ABSTRACT
Alginates are established among the most versatile biopolymers, used in a wide range of applications. The conventional use of alginate as an excipient in drug products generally depends on the thickening, gel-forming, and stabilizing properties. A need for prolonged and better control of drug administration has increased the demand for tailor-made polymers. Hydrocolloids like alginate can play a significant role in the design of a controlled-release product. At low pH hydration of alginic acid leads to the formation of a high-viscosity “acid gel.” Alginate is also easily gelled in the presence of a divalent cation as the calcium ion. Dried sodium alginate beads reswell, creating a diffusion barrier decreasing the migration of small molecules (e.g., drugs). The ability of alginate to form two types of gel dependent on pH, i.e., an acid gel and an ionotropic gel, gives the polymer unique properties compared to neutral macromolecules. The molecule can be tailor-made for a number of applications. So far more than 200 different alginate grades and a number of alginate salts are manufactured. The potential use of the various qualities as pharmaceutical excipients has not been evaluated fully, but alginate is likely to make an important contribution in the development of polymeric delivery systems. This natural polymer is adopted by Ph.Eur. It can be obtained in an ultrapure form suitable for implants. This review discusses the present use and future possibilities of alginate as a tool in drug formulation.
INTRODUCTION
The interest in formulated dosage forms, where the drug release can be controlled, has increased steadily during the last 50 years. In most cases the purpose is to make a product that maintains a prolonged therapeutic effect at a reduced dosing frequency. Although a large number of substances demonstrate pharmacological effects in vitro, the active principle must reach the site of action in a concentration large enough to initiate a pharmacological response to be useful as a compound in human medicine. The amount of the drug delivered to the site of action will depend on the administration dose, the rate and extent of absorption, and the distribution throughout the body. The clinical effect will last until the drug concentration falls below a minimum level due to excretion and metabolism. Drugs are almost never administered to a patient in an unformulated state. A dosage form generally consists of one or more active principles together with a varying number of other substances (excipients) that have been added to the formulation in order to facilitate the preparation and administration, promote the consistent release and bioavailability of the drug, and protect it from degradation. These excipients strongly influence the physicochemical characteristics of the final products. Excipients were considered to be inert in that they should not exert any therapeutical or biological action or modify the biological action of the drug substance. It is now recognized that excipients can potentially influence the rate and/or extent of absorption of a drug (e.g., by complex formation). The successful formulation of a stable and effective dosage form therefore depends on the careful selection of excipients. In this context it should be mentioned that the use of polymers as a formulation aid in controlled drug delivery systems has over the years become an important area of research and development.
The present trend points to an increasing interest in the use of natural ingredients in food, drugs, and cosmetics. The naturally occurring alginate polymers have a wide potential in drug formulation due to their extensive application as food additives and their recognized lack of toxicity. Alginates can be tailor-made to suit the demands of applicants in both the pharmaceutical and biomedical areas. This group of polymers possesses a number of characteristics that makes it useful as a formulation aid, both as a conventional excipient and more specifically as a tool in polymeric-controlled drug delivery.
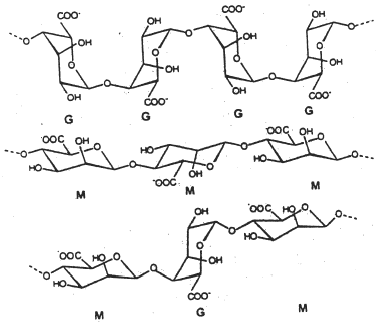
Alginates are natural polysaccharide polymers isolated from brown seaweed (Phaeophyceae) (). The seaweed is extracted with a dilute alkaline solution which solubilizes the alginic acid present. Free alginic acid is obtained on treatment of the resulting thick and viscous mass with mineral acids. The alginic acid can then be converted to a salt of which sodium alginate is the major form currently used. Alginic acid is a linear polymer consisting of d-mannuronic acid and l-guluronic acid residues that are arranged in the polymer chain in blocks. These homogeneous blocks (composed of either acid residue alone) are separated by blocks made of random or alternating units of mannuronic and guluronic acids Citation[1]. Alginates have been reported to undergo proton-catalyzed hydrolysis, which is dependent on time, pH, and temperature. Alginates from different sources vary in their proportions of blocks. Hydration of alginic acid leads to the formation of a high-viscosity “acid gel” due to intermolecular binding. After gelation the water molecules are physically entrapped inside the alginate matrix, but are still free to migrate. This is of great importance in many applications (e.g., alginate gels for cell immobilization/encapsulation). The water-holding capacity of the gel is due to capillary forces. Heat-stable gels can develop at room temperature.
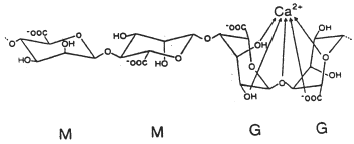
Monovalent metal ions form soluble salts with alginate whereas divalent and multivalent cations (except Mg2+) form gels or precipitates. The various cations show different affinity for alginate, and selective ion binding is the basis for the ability of alginate to form ionotropic hydrogels. Alginates with a high content of guluronic acid blocks give gels of considerably higher strength compared to alginates rich in mannuronate, as the G residues exhibit a stronger affinity for divalent ions than the M residues () Citation2-3.
Transmittancy, swelling, and viscoelasticity of alginate gel membranes are highly affected by the M/G ratio Citation4-5. The calcium alginate gels are the most extensively studied. The ability of alginate to form two types of gel dependent on pH, i.e., an acid gel and an ionotropic gel, gives the polymer unique properties compared to neutral macromolecules. The physicochemical properties of the polymer system and the swelling process to activate the release of drugs will be dependent on the type of gel formed.
Alginic acid and its sodium and calcium salts are regarded as generally non-toxic and biocompatible Citation[6]. These products are commercially available and over 200 different alginate grades, in addition to alginic acid and a number of corresponding salts, are manufactured. Alginates are widely used in the pharmaceutical, cosmetic, and food industry Citation7-8. However, since alginates are obtained from a natural source, a variety of impurities may potentially be present. These include heavy metals, proteins, and endotoxins. For pharmaceutical applications, particularly for parenteral administration, these impurities should be removed. A monograph for alginic acid is included in the European Pharmacopoeia (Ph.Eur.). Alginate approved by Ph.Eur. can now be obtained. Ultrapure grades of alginates have a controllable level of pyrogenicity and may be used as implants in combination with drugs.
Compounds used as excipients will often find more than one application, and that is also the case for alginic acid and its salts. Their application generally depends on the thickening, gel-forming, and stabilizing properties. As examples it can be mentioned that sodium alginate can be used as a binding and disintegrating agent in tablets, as a suspending and thickening agent in water-miscible gels, lotions, and creams, and as a stabilizer for emulsions. The potential lies, however, in the development of alginate-controlled drug delivery systems.
THE APPLICATION OF ALGINATE IN ORAL DOSAGE FORMS WITH SYSTEMIC EFFECT
Tablets and capsules are by far the most frequently used oral dosage forms. The products are often designed as immediate-release type, i.e., immediate release of drug for rapid absorption. Coating of the units can lead to sustained-release products, i.e., the release of the therapeutic agent is retarded. Controlled-release drug delivery systems are designed to give a reproducible and kinetically-predictable release of drug substance. Alginates may be utilized in dosage forms designed for either type of drug release.
Traditionally, sodium alginate has been used as a tablet binding agent, while alginic acid is used as a tablet disintegrant in compressed tablets designed for immediate drug release Citation[8]. The effect of sodium alginate on tablet properties is, however, dependent on the amount incorporated in the formulation and in some cases the alginate salt can promote disintegration.
Development of a Controlled-Release System
The oral dosage forms are often made according to one of the following principles: the entire drug dose is in the same physical unit or the dose is contained in an assembly of small sub-units. In the latter case the sub-units are filled into a capsule or compressed into a tablet. The formulations employ a chemical or physical “barrier” to provide a controlled release of the drug. Many formulation techniques have been used to build the barrier into the peroral dosage form, e.g., the coating of a core containing the active ingredient or the embedding of the active ingredient in a polymer matrix. Hydrocolloids like alginate can play a significant role in the design of a controlled-release product. The alginate molecule will undergo an almost immediate hydration to create a hydrocolloidal layer of high viscosity. This makes up a diffusion barrier decreasing the migration of small molecules (e.g., drugs). So far, alginate has mainly been applied in systems based on diffusion.
Diffusion Systems
Diffusion systems based on alginate can be divided into two main categories. In the polymer membrane system the drug formulation is encapsulated within a drug reservoir compartment. The drug formulation may exist as a solid or suspension, or in a solution. The drug release is controlled by the polymeric encapsulating membrane having a specific permeability. The encapsulation of drug is accomplished by various techniques, e.g., spray-coating or microencapsulation. Alginate has been applied in the preparation of gel capsules. In one study, the compound theophylline was encapsulated and the drug release rate was significantly reduced compared to the matrix-type alginate gel beads Citation[9]. The release rate became lower as the coat thickness increased. The release followed zero-order kinetics as expected. A further decrease in release rate can be obtained by incorporating additives such as carnauba wax into the drug reservoir. This is demonstrated for indomethacin, a non-steroidal anti-inflammatory drug which is highly irritating to the mucosa in the upper gastrointestinal (GI) tract Citation[10].
In the polymer matrix system the drug is homogeneously dispersed in a rate-controlling polymer matrix. The final product may be in the form of swellable microspheres or conventional tablets. When such systems are exposed to the dissolution medium, drug release is modulated by diffusion through matrix swelling and dissolution/erosion at the matrix periphery Citation[11]. The “swelling–dissolution–erosion” process is highly complex. In systems based on sodium alginate cross-linked with calcium chloride, the osmotic pressure gradient that exists between the alginate gel and the environment comprises an important factor in the swelling process. Under acidic conditions (e.g., in the stomach) swelling of the calcium alginate beads scarcely occurs. A drug is likely to be released by diffusion through the insoluble matrix. Under neutral conditions (e.g., intestine) the beads will swell and the drug release depend on the swelling and erosion process. The swelling behavior of calcium alginate has been thoroughly exploited for the development of a multiple-unit, controlled-release drug delivery system Citation12-13. Alginate gel beads seem to be most effective in retarding drugs at higher alginate concentrations Citation14-15 and when the alginates are rich in guluronic acid Citation[14], Citation[16]. The guluronic acid conformation gives a high degree of coordination of the calcium, and thereby forms more rigid gels that are less prone to swelling and erosion. By increasing the mannuronic acid content the gels become softer, more elastic, but less porous and they dissolve more easily. The situation may be different for drug molecules that strongly interact with alginate. Gentamicin sulfate was found to interact selectively on the mannuronic residues of alginate without competition with calcium ions involved in the polymer gelation. In this case a higher mannuronic acid content would lead to a higher binding capacity for drug molecules, and mannuronic-rich alginates may be preferred Citation[17]. The molecular weight and viscosity of the alginates did not affect the drug release of nicardipine HCl in neutral medium Citation[16]. Interestingly, the release of the basic drug pindolol was, however, demonstrated to be dependent on the alginate molecular size Citation[18]. The slowest in vitro release rate of pindolol (at neutral pH) was observed for the beads prepared by alginate of low molecular weight, although this showed the fastest in vivo absorption rate. The drug:alginate ratio and calcium chloride concentration affect the drug release. The release of nicardipine from alginate particles prepared in a ratio of 1:1 was delayed more than that from 1:2 particles Citation[19]. A high calcium content is favorable Citation[14], Citation19-21. The curing time seems to be of minor importance in some systems Citation[19], while a long gelling time is favorable under other experimental conditions Citation[14], Citation20-21. The cross-linker type and concentration seem to have a pronounced influence on the drug release Citation21-23. Calcium alginate beads displayed prolonged release profiles when compared to alginate beads prepared from other cross-link agents like Ba2+ and Sr2+ Citation[24]. It is interesting to notice that even calcium bound with the G block was displaced by monovalent cations at high salt concentration (>0.2 M), resulting in an increased swelling Citation[3]. Variation in bead size may be used as a formulation principle to change the release time onset. Release of dextran in a pulsative fashion is obtained by mixing different bead sizes Citation[25]. This may be of importance in the design of drug delivery systems intended to follow the circadian rhythm in the body.
Drug release from polymer matrix systems can be modulated by varying the material for encapsulation. Cross-linking with glutaraldehyde decreased the swelling of alginate microspheres while the loading of the water-soluble drug nimesulide increased Citation[26]. Ironcross-linked hydroxamated alginic acid has proved to be successful in prolonging drug release of a number of compounds Citation[27]. The drug release profiles of calcium alginate microspheres loaded with sulfaguanidine were affected by addition of various copolymers Citation28-29. It was demonstrated that some cellulose derivatives produced a marked aggregation of the microspheres. Of the cellulose derivatives only ethyl cellulose and hydroxypropyl cellulose resulted in a decrease in drug release rate Citation28-31. For these preparations the retardation in drug release was attributed to the aggregate formation. Addition of pectinate to alginate makes the coating more brittle Citation[32]. Alginate forms strong complexes with polycations like chitosan. Chitosan-treated alginate beads have been developed as an attempt to suppress the gel matrix erosion of alginate beads Citation[33]. The alginate beads are coated with chitosan by ionic interaction during preparation. A retarded release of highly-soluble drugs like nitrofurantoin and timolol maleate or a prodrug was observed with the chitosan-treated beads Citation34-36. An enteric protection of a preparation with the drug diclofenac was demonstrated by mixing the alginate with chitosan Citation[37]. The resulting alginate–chitosan beads showed a release behavior dependent on pH. The chitosan polymer is poorly soluble in water. In acidic medium, protonation of the amine groups improves solubility. The interpolymeric complex between alginate and chitosan exists in a gel form at low pH. At neutral pH the viscous complex will swell and the gel formed will slowly disintegrate, releasing the drug. The release rate is a function of the degree of cross-linking between both polymers. An interaction between chitosan and the drug molecule was also observed Citation37-38. Other coatings like Eudragit® will also modify the release of drugs from alginate beads Citation39-40.
Combinations of liposomes and alginate have been investigated in order to modify the release of drugs from such phospholipid vehicles and to stabilize the products. Alginate either served as a vehicle for the liposomes Citation[41] or formed a gel inside the liposome Citation[42] leading to stabilization and a delayed drug release respectively. Alginates are demonstrated to have antioxidative activity and will thereby further stabilize the liposome preparation Citation[43].
Alginate-based polymeric matrix systems can also be prepared as compressed tablets rather than beads. Drug release from hydrophilic matrix tablets is controlled by the formation of a hydrated viscous layer around the tablet, which acts as a barrier to drug release by opposing penetration of water into the tablet, and also the movement of dissolved solutes out of the matrix. A compressed alginate tablet will have a very closed structure compared to a gel bead, and the degree of sustained release effect will therefore be higher in the tablet. Water-soluble drugs are released primarily by diffusion of dissolved drug molecules across the gel layer, whilst poorly water-soluble drugs are released predominantly by erosion mechanisms. In a preparation made by direct compression of drug–alginate blends it has been demonstrated that drugs of high water solubility are released significantly faster in simulated gastric fluid than in simulated intestinal fluid, whereas the opposite effect is observed for drugs of poor solubility Citation[44]. This is explained in terms of the different hydration kinetics in these two media. Cationic drugs (e.g., lidocaine HCl) seem to be released more slowly than anionic drugs (e.g., sodium salicylate), possibly because of the negative charge of the matrices Citation[45]. By incorporating a pH-independent hydrocolloid gelling agent (e.g., cellulose polymers) in the tablet the release rate of a basic drug can be made independent of pH Citation[46]. Spray-dried composite particles of lactose and sodium alginate used as a filler of tablets can also modify the release properties. The release of acetaminophen from tablets containing spray-dried lactose–alginate particles was found to be slower in an acidic solution and more rapid in a neutral solution than release from a “conventional” sodium alginate matrix tablet Citation[47]. One explanation could be that the spray-dried particles had a much smaller particle size than an average sodium alginate particle, leading to a more effective gel network and thereby a more effective barrier. As the pH increases the lactose was more rapidly dissolved than the sodium alginate and a faster erosion occurred. In order to obtain specific delivery to the colon, lactose–sodium alginate particles were applied as the coating filler of dry-coated tablets. Addition of chitosan to the mixture further prolonged the induction period in the drug release process Citation[48]. Dry-coated tablets with alginate in the coating or alternatively alginate in the tablet core and a calcium-containing salt in the coating also showed a slow release rate Citation49-50. Gelatin capsules coated with alginate are demonstrated to remain intact as long as they are retained in the stomach, allowing for drug delivery selectively to the intestine Citation[51]. In one study acrylic polymer microspheres containing a highly water-soluble drug (acebutolol hydrochloride) were powder-coated with sodium alginate and formulated into capsules or tablets Citation52-54. This caused a prolonged release because of the gelled matrix structures formed during dissolution. The same effect has also been demonstrated when the drug substance (indomethacin) is cross-linked with sodium alginate and compressed into tablets Citation[55].
An oral sustained-delivery formulation based on in situ gelation of sodium alginate has been reported Citation[56]. The formulation depends for its action on in situ gelling induced by the sequential administration of two solutions, the first containing sodium alginate immediately followed by administration of a solution containing calcium ions in a complexed form. The acidic environment in the stomach causes the calcium ions to be released, allowing the gellation to take place. This formulation principle leads to a significant increase in the bioavailability of theophylline compared to a proprietary oral sustained-release formulation, although no increase in the mean residence time could be observed.
Drug molecules with low water solubility can show poor bioavailability when formulated into solid dosage forms. Low-molecular alginate, i.e., hydrolysis products of the polymer, may be used as a carrier to enhance the dissolution rate of acidic, basic, or neutral drugs. Kneaded mixtures of various drug substances and low-molecular alginate were investigated, and a significant increase in dissolution rate was observed Citation57-58. This may be due to an improvement of wettability and to changes of the crystallinity, microcrystal size, and shape. Alginate coating of the drug substance prior to compression is another approach to make the particles more hydrophilic Citation[59]. Microcrystalline cellulose coprocessed and partly surface-coated with an alginate calcium/sodium salt complex (Avicel® AC-815) provides excellent suspending agents for water-insoluble drugs Citation[60]. Highly lipophilic drugs can be incorporated into alginate microspheres by use of a multiple-phase emulsion technique Citation[61]. The drug is dissolved in an oil (e.g., soybean oil) and the resulting alginate microspheres contain immobilized drug-containing oil microdroplets. The microspheres can be coated further, allowing a pH-dependent release.
Modulation of Gastrointestinal Transit Time
A variation in gastric emptying time may strongly influence the absorption of drugs, especially of compounds with an “absorption window” or with poor bioavailability from the lower regions of the digestive tract. A buoyant capsule formulation has been prepared for the pH-independent controlled release of a basic drug. This is obtained by the preparation of a powder consisting of the drug substance in combination with alginate and a pH-independent hydrocolloid gelling agent (e.g., hydroxypropylmethyl cellulose). The powder is filled into hard gelatin capsules Citation[62]. The preparation does not contain calcium ions. Neither does it involve gas generation. In the stomach, water penetrates the capsule shell, initiating surface hydration of the pH-independent polymer, leading to the formation of a gel layer. Air is trapped inside the less dense powder bulk to account for the buoyant behavior of the capsule. At this low pH, alginic acid is formed from alginate and this further modifies the gel layer. Erosion of the gel layer gradually exposes more dry matrix that hydrates at the same time as drug dissolves in the gel and diffuses out to the surrounding aqueous environment. After buoyancy is lost the dosage form is emptied from the stomach followed by an increase in pH. The gelled powder plug changes structure and becomes more porous as the alginic acid turns into a more soluble salt. The drug can diffuse more readily through the matrix and this compensates for the lower dissolution of basic substances at higher pH.
Another potential approach to extend the gastrointestinal residence time is to prepare a bio(muco)adhesive drug delivery system. Specialized cells located in the stomach, duodenum, and transverse colon continuously secrete a large amount of mucin-containing mucus to protect the surface epithelium against the acidic environment and protein-splitting enzymes present in the GI tracts. Mucin consists of an oligosaccharide chain with terminal sialic acid (pKa 2.6). The drug delivery systems should contain a mucoadhesive component that binds to mucin. It appears that polyanions, especially polymers containing carboxylic groups and with a high charge density, are highly active.
Alginate is demonstrated to have excellent bioadhesive properties. The addition of a chitosan coating to alginate beads increased the adhesive properties significantly Citation[13], Citation[63]. Both the coated and the uncoated microcapsules showed the strongest affinity for the stomach mucosa. The adhesive properties increased with an increase in homogeneity of the beads. Alginate/chitosan tablets have been prepared for adhesion to human cheek mucosa Citation64-65.
THE APPLICATION OF ALGINATE IN DELIVERY SYSTEMS FOR BIOMOLECULES
Alginate has several unique properties that have enabled it to be used as a matrix for the entrapment and/or delivery of biomolecules like DNA, proteins, and cells. A relatively mild gelation process free of organic solvents enables biomolecules and cells to be incorporated into the matrices with the retention of the three-dimensional structure (i.e., full biological activity) Citation[66]. The aqueous environment within the matrix is quite inert, and may consist of distilled water or sucrose solutions Citation67-68. The porosity of the gel allows for acceptable diffusion rates of macromolecules or low molecular weight drugs bound to macromolecules Citation[69]. The diffusion rate can be further controlled by coating of the beads Citation[70]. Alginate gels are stable in the temperature range 0–100°C and may be autoclaved under special conditions Citation[71]. Freeze-drying of alginate beads containing cells or biomolecules is a possibility to keep their secondary structure and to ensure their stability during storage Citation[72].
Biocompatibility or/and immunogenicity of alginate is demonstrated to vary with factors like M/G ratio and mitogenic impurities. Capsules composed of G-rich alginates in combination with polylysine proved to induce a severe inflammatory response Citation[73], but in general the G-rich alginates seem to possess a higher biocompatibility than the M-rich polymers Citation[74]. It is however apparent that other factors like form and size of the beads, and smoothness, composition, and viscosity of the membrane can influence alginate immunogenicity.
A large number of proteins have been encapsulated in alginate microbeads Citation74-78. Positively charged proteins can potentially compete with calcium ion for available carboxylic acid sites on the alginates, resulting in a reduction in diffusion rate or protein inactivation. It may therefore be necessary to include additives (e.g., polyacrylic acid) which protect the active agent from the alginate polymer Citation[79]. Protein diffusion within alginate gels is demonstrated to be greatest for gels prepared from sodium alginate of low guluronic acid content Citation[80]. Magnetically triggered delivery of insulin from a formulation based on alginate spheres has been evaluated Citation[81]. The magnetic field characteristics and the mechanical properties of the polymer matrix were factors of great importance in controlling the release rate. A higher release rate was observed for less rigid matrices.
The mucoadhesive feature of alginate may aid in its utility as a potential delivery vehicle for biomolecules to mucosal tissues (other than the GI tract), thereby improving overall drug effectiveness and bioavailability. Attachment of lectins to the surface of spermine-modified alginate beads allows for adhesion to epithel rather than mucosa Citation[82]. Adhesion to surfaces is important in the development of non-parenteral vaccines, e.g., oral, intranasal, or vaccines to the upper respiratory tract. Recent work has shown promising results for intranasal or oral administration of antigens, or delivery of BCG virus to the lung by inhalation Citation[74], Citation[83]. Encapsulation of cells or DNA in the alginate matrix is another field of growing interest. So far, a number of cells and DNA have successfully been encapsulated in alginate matrices, which may be of importance in the treatment of a number of chronic diseases (e.g., diabetes, Parkinson's disease, cystic fibrosis, cancer) Citation71-72, Citation[74], Citation84-87. The application of alginate in parenteral preparations, e.g., a subcutaneous injection for chemotherapy, has also been evaluated Citation[88].
THE APPLICATION OF ALGINATE IN PREPARATIONS FOR LOCAL ADMINISTRATION
Alginate-based raft-forming formulations have a wide application in the treatment of heartburn and esophagitis Citation[89]. The foam makes a barrier against reflux and adds to the mucosa, providing a protective coating against stomach acid. The raft-forming formulations can be used in combination with antacids or cimetidin (H2 blocker). Recently, a liquid preparation of sodium alginate in combination with a calcium-containing solution has been evaluated for eradication of Heliobacter pyroli (HP), a bacterium closely associated with chronic gastritis and peptic ulcers. Although HP is highly sensitive to most antibiotics, it is difficult to obtain minimum inhibitory concentrations in the gastric mucus, in which HP colonizes, by systemic treatment. The alginate preparation will spread in the stomach and release the incorporated drugs to the gastric wall rather than the gastric lumen by gel formation on the surface of the preparation Citation[90].
Alginates are used in various types of wound dressings, e.g., powder-, film-, or fiber-based Citation[8], Citation[91]. The hydrocolloid may be used on dry wounds provided that the dressing is premoistened with saline. Alginates are, however, best suited for moist exudative wounds. Sodium from the wound's exudate and calcium from the alginate undergo ion exchange, forming a soluble sodium alginate gel. The generation of free calcium ion amplifies the clotting cascade, endowing the dressing with significant hemostatic properties. The dressing is non-adherent to the wound. The gel can also serve as a vehicle for drugs. Further, alginate fibers can be used in woven, non-woven, and gauze-type dressings. Alginates high in guluronic acid form highly absorbent gels, while mannuronic acid-rich alginates make softer fibers which form less absorbent gels.
The bioavailability of ocular administered drugs is low (1–3%) due to dilution and a high drainage rate. Macromolecular excipients offer the possibility of prolonging the contact time on the cornea by an increase in viscosity and by mucoadhesion. Sodium alginate is demonstrated to undergo in situ gelation in the eye without the addition of calcium ions Citation[92]. Instant gel formation was observed from alginates with G contents more than 65%. Pilocarpine incorporated into the gel was released over a period of 24 hr. A decrease in gel viscosity was, however, observed in contact with tear fluid Citation[93].
OTHER APPLICATIONS OF ALGINATES AS DRUG EXCIPIENTS
Many drug substances have a bitter or unpleasant taste that makes them unsuitable for certain oral dosage forms. Sodium alginate has been used in tablets to mask the bitter taste of amiprilose hydrochloride as a model drug Citation[94]. A core tablet was undercoated with sodium alginate and overcoated with calcium gluconate in order to form a gel on the tablet surface in the mouth at oral administration. The method was found useful for taste masking of oral compressed formulations.
CONCLUSION
Alginates have a wide application as rate-controlling excipients in drug delivery systems, as a matrix for biomolecules, and as an excipient in pharmaceutical preparations for local administration. Critical considerations of pharmaceutical excipients are compatibility, physicochemical properties, toxicology, and formulation issues (e.g., sterilization, bulk flow, compressibility, stability). This specific polymer can be prepared in a neutral or charged form, which makes it compatible with a broad variety of substances. The ability to form two types of gel depending on the pH of the medium results in a large variation in physicochemical properties. It is suitable for freeze-drying and direct compression (tablets). Alginate has been widely used in food products for several decades and is generally non-toxic. The acceptable daily intake (ADI) is “not specified”, which is the highest possible classification for food additives. Being polysaccharides, alginates are susceptible to hydrolysis or degradation in strong acid or alkali, especially at elevated temperatures. Under neutral conditions alginate is generally quite stable at room temperature. Standard alginate grades will precipitate in acid conditions. The excipient can be obtained in an ultrapure form. Sterilization may be performed by sterile filtration or by heat treatment under special conditions. Alginate is further adopted by Ph.Eur. The pharmacopeial requirements may change in the future according to specific applications of a polymer. This may lead to several alginate monographs, and thereby official status for new alginate grades. The molecule can be tailor-made for a number of applications. Polymer-controlled drug delivery is still in the developmental stage. A new approach in the field is the development of systems that are capable of adjusting drug release according to physiological needs (e.g., pH-responsive systems based on polymer swelling, magnetically triggered delivery systems). Alginate would also possess the physicochemical properties required to make it an important contributor to this area of future research.
REFERENCES
- Smidsrød O., Draget K.I. Carbohydr. Eur. 1996; 14: 6–13
- Draget K.I., Skjåk-Bræk G., Smidsrød O. Carbohydr. Polym. 1994; 25: 31–38
- Wang X., Spencer H.G. Polymer 1998; 13: 2759–2764
- Draget K.I., Skjåk-Bræk G., Christiansen B.E., Gåsrød O., Smidsrød O. Carbohydr. Polym. 1996; 29: 209–215
- Inukai M., Yonese M. Chem. Pharm. Bull. 1999; 47: 1059–1063
- King A.H. Food Hydrocolloids, M. Glicksman. CRC Press, Boca Raton, FL 1983; Vol. II: 115–154
- Espevik T., Skjåk-Bræk G. Carbohydr. Eur. 1996; 14: 19–25
- Onsøyen E. Carbohydr. Eur. 1996; 14: 26–31
- Tomida H., Nakamura C., Yoshitomi H., Kiryu S. Chem. Pharm. Bull. 1993; 41: 2161–2165
- Joseph I., Venkataram S. Int. J. Pharm. 1995; 126: 161–168
- Takka S., Ocak, Ö H., Acartürk F. Eur. J. Pharm. Sci. 1998; 6: 241–246
- Østberg T. Thesis. University of Oslo, OsloNorway 1994
- Gåserød O. Thesis. Norwegian University of Science and Technology (NTNU), TrondheimNorway 1998
- Østberg T., Vesterhus L., Graffner C. Int. J. Pharm. 1993; 97: 183–193
- Pillay V., Dangor C.M., Govender T., Moopanar K.R., Hurbans N. Drug Del. 1998; 5: 25–34
- Takka S., Acartürk F. J. Microencaps. 1999; 16: 275–290
- Iannuccelli V., Coppi G., Cameroni R. Int. J. Pharm. 1996; 143: 195–201
- Imai T., Kawasaki C., Nishiyama T., Otagiri M. Pharmazie 2000; 55: 218–222
- Acartürk F., Takka S. J. Microencaps. 1999; 16: 291–301
- Pillay V., Dangor C.M., Govender T., Moopanar K.R., Hurbans N. J. Microencaps. 1998; 15: 215–226
- Aslani P., Kennedy R.A. J. Contr. Rel. 1996; 42: 75–82
- Al-Musa S., Fara D.A., Badwan A.A. J. Contr. Rel. 1999; 57: 223–232
- Remunán-López C., Bodmeier R. J. Contr. Rel. 1997; 44: 215–225
- Takka S., Acartürk F. Pharmazie 1999; 54: 137–139
- Kikuchi A., Kawabuchi M., Sugihara M., Sakurai Y., Okano T. J. Contr. Rel. 1997; 47: 21–29
- Kulkarni A.R., Soppimath K.S., Aralaguooi M.I., Aminabhavi T.M., Rudzinski W.E. Drug Dev. Ind. Pharm. 2000; 26: 1121–1124
- Taha M.O., Aiedeh K. Pharmazie 2000; 55: 663–667
- Chan L.W., Heng P.W.S., Wan L.S.C. J. Microencaps. 1997; 14: 545–555
- Chan L.W., Heng P.W.S. J. Microencaps. 1998; 15: 409–420
- Chun K.H., Kwon I.C., Kim Y.H., La S.B., Sohn Y.T., Jeong S.Y. Arch. Pharm. Res. 1996; 19: 106–116
- Gürsoy A., Karakus D., Okar I. J. Microencaps. 1999; 16: 439–452
- Pillay V., Fassihi R. J. Contr. Rel. 1998; 59: 243–256
- Murata Y., Maeda T., Miyamoto E., Kawashima S. Int. J. Pharm. 1993; 96: 139–145
- Filipovic-Grcic J., Maysinger D., Zorc B., Jalsenjak I. Int. J. Pharm. 1995; 116: 39–44
- Hari P.R., Chandi T., Sharma C.P. J. Microencaps. 1996; 13: 319–329
- Sezer A.D., Akbuga J. J. Microencaps. 1999; 16: 687–696
- Fernández-Hervás M.J., Holgado M.A., Fini A., Fell J.T. Int. J. Pharm. 1998; 163: 23–34
- Murata Y., Miyamoto E., Kawashima S. J. Contr. Rel. 1996; 38: 101–108
- Lee B.-J., Min G.-H. Arch. Pharm. Res. 1995; 18: 183–188
- Lee B.-J., Cui J.-H., Kim T.-W., Heo M.-Y., Kim C.-K. Arch. Pharm. Res. 1998; 21: 645–650
- Takagi I., Nakashima H., Takagi M., Yotsuyanagi T., Ikeda K. Chem. Pharm. Bull. 1997; 45: 389–393
- Monshipouri M., Rudolph A.S. J. Microencaps. 1995; 12: 117–127
- Xue C., Yu G., Hirata T., Terao J., Lin H. Biosci. Biotechnol. Biochem. 1998; 62: 206–209
- Hodson A.C., Mitchell J.R., Davies M.C., Melia C.D. J. Contr. Rel. 1995; 33: 143–152
- Park H.-Y., Choi C.-R., Kim J.-H., Kim W.-S. Drug Del. 1998; 5: 13–18
- Howard J.R., Timmins P., U.S. Patent 4,792,452, 1988
- Takeuchi H., Yasuji T., Hino T., Yamamoto H., Kawashima Y. Int. J. Pharm. 1998; 174: 91–100
- Takeuchi H., Yasuji T., Yamamoto H., Kawashima Y. Pharm. Res. 2000; 17: 94–99
- Sirkiä T., Salonen H., Veski P., Jürjenson H., Marvola M. Int. J. Pharm. 1994; 107: 179–187
- Kaneko K., Kanada K., Miyagi M., Saito N., Ozeki T., Yuasa H., Kanaya Y. Chem. Pharm. Bull. 1998; 46: 728–729
- Narayani R., Rao P. J. Biomater. Sci. Polym. Edn. 1995; 7: 39–48
- Cui F., Kawashima Y., Takeuchi H., Niwa T., Hino T. Chem. Pharm. Bull. 1996; 44: 837–842
- Veski P., Marvola M. Pharmazie 1993; 48: 757–760
- Veski P., Marvola M., Smal J., Heiskanen I., Jürjenson H. Int. J. Pharm. 1994; 111: 171–179
- Pillay V., Dangor C.M., Govender T., Moopanar K.R., Hurbans N. Drug Del. 1998; 5: 35–46
- Miyazaki S., Kubo W., Attwood D. J. Contr. Rel. 2000; 67: 275–280
- Shiraishi S., Arahira M., Imai T., Otagiri M. Chem. Pharm. Bull. 1990; 38: 185–187
- Shiraishi S., Imai T., Iwaoka D., Otagiri M. J. Pharm. Pharmacol. 1991; 43: 615–620
- Bodmeier R., Paeratakul O. J. Pharm. Sci. 1989; 78: 964–967
- Selinger E., Dell S.M., Colliopoulos J.A., Reilly W.J., U.S. Patent 5,840,768, 1998
- Ribeiro A.J., Neufeld R.J., Arnaud P., Chaumeil J.C. Int. J. Pharm. 1999; 187: 115–123
- Dennis A., Timmins P., Kevin L., U.S. Patent 5,169,638, 1992
- Gåserød O., Jolliffe I.G., Hampson F.C., Dettmar P.W., Skjåk-Bræk G. Int. J. Pharm. 1998; 175: 237–246
- Miyazaki S., Nakayama A., Oda M., Takada M., Attwood D. Biol. Pharm. Bull. 1994; 17: 745–747
- Miyazaki S., Nakayama A., Oda M., Takada M., Attwood D. Int. J. Pharm. 1995; 118: 257–263
- Patil R.T., Speaker T.J. J. Microencaps. 1997; 14: 469–474
- Nussinovitch A., Gershon Z., Nussinovitch M. Food Hydrocoll. 1996; 10: 21–26
- Nussinovitch A., Gershon Z., Nussinovitch M. Food Hydrocoll. 1997; 11: 209–215
- Bhardwaj T.R., Kanwar M., Lal R., Gupta A. Drug Dev. Ind. Pharm. 2000; 26: 1025–1038
- Okhamafe A.O., Amsden B., Chu W., Goosen M.F.A. J. Microencaps. 1996; 5: 497–508
- Daigle D.J., Cotty P.J. Biocontrol Sci. Technol. 1997; 7: 3–10
- Esquiabel A., Hernández R.M., Igartua M., Gascón A.R., Calvo B., Pedraz J.L. J. Microencaps. 1997; 14: 627–638
- de Vos P., de Haan B., van Schilfgaarde R. Biomaterials 1997; 18: 273–278
- Gombotz W.R., Wee S.F. Adv. Drug Del. Rev. 1998; 31: 267–285
- Polk A., Amsden B., de Yao K., Peng T., Goosen M.F.A. J. Pharm. Sci. 1994; 83: 178–185
- Dashevsky A. Int. J. Pharm. 1998; 161: 1–5
- Bomberger D.C., Catz P.G., Smedley M.I., Stearns P.C., U.S. Patent 5,879,712, 1999
- Shinya E., Dervillez X., Edwards-Lévy F., Duret V., Brisson E., Ylisastigui L., Lévy M.C., Cohen J.H.M., Klatzmann D. Biomed. Pharmacother. 1999; 53: 471–483
- Mumper R.J., Hoffman A.S., Puolakkainen P.A., Bouchard L.S., Gombotz W.R. J. Contr. Rel. 1994; 30: 241–251
- Amsden B., Turner N. Biotechnol. Bioeng. 1999; 65: 605–610
- Saslawski O., Weigarten C., Benoit J.P., Couvreur P. Life Sci. 1988; 42: 1521
- Sultzbaugh K.J., Speaker T.J. J. Microencaps. 1996; 13: 363–375
- Cho N.-H., Seong S.-Y., Chun K.-H., Kim Y.-H., Kwon I.C., Ahn B.-Y., Jeong S.Y. J. Contr. Rel. 1998; 53: 215–224
- Mrsny R.J., Daugherty A.L., Short S.M., Widmer R., Siegel M.W., Keller G.-A. J. Drug Target. 1996; 4: 233–243
- Brissová M., Lacík I., Powers A.C., Anilkumar A.V., Wang T. J. Biomed. Mater. Res. 1998; 39: 61–70
- Liu L.-S., Liu S.-Q., Ng S.Y., Froix M., Ohno T., Heller J. J. Contr. Rel. 1997; 43: 65–74
- Quong D., Neufeld R.J. J.Microencaps. 1999; 16: 573–585
- Yoshikawa Y., Komuta Y., Nishihara T., Itoh Y., Yoshikawa H., Takada K. J. Drug Target. 1999; 6: 449–461
- Mandel K.G., Daggy D.A., Brodie D.A., Jacoby H.I. Aliment. Pharmacol. Ther. 2000; 14: 669–690
- Katayama H., Nishimura T., Ochi S., Tsuruta Y., Yamazaka Y., Shibata K., Yoshitomi H. Biol. Pharm. Bull. 1999; 22: 55–60
- Kannon G.A., Garrett A.B. Dermatol. Surg. 1995; 21: 583–590
- Cohen S., Lobel E., Trevgoda A., Peled Y. J. Contr. Rel. 1997; 44: 201–208
- Hartmann V., Keipert S. Pharmazie 2000; 55: 440–443
- Kaneko K., Kanada K., Yamada T., Miyagi M., Saito N., Ozeki T., Yuasa H., Kanaya Y. Chem. Pharm. Bull. 1997; 45: 1063–1068