the difference with monotherapy of any importance?
Nannini pooled data of the fluticasone proportionate salmeterol (FPS) combination vs. the comparators, salmeterol, fluticasone, or placebo Citation[[62]]. When comparing FPS vs. placebo, data pooled from Mahler 2002 and Hanania 2003 gave a weighted mean difference in trough FEV1 of 0.16 liters (95% confidence, 0.12–0.20). There was a significant difference between the albuterol reversible groups of patients, weight mean difference of 0.20 L (95% confidence, 0.15–0.25), and the irreversible patients, weight mean difference of 0.12 L (95% confidence, 0.07–0.17).
When comparing FPS to salmeterol, the difference was a weighted mean difference of trough FEV1 of 0.06 L (95% confidence, 0.02–0.10) with no heterogeneity between the reversible and nonreversible participants. When looking at FPS versus FP, a weighted mean difference of 0.05 L (96% confidence, 0.02–0.0.9) with no heterogeneity between reversible and nonreversible participants. However, the combination FPS did not significantly reduce exacerbations compared with either of its components.
When the combination of budesonide formoterol (BDF) was compared to monotherapy with the individual components, there was a significant difference with BDF verses placebo, WMD 14.40% (95% CI 11.91to 16.90), verses BD; WMD10.17% (95%CI 7.71 to 12.62) but not for F, WMD 3.06 (95%CI − 0.86 to 6.97). Therefore, the combination was superior to BD but not for lung function, but the opposite was true for exacerbations where BD was not inferior to the combination but formoterol was. Therefore, when determining MCID for trough FEV1, changes of greater than 100 mL seen in the placebo comparisons appear meaningful but the changes of 30–50 mL verses monotherapy with either a LABA or ICS cannot differentiate any benefits of the combination Citation[[62]].
Combination Therapy: Bronchodilators
The 3 classes of bronchodilators that physicians frequently combine include beta agonists, anticholinergics, andtheophyllines to take advantage of the different mechanisms of action, increase efficacy, and lower adverse events. What constitutes a minimal clinically important difference in FEV1 when combining bronchodilator drugs? The combination of albuterol/ipratropium is the most frequently prescribed agent for COPD in the United States. The increase in peak FEV1 was 31–34% for the combination, 24 to 24% for ipratropium, and 24–27% for albuterol alone. The FEV1 AUC0-4 also differed but there was no difference in symptoms Citation[[63]].
A similar study was repeated with the nebulized solution of Ipratropium, albuterol, and the combination Citation[[64]]. Here the peak increases in FEV1 were: 370 mL with the combination, 290 mL with IB, and 310 mL with albuterol. These differences in FEV1 had no effects on quality of life scores, global evaluation, and symptom scores yet the combination was approved and is widely used. Sin et al., reviewed 3 trials of 1399 patients with advanced COPD and the combination reduced exacerbations 32% (95% CI, 9to49%) compared to a beta agonist alone but was not superior to ipratropium alone Citation[[55]]. However, the combination did seem to lower the total treatment costs and improved cost effectiveness Citation[[65]].
This combination is widely used in acute asthma where a pooled analysis of 3 trials in 1064 patients revealed an improvement in FEV1 of 43 mL (95% CI-20, 107) over albuterol alone with modest improvement in clinical outcomes Citation[[66]].
Karpel added a third drug, theophylline, to the other two and the improvement at 1 h was 100 mL with IB, 200 mL with both theophylline and albuterol, and 300 mL with triple drug therapy, but at 5 h, only the triple drug and 2 drug combinations were 100 mL over placebo Citation[[67]].
Are these types of changes sufficient to prescribe triple therapy with the possibility of more side effects? More exacerbation and symptom data would be useful in the determination of an MCID for FEV1 that could guide therapeutic decisions about combinations.
What about longer acting agents? Salmeterol was combined with theophylline () Citation[[68]], and the changes in FEV1 are plotted verses time. Does the change in predose FEV1 of 180 mL with theophylline and salmeterol combined verses 90 with salmeterol alone warrant dual therapy? This change in FEV1 correlated with a TDI change of 1.9. Salmeterol has been combined with ipratropium bromide with similar levels of improvement over monotherapy Citation[[69]]. All these studies report efficacy as increases in FEV1 but clearly no attempt at defining an MCID has been made and correlations with other clinical outcomes is often lacking. Therefore, at present, there is no definite standard or MCID for FEV1 to guide decision making when combining medications in COPD.
Other Clinical Consideration
Tolerance: MCID in FEV1 When Does a Decline in FEV1 Mean Something?
The development of tolerance with bronchodilators is controversial. In asthma, there is loss of bronchoprotection and, less commonly, loss of bronchodilation with regular use ofshort-acting agents especially in those with beta receptor polymorphism such as ARG/ARG at position 16. These changes in FEV1 occur early and then plateau. In COPD, there appears to be less tolerance especially with long-acting agents Citation[[70]].
As of now there are no accepted criteria for clinical significance to loss of lung function. Just as determining a MCID for improvement in FEV1 with therapy is needed, so too it is necessary to determine the significance of loss of FEV1. The six month comparison of tiotropium to salmeterol and placebo provided such an opportunity (). On day one, the increase in FEV1 with the two long-acting bronchodilators was similar. However, over the course of the study, there was a decline in the efficacy in the salmeterol arm. Over the course of the study, the changes in the mean (SE) FEV1 with salmeterol occurred primarily in the first 15 days, but smaller changes did occur subsequently. There was a decline in the trough FEV1 of 35 (13) mL, peak FEV1 of 56 (15) mL, average FEV1 of 0–4 hours; 50 (14) mL average FEV1 of 4–8 h of 68 (14) mL; average FEV1 of 8–12 h of 66 (13) mL and average FEV1 of 0–12 h of 61 (13) mL with salmeterol while smaller declines with placebo and small increases with tiotropium were seen. Moreover, the loss in the FEV1 was below a threshold of 100 mL and no clinical significant differences were noted in other outcomes. The study was not designed to test for tolerance, and careful observations of other outcomes over the 12 h study periods were not performed. There has been much speculation and controversy on the topic of tolerance to beta agonists. Rigorous attention to the spirometric indices and careful correlations with symptoms may establish an MCID that will inform decision making concerning tolerance in the future.
Table 8. Response of FEV1 to Tiotropium, Salmeterol and Placebo.
Is There a MCID for the Magnitude of FEV1 Vs. FVC Response to Albuterol that Can Distinguish Asthma from COPD
Many try to distinguish asthma from COPD by evaluating bronchodilator reversibility by the magnitude of the FEV1 response. However, this approach is flawed because of overlap in FEV1 in both conditions and different patterns of response in individual patients. In a comparison of the bronchodilator response to albuterol in asthma and COPD using the standard cut off that 12% and 200 mL improvement following bronchodilator indicates reversibility, Chhabra reported that the average increase in FEV1 in asthmatics was 307 mL while it was 120 mL in COPD Citation[[70]]. In comparing the bronchodilator response in asthma and COPD, 3 patterns were noted: FVC alone; FEV1 alone; or both. In asthma, most showed an increase in both FVC and FEV1, where COPD, FVC alone was the most common. Thus, there is a bronchodilator response that differs both quantitatively and in the pattern. Similarly, Kesten tried to answer the same question but concluded that the sensitivity and specificity of post bronchodilator changes in FEV1 were not generally sufficient to diagnose or exclude asthma reliably Citation[[72]]. Clearly, an asthmatic response to bronchodilator is usually greater than that seen in COPD; however, there is no absolute cut off in FEV1 that allows separation of COPD from asthma. Furthermore, when evaluating tests of lung function for MCID, investigators may need to look at both FEV1 and FVC in determining changes that are clinically important in COPD.
Implications for the MCID FEV1: Dose Response and Duration
Some medications for patients with COPD appear to have a dose response as determined by lung function. This is true for formoterol, ipratropium, albuterol, and theophylline. Others, such as salmeterol, inhaled and oral corticosteroids, combinations of ICS/LABA, and tiotropium, do not. How do physicians evaluate this phenomenon, and what are the implications for MCID FEV1?
Gross evaluated a dose response to ipratropium as a nebulized solution in patients with COPD Citation[[23]]. Five doses from 0.05 to 0.6 mg of ipratropium plus placebo and MDI (40 mcg) were studied. At the peak, FEV1 at one to two hours, the two higher doses increased approximately 440 ± 194 mL (mean ± SD) over placebo and increased the duration of effect up to 8 hours ().
Figure 3. FEV1 response to increasing doses of ipratropium in COPD. Mean FEV1 values are shown at baseline (0 h) and during the 8 h after administration of each of seven agents in 42 subjects with COPD (open circles = placebo; closed circles = 0.05 mg nebulized ipratropium; open triangles = 0.1 mg nebulized ipratropium; closed triangles = 0.2 mg nebulized ipratropium; open squares = 0.4 mg nebulized ipratropium; closed squares = 0.6 mg nebulized ipratropium; open inverted triangles = 40 µg ipratropium by metered-dose inhaler). Reprinted from Ref. Citation[[23]].
![Figure 3. FEV1 response to increasing doses of ipratropium in COPD. Mean FEV1 values are shown at baseline (0 h) and during the 8 h after administration of each of seven agents in 42 subjects with COPD (open circles = placebo; closed circles = 0.05 mg nebulized ipratropium; open triangles = 0.1 mg nebulized ipratropium; closed triangles = 0.2 mg nebulized ipratropium; open squares = 0.4 mg nebulized ipratropium; closed squares = 0.6 mg nebulized ipratropium; open inverted triangles = 40 µg ipratropium by metered-dose inhaler). Reprinted from Ref. Citation[[23]].](/cms/asset/76003e21-8d35-4138-a15d-6dc41dbc5828/icop_a_53377_uf0003_b.gif)
However, the MDI achieved only 63–73% of the bronchodilation with the 2 highest doses. Extrapolating from the dose response curves presented, it appears that the lower doses were similar to the 2 highest doses in improving FEV1 at the peak but differed by up to 100 mL at 2 h and then by 200 mL at each measurement until 8 h, the termination of the study. The FVC AUC (0–8) was greater with the 2 higher doses. The higher (0.5 mg) dose was selected and approved by the FDA and is the standard dose used with the nebulized solution. No significant adverse events occurred with any of the doses, and no attempt to evaluate other outcomes weremade. In the future, a consideration of MCID for postdosing FEV1 correlated to other outcomes could be useful in selecting an optimal dose for clinical use.
Increasing the dose of salmeterol from 50 to 100 mcgs increases FEV1, but Health Status-QOL declines Citation[[73]]. Similarly, there is a log-dose response for theophylline on FEV1 but side effects negate the advantages. Thus, an MCID for FEV1 must be anchored to a global or overall assessment when evaluating dose-response.
The Newer Agents
Newer agents, such as Phosphodiesterase E4 Inhibitors (PDE4) inhibitors, have been studied in patients primarily with modest reversibility. These agents are anti-inflammatory and are weak bronchodilators. In the most positive study so far, Compton showed that the mean trough pre-bronchodilator FEV1 was greater than 100 mL for 15 mg twice daily (BID) of cilomilast vs. placebo Citation[[74]] The maximum increase in post-bronchodilator FEV1with cilomilast vs. placebo was 0.16 L. However, most studies with the PDE4 class have showed substantially lower improvement in FEV1and inconsistent effect on other outcomes. In fact, the increase in FEV1 in the cilomast data presentation for FDA approval was less than 50 mL. Data on FEV1 and exacerbations in patients with COPD with once a day roflumilast are not available at this time. It appears that PDE4 and ICS will have weak effects on trough FEV1 (50–60 mL) but might reduce exacerbations and improve dyspnea. Does this indicate that the MCID for FEV1 should be 50 mL?
Expert Opinion on the MCID FEV1
The opinions of thought leaders on MCID FEV1 is potentially useful.
Recently, a small group was posed the question of what is the MCID for FEV1? None selected 50 mL, 4 selected 100 mL, 4 selected 150 mL, 5 selected none of the above for an MCID. A more formal questionnaire will be developed and tested in a large group of specialists to evaluate expert opinion on MCID FEV1.
Proposals
Since comparative and quantitative data are often lacking, determination of a general level of improvement in FEV1 that is both perceptible and clinically meaningful has proven difficult. The following are proposed:
Review actual quantitative data on predose, postdose, peak, mean, FEV, AUC12, AUC24, and standard deviations from large well designed clinical trials that should be online in all published clinical trials.
Obtain additional data on lung volumes both in stable patients and during exercise when evaluating new modalities. Also try to correlate FEV1 with functional indices such as BODE index, which gives a more complete picture.
Develop distributional approaches and statistical methods similar to those developed for quality of life in COPD that can be used with FEV1.
Distribute questionnaires and scenarios to stakeholders such as specialists, primary care givers, industry personnel, regulatory personnel and patients to try to establish a consensus on what is the MCID for FEV1.
Conclusions
The MCID for FEV1 and other lung function parameters is unknown. The predose or trough FEV1improvement of about 100 mL does correlate with other important clinical outcomes when using anchoring techniques. However, issues such as placebo effect, reproducibility of measurement, and variability must be considered for the noise effects can exceed 100 mL. The FEV1is less important to patients than is survival, dyspnea, health status, and exacerbations. Since the correlations of FEV1 and survival are inconsistent, an attempt was made to anchor FEV1 to patient perception, exacerbation, and clinical trials. A change of 100 mL in FEV1 is anchored to exacerbations, patient perception, and two year decline in lung function. This level of improvement in trough FEV1 is typical of the magnitude of change in clinical trials of FDA approved bronchodilators and ICS/LABA combinations for COPD. Since dyspnea is more anchored to hyperinflation than FEV1, it may be more meaningful to attempt to correlate changes in FEV1 with Borg Scale change and with changes in residual volume and inspiratory capacity. A change in 100 mL in predose FEV1 is more meaningful to the typical patient seen in practice who presents with an FEV1 below 50% than one with higher levels. The MCID in FEV1 remains an important but still undetermined issue for patients with COPD.
| Glossary | ||
| FEV1: | = | = Forced Expiratory Volume in one second |
| FVC: | = | = Forced Vital Capacity |
| RV: | = | = Residual Volume |
| FRC: | = | = Functional Residual Capacity |
| IC: | = | = Inspiratory Capacity |
| ICS: | = | = Inhaled Corticosteroids |
| SABA: | = | = Short-Acting Beta Agonist |
| LAAC: | = | = Long-Acting Anti cholinergics |
| LABA: | = | = Long-Acting Beta Agonist |
REFERENCES
- Celli B R, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23:932–946. [PUBMED], [INFOTRIEVE]
- Fabbri L, Pauwels R A, Jurd S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary updated 2003. COPD 2004; 1:105–141.
- Jaesche R, Singer J, Guyatt G H. Measurement of health status ascertaining the minimal clinically important difference. Control Clin Trials 1989; 10:407–415., [CROSSREF]
- Beaton D E, Boers M, Wells G A. Many faces of the minimal clinically important differences (MCID): a literature review and directions for future research. Curr Opin Rheumatol 2002; 14:109–114. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Wyrwich K W, Tierney W M, Wolinsky F D. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol 1999; 52(9):861–873. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- American Thoracic Society. Standardization of spirometry 1994 update. Am J Respir Crit Care Med 1995; 152:1107–1136. [CSA]
- Sutherland E R, Allmers H, Ayas N T, Venn A J, Martin R J. Inhaled corticosteroids reduce the porgession of airflow limitation in chronic obstructive pulmonary disease: a meta-analysis. Thorax 2003; 58(11):937–941. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Vestbo J, TORCH Study Group. The TORCH (towards a revolution in COPD health) survival study protocol. Eur Respir J 2004 Aug; 24(2):206–210. [CROSSREF]
- Decramer M, Celli B, Taskin D P, Pauwels R A, Burkhart D, Cassino C, Kesten S. Clinical trial design considerations in assessing long term functional impacts of tiotropium in COPD, the uplift trial. J COPD 2004; 1(2):303–312.
- Decramer M, Dekhuijzen P N, Troosters T, van Herwaarden C, et al. The bronchitis randomized on NAC Cost-utility study (BRONCUS): hypothesis and design. BRONCUS-trial committee. Lancet 2005, In press.
- The Alpha 1-Antitrypsin Deficiency Registry Study Group. Survival and FEV1 decline in individuals with severe deficiency of alpha 1-antitrypsin. Am J Respir Crit Care Med 1998; 158:49–59. [CSA]
- Scanlon P D, Connett J E, Waller L A, Altose M D, Bailey W, Buist S S, Tashkin D P, Lung Health Study Research Group. Smoking Cessation and lung Function in Mild-to-Moderate Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2000; 162:381–390.
- Celli R B, Cote C G, Martin J M, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Eng J Med 2004; 350:1005–1012. [CROSSREF]
- Nishamura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002; 121:1434–1440. [CROSSREF]
- Hansen E F, Phanareth K, Laursen L S, Kok-Jensen A, Dirksen A. Reversible and irreversible airflow obstruction as predictor of overall mortality in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 159:1267–1271. [PUBMED], [INFOTRIEVE], [CSA]
- Casabari R, Briggs D D Jr, Donohue J F, Serby C W, Menjoge S S, Witek T J Jr, US Tiotropium Study Group. The spirometric efficacy of once-daily dosing with tiotropium in stable COPD: a 13-week multicenter trial. Chest 2000; 118:1294–1302. [CROSSREF]
- Donohue J F, van Noord J A, Bateman E D, Langley S J, Lee A, Witek T J Jr, Kesten S, Towse L. A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest 2002; 122:47–55. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Donohue J F, Kalberg C, Emmett A, Merchant K, Knobil K. A short-term comparison of fluticasone propionate/salmeterol with ipratropium bromide/albuterol for the treatment of COPD. Treat Respir Med 2004; 3(3):173–181. [PUBMED], [INFOTRIEVE], [CSA]
- Hanania N A, Darken P, Horstam D, Reisner C, Lee B, Davis S, Shah T. The efficacy and safety of fluticasone propionate (250 µg/ salmeterol [50 µg]) combined in the diskus inhaler for the treatment of COPD. Chest 2003; 124(3):834–843. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Mahler D A, Wire P, Horstman D, Chang C N, Yates J, Fischer T, Shah T. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002; 166:1084–1091. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Vincken W, van Noord J A, Greefhort A PM, Bantje T A, Kesten S, Korducki L, Cornelissen P JG, Dutch/Belgian Tiotropium Study Group. Improved health outcomes in patients with COPD during 1 yr's treatment with tiotropium. Eur Respir J 2002; 19(2):209–216. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax 2003; 58:399–404. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Gross N J, Petty T L, Friedman M, Skordin M S, Silvers G W, Donohue J F. Dose response to ipratropium as a nebulized solution in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis May 1989; 139(5):1188–1191. [PUBMED], [INFOTRIEVE]
- Rossi A, Kristufek P, Levine B E, Thomson M H, Till D, Kottakis J, Cioppa G D, Formoterol in Chronic Obstructive Pulmonary Disease (FICOPD) II Study Group. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest 2002; 121:1058–1069. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Quanjer P H, Tammeling G J, Cotes J E, Pedersen O F, Peslin R, Yernault J C. Lung volumes and forced ventilatory flows, report working party standardization of lung function tests. Official statement of the European respiratory society. Eur Respir J 1993; 16(suppl):5–40.
- American Thoracic Society. Standardization of spirometry; 1987 update. Am Rev Respir Dis 1987; 136:1286–1296.
- Hankinson J L, Bang K M. Acceptability and reproducibility criteria of the American thoracic society as observed in a sample of the general population. Am Rev Repsir Dis 1991; 143:516–521.
- Schermer T R, Jacobs J E, Chavannes N H, Hartman J, Folgering H T, Bottema B J, van Weel C. Validity of spirometric testing in a general practice population of patients with chronic obstructive pulmonary disease (COPD). Thorax 2003 Oct; 58(10):861–866. [CROSSREF]
- Townsend M C, Hankinson J L, Lindesmith L A, Slivka W A, Stiver G, Ayres G T. Is my lung function really that good? Flow-type spirometer problems that elevate test results. Chest 2004; 125(5):1902–1909. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Santus P, Pecchiari M, Carlucci P, Boveri B, Di Marco F, Castagna F, Centanni S. Bronchodilation test in COPD: effect of inspiratory manoeuvre preceding forced expiration. Eur Respir J 2003; 21(1):82–85. [PUBMED], [INFOTRIEVE]
- Sharafkhanek A, Officer T M, Goodnight-White S, Rodart J R, Borek A M. Novel method for measuring effects of gas compression on expiratory flow. Am J Physiol Regal Integr Comp Physiol 2004; 287:R479–R484.
- Medical Research Council. Definition and classification of chronic bronchitis for clinical and epidemiological purposes. Lancet 1965; 1:775–779.
- Freedman S, Prowse K. How many blows make an FEV1? Lancet 1996; 2:618–619.
- Joyce D P, Jackevicius C, Chapman K R, McIvor R A, Kesten S. The placebo effect in asthma drug therapy trials: a meta-analysis. J Asthma 2000 Jun; 37(4):3003–3018.
- Enright P L, Beck K C. Sherrill, repeatability of spirometry in 1,800 adult patients. Am J Respir and Crit Care Med 2004; 169:235–238. [CSA], [CROSSREF]
- Tweeddale P M, Alexander F, McHardy G JR. Short term variability in FEV1 and bronchodilator responsiveness in patients with obstructive ventilatory defects. Thorax 1987; 42:487–490. [PUBMED], [INFOTRIEVE]
- Wise R A, Connett J, Kurnow K, Grill J, Johnson L, Kanner R, Enright P, Lung Center Study Group. Selection of spirometric measurements in a clinical trial, the lung health study. Am J Respir Crit Care Med 1995; 151:1675–1681. [CSA]
- Brand P L, Quanjer P H, Postma D S, Kerstjens H A, Koeter G H, Dekhuijzen P N, Sluiter H J. Interpretation of bronchodilator response in patients with airways disease. The Dutch chronic non-specific lung disease (CNSLD) study group. Thorax 1992; 47(6):429–436. [PUBMED], [INFOTRIEVE]
- Hankinson J L, Crapo R O, Jensen R L. Spirometric referencevalues for the 6-s FVC maneuver. Chest 2003 Nov; 124(5):1805–1811. [CROSSREF]
- Swanney M P, Jensen R L, Crichton D A, Beckert L E, Cardno L A, Crapo R O. FEV6 is an acceptable surrogate for FVC in the spirometric diagnosis of airway obstruction and restriction. Am J Respir Crit Care Med 2000; 162(3):917–919. [PUBMED], [INFOTRIEVE], [CSA]
- Enright P L, Connett J E, Bailey W C. The FEV1/FEV6 predicts lung function decline in adult smokers. Respir Med 2002; 96:444–449. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- O'Donnell D E, Lam M, Webb K A. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 158:1557–1565. [PUBMED], [INFOTRIEVE], [CSA]
- Di Marco F, Milic-Emili J, Boveri B, Carlucci P, Santus P, Casanova F, Cazzola M, Centanni S. Effect of inhaled bronchodilators on inspiratory capacity and dyspnoea at rest in COPD. Eur Respir J 2003; 21:86–94. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Newton M F, O'Donnell D E, Forkert L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest 2002; 121:1042–1050. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Redelmeier D A, Goldstein R S, Min S T, Hyland R H. Spirometry and dyspnea in patients with COPD: when small differenced mean little. Chest 1996; 109:1163–1168. [PUBMED], [INFOTRIEVE]
- Santanello N C, Zhang J, Seidenberg B, Reiss T F, Barber B L. What are minimal important changes for asthma measures in a clinical trial?. Eur Respir J 1999; 14(1):23–27. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Niewoehner D E, Collins D, Erbland M L, Deaprtment of Veterans Affairs Cooperative Study Group. Relation of FEV1 to clinical outcomes during exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161:991201–991205.
- Singh J M, Palda V A, Stanbrook M B, Chapman K R. Corticosteroids therapy for patients with acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med 2002; 162:2527–2536. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Davies L, Angus R M, Calverley P M. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomized controlled trial. Lancet 1999; 354:456–460. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Aaron S D, Vandemheen K L, Herbert P, Dale R, Stiell K T, Ahuja J, Dickinson G, Brison R, Rowe B H, Dreyer J, Yetisir E, Cass D, Well G. Outpatient oral prednisone after emergency treatment of chronic obstructive pulmonary disease. N Engl J 2003; 26:2618–2625. [CROSSREF]
- Fletcher C, Peto R, Tinker C. The Natural History of Chronic Bronchitis and Emphysema. New York: Oxford University Press, 1976:1–272.
- Antonison N R, Connett J E, Killey J P, Bailey W C, Buist A S, Conway W A Jr, Enright P L, Kanner R E, O'Hara P, Owens G R, Scanlon P D, Tashkin D P, Wise R A. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator of the rate of decline in FEV1, the lung health study. JAMA 1994; 272(19):1497–1505.
- Highland K B, Strange C, Heffner J E. Long-term effects of inhaled corticosteroids on FEV1 in patients with chronic obstructive pulmonary disease. Ann Intern Med 2003; 138:969–973. [PUBMED], [INFOTRIEVE]
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ 2004; 328:1519. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Sestine P, Renzoni E, Robinson S, Poole P, Ram F S. Short-acting β2-agonists for stable chronic obstructive pulmonary disease (Cochran review). Cochrane Database Syst Rev (database online) 2003; (2), Accessed July 10, 2003.
- Calvery P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsrik A. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003; 361(9356):449–456. [CROSSREF]
- Sin D D, McAlister F A, Man S FP, Anthonisen N R. Contemporary management of chronic obstructive pulmonary disease. JAMA 2003; 290(17):2301–2316. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Appleton S, Poole P, Smith B, Veale A, Bara A. Long-acting β2-anonists for chronic obstructive pulmonary disease patients with poorly reversible airflow limitation (Cochrane review). Cochrane Database 2003; (2), Accessed July 10, 2003. [CSA]
- van Noord J A, Smeets J J, Custers F LJ, Korducki L, Cornelissen P J. Pharmacodynamic steady state of tiotropium in patients with chronic obstructive pulmonary disease. Eur Respir J 2002; 19:639–644. [PUBMED], [INFOTRIEVE], [CROSSREF]
- O'Donnell D E, Fluge T, Gerken F, Hamilton A, Webb K, Aguilanice B, Make B, Magnussen H. Effects of tiotropium on lung hyperinflation, dyspnea and exercise tolernace in COPD. Eur Respir J 2004; 23:832–840. [PUBMED], [INFOTRIEVE]
- Burge P S, Calvery P MA, Jones P W, Spencer S, Anderson J A, Maslen T K. On behalf of the ISOLDE study investigators. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 2000; 320:1297–1305. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Hanania N A, Darken P, Horstam D, Reisner C, Lee B, Davis S, Shan T. The efficacy and safety of fluticasone propionate (250 µg/ salmeterol [50µg]) combined in the diskus inhaler for the treatment of COPD. Chest 2003; 124(3):834–843. [PUBMED], [INFOTRIEVE], [CROSSREF]
- COMBIVENT Inhalation Aerosol Study Group. In chronic obstructive pulmonary disease, a combination of ipratropium and albuterol is more effective than either agent alone. An 85-day multicenter trial. Chest 1994 May; 105(5):1411–1419.
- COMBIVENT Inhalation Aerosol Study Group. Routine nebulized ipratropium and albuterol together are better than either alone in COPD. Chest 1997 Dec; 112(6):1514–1521.
- Friedman M, Serby C W, Menjoge S S, Wilson J D, Hilleman D E, Witek T J Jr. Pharmacoeconomics evaluation of a combination of ipratropium plus albuterol compared with ipratropium alone and albuterol alone in COPD. Chest 1999; 115:635–641. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Lanes S F, Garrett J E, Wentworth C E, Fitzgerald J M, Karpel J P. The effect of adding ipratropium bromide to salmeterol in the treatment of acute asthma: a pooled analysis of three trials. Chest 1998 Aug; 114(2):365–372.
- Karpel J P, Kotch A, Zinny M, Pesin J, Alleyne W. A comparison of inhaled ipratropium, oral theophylline plus inhaled beta-agonist, and the combination of all three in patients with COPD. Chest 1994 Apr; 105(4):1089–1094.
- Zuwallach R L, Mahler D A, Reilly D, Church N, Emmett A, Rickard K, Knobil K. Salmeterol plus theophylline combination therapy in the treatment of COPD. Chest 2001; 119:1661–1670. [CROSSREF]
- van Noord J A, de Munck A J, Bantje T A, Hop W CJ, Akveld M LM, Bommer A M. Long-term treatment of chronic obstructive pulmonary disease with salmeterol and the additive effect of ipratropium. Eur Respir J 2000; 15:878–885. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Donohue J F, Menjoge S, Kesten S. Tolerance to bronchodilating effects of salmeterol in COPD. Respir Med 2003; 97:1014–1020. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Chabra S K, Bhatnagar S. Comparison of bronchodilator responsiveness in asthma and chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci 2002; 44:91–97. [CSA]
- Kesten S, Rebuck A S. Is the short-term response to inhaled β-adrenergic agonist sensitive or specific for distinguishing between asthma and COPD? Chest 1994; 105:1042–1045. [PUBMED], [INFOTRIEVE]
- Jones P W, Bosh T K. Quality of life changes in COPD patients treated with salmeterol. Am J Respir Crit Care Med 1997; 155:1283–1289. [PUBMED], [INFOTRIEVE], [CSA]
- Compton C H, Gubb J, Nieman R, Edelson J, Amit O, Bakst A, Ayers J G, Creemers J P, Schultze-Werninghaus G, Brambilla C, Barnes N C. Cilomilast, a selective phosphodiesterase-4 inhibitor for treatment of patients with chronic obstructive pulmonary disease: a randomised, dose-ranging study. Lancet 2001; 358:265–270. [PUBMED], [INFOTRIEVE], [CROSSREF]