Abstract
The aim of this work is to study the temporary variation of oxidative stress in renal transplants, both in plasma and in erythrocytes (CR). In order to do so, we determined total glutathione (GST) levels, both oxidized (GSSG) and reduced (GSH), and the activity of enzymes, glutathione peroxidase (G-px), glutathione reductase (G-red) and glutathione transferase (GSt), in renal transplant patients. Determinations were made 48 h before the transplant 1 week and 2 weeks after the renal transplant. The results obtained confirm a high “oxidative stress” rate, resulting from the equilibrium between the production of free radicals and the activity of antioxidants, the former being higher proportionally. Immediately after the transplant there is an increase of oxidative stress, which results in an increase of G-red, a marked decrease of G-px in plasma and in erythrocytes (CR) and an abrupt drop both in GST levels in plasma and in GSG (as well as in the [GSH]/[GSSG] relationship). As times goes on, after the transplant, there is a significant improvement in the activity of antioxidant enzymes, but there is no normalization, which is easily seen in the fact that total glutathione levels and the activity of the various enzymes approach the average values of the control group.
INTRODUCTION
Free radicals are molecules or molecular fragments that contain one or more unpaired electrons in atomic orbitals or molecules that provide a great chemical reactivity, which also accounts for the great number of harmful effects. The most relevant oxygen free radicals are O2 superoxide ion, HO hydroxide ion, and peroxide ion.
The three radicals are molecules that intervene in the cellular respiratory process, where oxygen is reduced to water in a series of uni-electronic stages, by cytochrome oxidase.
Glutathione is a special molecule that participates in a great variety of metabolic processes, transport and detoxification. Its special characteristics facilitate its participation in many biological activities, among them, protection against free radicals damage.
It may be found as reduced glutathione (GSH) and oxidized glutathione (GSSG), and the proportion between both species shows the capacity the cell has to cope with oxidative attacks.
Their physiological functions are very varied: regulating protein synthesis, modifying enzymatic activity and protection against oxidative damage.
Glutathione peroxidases (G-pxs) are cellular enzymes that catalyze hydroperoxides decomposition, reducing them to stable forms, hydroxides, using GSH specifically as electrons provider.
Plasmatic G-px is thought to be synthesized directly by nephron, with the kidney as the main source of production.
Although it has not been completely demonstrated, a protective function has been attributed to this plasmatic form, against lipoprotein peroxidation, principally because of the reduction of phosphatidalcholine.Citation[[1]] A local mechanism has also been attributed to it that prevents the damage of the glomerulus basal membrane, caused by free radicals, similar to other dump molecules, principally polyanionic glycosaminoglycans. Its activity seems to reflect the amount of enzymes synthesized by the kidney, as an index, therefore, of renal function.Citation[[2]] This activity is negatively related to renal damage.
The relationship between [GSH]/[GSSG] in normal red blood corpuscles keeps high near 10.Citation[[3]] This suggests the existence of a mechanism that reduces GSSS to GSH.
The enzymes that carry out this function are glutathione-reductase enzymes.
Glutathione reductase (G-red) catalyses the reduction of an equivalent of GSSG to GSH,Citation[[4]] by using NADPH resulting from phosphate pentose. The main function of enzymes belonging to the family of glutathione-S-transferaseCitation[[5]], Citation[[6]], Citation[[7]] is to protect cells against potential toxicity of lipid hydroperoxides.
Patients suffering from chronic renal failure show a high oxidative stress.Citation[[8]] This work aimed at establishing how this stress is modified with renal transplant.
MATERIAL AND METHODS
A total of 10 patients were studied, before their renal transplant, 48 h after it, 1 week and 2 weeks after the transplant, and a control group that consisted of 20 healthy men and women.
In order to determine the enzymes, the red cells were separated from plasma by means of centrifugation at 3.000 r.p.m. Plasma was collected in Eppendorf for further studies and cells were washed three times with a NaCl isotonic solution. These cells were softened by adding 0.1 mL of them and 0.9 mL of distilled water. Finally, in order to withdraw the membranes, they were centrifuged at 11.000 r.p.m. for 5 min.
G-px activity that depends on Selenium both in plasma and in red cells was determined by using the spectrophotometric method suggested by Paglia and Valentine.Citation[[9]]
We report on the decrease in absorbance at 340 nm due to NADPH oxidation, in the presence of an excess of G-red, with the following coupled reaction:
G-reductase catalyses the reduction of oxidized glutathione by using NADPH as a co-substrate.
The activity of this enzyme may be determined by measuring the decrease in the concentration of HADPH by means of a spectrophotometer, since this molecule absorbs 340 nm and is related to the concentration through the law of Lambert–Beer:
GSt catalyze a great number of reactions in which glutathione's thiolate anion participates as a nucleophiles. Then by using an electrophile substrate liable to vary (increase or decrease) its absorbance in the presence of GSH (substrate of the enzyme under study), we will be able to know the activity of the enzyme present in the sample.
CDNB is chosen as electrophiles in Habig and Jacoby's method,Citation[[10]] (1-chloro-2-4-dinitrobenzene), since this species in the presence of glutathione absorbs at 340 nm.
The definition of glutathione transferase (GSt) unit is the quantity resulting from the combination of 1 µmol of CDNB with GSH in just 1 min, while pH = 6.5 and the temperature is 25°C.
This study will be carried out measuring the increase of absorbance at 340 nm every 10 s, for 3 min (maximum), choosing afterwards the minute where the variation is more linear. This value will be (A/min) sample.
For glutathione, blood is collected in tubes with heparin and homogenized in acid medium, adding one volume of perchloric acid (6%) equal to the one in blood, which provokes a precipitation of proteins. It is centrifuged at 3.000 r.p.m. and the precipitate is rejected. Floating is centrifuged in Eppendorf at 11.000 r.p.m. Ellman's method is used for glutathione determination; it is a clinical trial to record the formation of 2-nitro-5-benzoic acid.Citation[[11]]
Total glutathione GST and GSSG are determined and by the difference we calculate the GSH. Oxidized glutathione determination is based on the reduction of HADPH by means of the reaction catalyzed by G-red. NADPH absorbs without interference at 340 nm, which is why we can observe its disappearance all through the reaction.
In order to determine GST, the whole glutathione must be in the form of GSH, which is achieved by the previously mentioned reaction. After this ditiobisnitrobenzoic acid (DTNB) is added, which is easily reduced because of GSH, resulting in a kind of chromophore (TNB+2), which absorbs at 412 nm.
RESULTS
Urea and creatinine plasmatic figures 2 weeks after the renal transplant () show the normalization of renal function. All values recorded may be found in .
Table 1. Urea and Creatinin Values Two Weeks After the Transplant
Table 2. Average Values ± Standard Deviation of All Determinations Carried Out
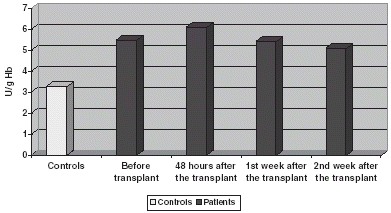
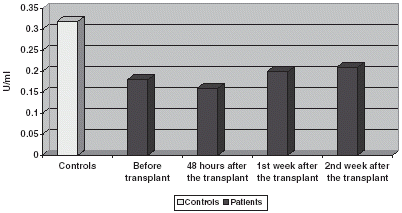
GST and GSH levels are significantly very reduced in relation to controls, decreasing slightly 48 h later and experimenting an increase on the 1st and 2nd week after it. ().
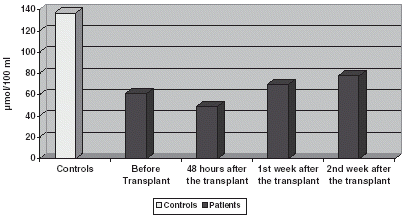
GSSG figures are slightly decreased before the transplant, without any significant modification during the study () GSH/GSSG relationship is significantly decreased, increasing on the 1st and 2nd weeks ().
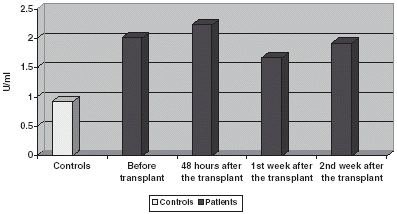
GSt is significantly increased if compared with controls both in plasma and blood corpuscles, experimenting an increase 48 h after the transplant to return to prior to transplant values on the 1st and 2nd week after it ( and ).
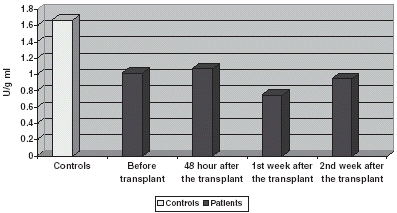
G-red values are reduced compared with controls, decreasing in plasma () during the 1st and 2nd weeks, having a slight modification in blood corpuscles ().
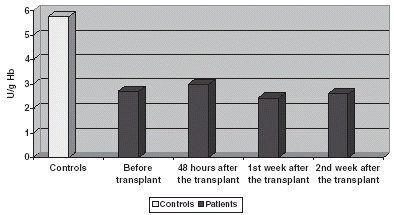
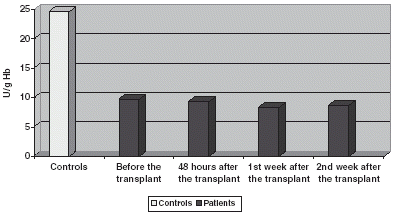
There is a decrease in G-px compared with controls both in plasma and in blood corpuscles, keeping a low profile all the time ( and ). In Plasma there is a slight increase on the 1st and 2nd week ().
DISCUSSION
The results obtained confirm a high “oxidative stress” rate, resulting from the equilibrium between the production of free radicals and the activity of antioxidants, the former being higher proportionally. It has been proved that there is a super-production of oxidants and a weakened defensive activity against them at the same time. This increase of oxidative stress and the role of free radicals in chronic renal patients have already been described in other studies.Citation[[1]], Citation[[8]], Citation[[12]]
After the transplant there is an increase of oxidative stress, which results in an increase of G-red, a strong decrease of G-px in plasma and in CR and an abrupt drop both in GST levels and in GSH (as well as in the [GSH]/[GSSG] relationship). Generally speaking there is a significant improvement in the activity of antioxidant enzymes, but there is no normalization, observing that, as times goes on, glutathione levels and the activity of the enzymes involved approach the average values of the control group.
Lipid peroxidation is the principal damage caused by free radical. Therefore, malondialdehyde (MDA), one of the final products in the peroxidation process, is a clear guide to calculate the amount of free radicals. It was the goal of a previous studyCitation[[13]] to measure MDA levels, proving to be higher in renal transplant patients from the 1st week after the transplant onwards. Other authorsCitation[[14]] show a high lipid peroxidation in transplanted patients.
GST and GSH levels proved to be significantly lower in patients than in control groups, which might be due to several factors, such as: a high oxidative stress rate that provokes a decrease in the levels of GSH; a failure in GSH regeneration. The G-red enzyme whose activity is lower in transplanted patients carries out the latter. GSH eliminates H2O2 and other peroxides through a reaction catalyzed by G-px, turning into GSSG. The uncontrolled appearance of peroxides in the erythrocyte causes a premature lysis in the cell. Therefore, the integrity of the membrane in the erythrocyte requires a great supply of reduced glutathione, or an increase in the active excretion of GSSG. Besides regenerating GSH, red cells are prevented from an intracellular accumulation of GSSG due to a process of active excretion that may account for the decrease of GST levels.
The relationship of [GSH]/[GSSG] decreases in transplanted patients compared with control groups. This means that, both the regeneration capacity of GSH and the store against an oxidative attack decrease. Oxidative stress is very high 48 h after the transplant. The storage in cold conditions and the ischaemia suffered by the kidney favor the stress state.
Total glutathione increases after the 1st and 2nd week. This may suggest that there is a decrease of oxidative stress and that the regeneration capacity of GSH is re-established by G-red, which in spite of being smaller in controls also increases in the long term.
A lower activity has been reported in G-px enzyme in transplanted patients compared with the control group. It has proved to be much lower 48 h after the transplant. This suggests a blockage due to products resulting from the attack of free radicals such as malondialdehyde, indolacetic acid, indoxyl-sulphate or a decrease in the levels of selenium (Se), since Se is a part of the active centre of G-px, and therefore, a deficiency in it will lead to a loss in the activity of the enzyme.
Both the concentration of reducing molecules with low molecular weight (glutathione), and the activity of antioxidant enzymes (G-px), are lower than in controls, which would mean that the defensive system against oxidation has been weakened in these patients.
The decrease observed in the activity of G-red seems to bear out the hypothesis that these patients have the pentose route blocked.Citation[[4]] GSH regenerates by the action of G-red, requiring NADPH to work, which comes from the pentose route. The enzyme that catalyses the first stage in this route is glucose-6-phosphate-deshydrogenase, whose activity is regulated by the concentration of NADP+.
When the cell takes up NADPH, the NADP+ concentration increases and there is an increase in the speed of the reaction catalyzed by G-6-P-dehydrogenase, stimulating then the production of NADPH to regenerate GSH. Therefore, the decrease of the G-red activity and the GSH levels proves a clear correlation between the two.
There is an increase in the activity of enzyme GSt, unlike the former enzymes, before the transplant compared with the control group and it is even higher 48 h after the transplant. This increase in the activity probably takes place to counteract the oxidative attacks, having its peak value 48 h later, coinciding with the maximum oxidative stress. Besides, it should also be noticed that its activity decreases as time goes on, which may be explained by the fact that it does not have to counteract such a high oxidative stress as immediately after the transplant.
We have observed that the percentage of GSSG is higher after the transplant than before it and the same happens with activity of G-px, 1 or 2 weeks after it. This is logical since G-px eliminates the peroxides resulting from “oxidative stress” caused by the transplant, at the expense of oxidizing GSH into GSSG. Therefore, the correlation between the two parameters is evident. This suggests using these parameters – [GSH], [GST], G-px activity – to evaluate renal damage, which is also backed up by other authors.Citation[[2]] The activity of G-px in plasma shows a significant correlation with plasmatic levels of creatinine and urea.
The increase in the activity of G-px 1 or 2 weeks after the transplant, mainly in plasma, may be due to the fact that the kidney is the highest synthesis source of this activity.
REFERENCES
- Halliwell; Gutteridge M.C. Free Radicals in Biology and Medicine, 2nd. Ed. Karger Basel 1998
- Schiavon G.L., Biasioli S., De Fanti E., Targa L. Plasma Glutathione Peroxidase Activity as an Index of Renal Function. Eur. J. Chem. Clin. Biochem. 1994; 32: 759–765
- Kosolver N.S., Em Kosolver. The Glutathione Status of Cells. Intl. Rev. Cytol. 1978; 54: 109–115
- López Barea J., Bárcena J.A., Bocanegra J.A., Florindo J. Glutathione Metabolism and Physiological Functions. Press, Boca Ratón, FL 1990; vol. 1: 105–116
- Lawrence R.A., Burk R.F. Biochem. Biophys. Commun. 1996; 71: 952–1050
- Dipolock A.T. Antioxidant Enzymes. Biochem. J. 1985; 227: 823–832
- Pierce S., Tappel A.L. Glutathione Metabolism Biochem. Biophys. Acta 1978; 523: 27–34
- Martín-Mateo M.C., Sánchez Portugal M., Iglesias S., De Paula A., Bustamante J. Oxidative Stress in Chronic Renal Failure. Renal Failure 1999; 21: 155–167
- Paglia, Valentine W.N. Studies on the Quantitative and Analytical Characterization of Erythrocyte Glutathione Peroxidase. J. Lab. Clin. Med. 1967; 70: 159–163
- Habig W.H., Jacoby W.B. Assays for the Differentiation of Glutathione-S-Transferase. Enzymol. 1981; 77: 398–405
- Tiezte E. Glutathione Determination. Anal. Biochem. 1969; 27: 502–510
- Ziegler D.M. Oxidative stress in renal failure. Ann. Rev. Biochem. 1985; 54: 305–309
- DaVenport A., Hopton M., Bolton C. Measurement of Malondialdehyde as a Marker of Oxygen Free Radical Production During Allograft Transplantation and the Effect on Easily Graph Function. Clin. Trasplant 1995; 9: 171–175
- Sherke R.L., Balasubramaniam K.A., John G.T., Jacob C.K., Shastry J.C. Free Radical Injury in Human Renal Allograft Rejection. Indian J. Med. Res. 1994; 1000: 70–72
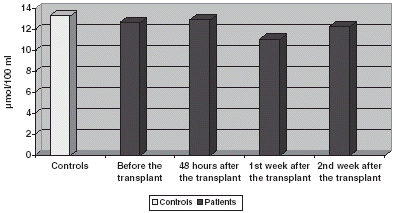
![Figure 3. Comparison of the value of [GSH]/[GSSG] relationship.](/cms/asset/10bfebaa-64f3-491b-90ca-446842a303d0/irnf_a_11379050_uf0003_b.gif)