Abstract
Introduction: For the long-term monitoring of kidney function, polytraumatized patients were examined and routine as well as specialized parameters were compared. Materials and methods: 30 patients of the Surgical Intensive Care Unit (ICU) were examined daily over the entire period they stayed in the ICU. The patients were retrospectively classified as either survivors or deceased patients. Group 1 consisted of 20 patients who resided in the ICU for 11–15 (Median 14) days before they could be transferred to a normal hospital unit. Group 2 consisted of 10 patients who had passed away after 13–18 (Median 16) days in the ICU. In addition to the routine parameters diuresis, serum creatinine and serum urea, specialized parameters for kidney function including the excretion rates of α1-microglobulin (α1-MG), N-Acetyl-β-d-glucosaminidase (NAG), angiotensinase A (ATA) and immunoglobulin G (IgG) were determined. Results: Similar biometric data were shown by all patients at admission into the ICU, but differences did exist regarding the Revised Trauma Score, Injury Severity Score and the APACHE-II-Score. In the period between the 5th and 8th day of intensive treatment almost all patients showed pathological excretion rates of tubular and glomerular parameters whereby no increased frequency of unusual events could be determined at these time-points. Conclusion: During treatment in the ICU, all examined patients showed at times pathological excretion rates of specialized kidney function parameters. Such transient damage was only apparent in a few of the patients when the standard parameters serum creatinine and serum urea were employed. In 90% of the surviving patients the kidney parameters had normalized until the time they were transferred, indicating that such parameters reflected the general state of health of these patients.
INTRODUCTION
Amongst the problems which patients develop after severe trauma include an increased frequency of organ failure in the lungs, the liver, and last but not least the kidney; such events are brought about by hypoxia, hemodynamic alterations or infections. Acute renal failure (ARF) still remains a frequent complication, which entails severe consequences for intensive-care patients. While solitary renal failure is treatable and the anuric period can be overcome comparatively well, the mortality of ARF is increased during multiple organ failure.Citation[[1]], Citation[[2]] Limited renal function is identified primarily by measuring creatinine clearance, diuresis and the serum concentrations of creatinine and urea. These parameters, however, only become pathological during the later course of a kidney disease, at a time when the noxious agent (poisons, hypoxia, microembolism) is not necessarily active anymore. Post-traumatic intensive care treatment occurs in phases with many patients. After surviving the acute phase, organ complications start to appear after different periods of time.
This study was designed to study renal function in surviving and deceased patients during these phases by long term monitoring with the aid of sensitive parameters.
MATERIALS AND METHODS
In total, 30 patients of the Surgical Intensive Care Unit were examined after approval by the local ethical committee. The patients were divided retrospectively into 2 groups. Twenty patients survived the intensive phase while 10 passed away despite the application of a maximal intensive therapy.
All patients showed no clinical or laboratory signs of any renal functional insufficiency at admission to the intensive care unit (ICU). Furthermore, any history of renal damage was excluded by employing contrast media.
The spectrum of parameters examined included diuresis and the serum concentrations of creatinine and urea. α1-microglobulin (α1-MG) was employed as a specific parameter for tubular function, while N-acetyl-β-d-glucosaminidase (NAG) served as a specific marker for lysosomal structures. Immunoglobulin G (IgG) and angiotensinase A (ATA) were recorded as specific markers for glomerular function.
With all patients, the Revised Trauma Score and Injury Severity Scores were assessed at admission into the clinic.Citation[[3]], Citation[[4]], Citation[[5]] All patients were examined daily in the ICU at predetermined time-points. The medication and ventilation therapy were also documented, and the APACHE-II-score was measured. The plasma and urine samples were withdrawn from arterial or indwelling urine catheters, respectively. The withdrawn urine samples were centrifuged for 10 min at 3000 rpm in a Hettich Rotixa/KS centrifuge. Samples were then separated into aliquots and either frozen at −20°C (NAG) or stored at 4°C for a maximum of 7 days (ATA, α-1-MG, IgG).
Serum Creatinine and Urea
Serum concentrations of creatinine were measured using an autoanalyzer (Model 30 R, WAKO Chemicals, Neuss, Germany). Measurement of urea in the serum samples was performed using a kinetic ultraviolet assay.
1-Microglobulin (α1-MG) and Immunoglobulin G (IgG)
Urinary concentrations of α1-MG and IgG were assessed by immunonephelometry (Behring nephelometer analyzer, Marburg, Germany).Citation[[6]], Citation[[7]] The proteins to be determined form immune complexes with their corresponding antisera. The amount of light scattered by an immune complex from a primary beam of light determines the concentration of the immune complex.
N-Acetyl-β-d-Glucosaminidase (NAG)
3-cresolsulfonephthaleinyl-N-acetyl-β-d-glucosaminide is hydrolytically cleaved by N-acetyl-β-d-glucosaminidase (NAG) so that 3-cresolsulfonephthalein is released which is then measured photometrically at 580 nm. Addition of sodium carbonate arrests the reaction.Citation[[8]]
Angiotensinase A (ATA)
A spectrophotometric color test was used for determining urinary ATA. l-α-glutamyl-4-nitroanilide and H2O are cleaved by angiotensinase A into 4-nitroaniline and glutamic acid and the nitroaniline, which arise are measured spectrophotometrically at 405 nm.Citation[[9]]
Statistics
Data are expressed as means ± standard deviations. Inter-group comparisons of patient characteristics, hemodynamic data etc. were performed using one-way analysis of variance. For inter- and intra-group comparisons of laboratory data, two-way repeated measured analysis of variance and post-hoc tests were used. Probability value less than 0.05 was considered significant.
RESULTS
Patients, Scores, and Treatment Regimes
The patterns of injury amongst patients in both groups were comparable, although there were differences concerning the Revised Trauma Score and the Injury Severity Score (). Regarding the biometric data, the hemodynamic parameters MAP, CVP as well as the APACHE-II-Scores were also comparable at admission to the ICU (). None of the patients investigated required hemofiltration or hemodialysis.
Table 1. Patients' Biometric Data, Hemodynamics, Score Values, Operation, and Trans-fusion Data
Diuresis, Creatinine, and Urea
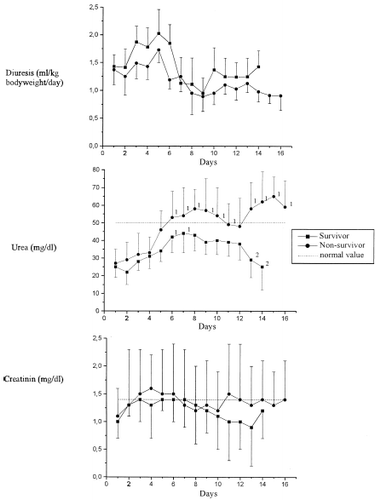
Normal diuretic rates were shown at admission for all patients, and these decreased between the 7th and 8th days in the ICU for both groups (). These values normalized again at transfer of the surviving patients, while in those who died the values had remained clearly reduced. Plasma creatinine and urea concentrations were within the normal range at admission for all patients: Maximum levels were achieved between the 4th and 8th day of care, whereby normalization was only seen amongst the survivors ().
1-Microglobulin and N-Acetyl-β-d-glucosaminidase (NAG)
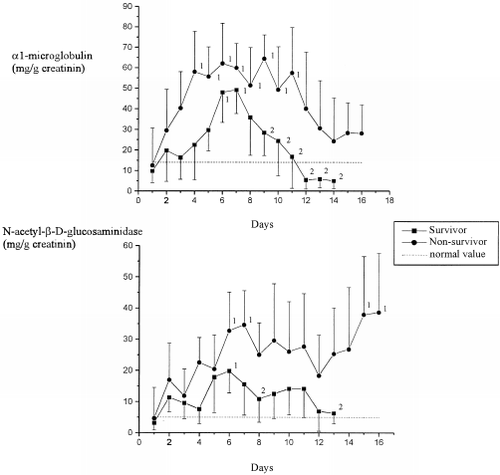
Amongst 10% of all the patients examined there were increased elimination rates of the tubular marker α1-MG and NAG at admission (). All examined patients showed raised excretion rates at some time during the intensive medical care. The maximum elimination rate was seen between the 5th and 7th days of care in each of the groups. While the excretion rate had normalized in the survivors by the time they were transferred to a normal ward, these levels remained pathological in the deceased patients.
Immunoglobulin G (IgG) and Angiotensinase A (ATA)
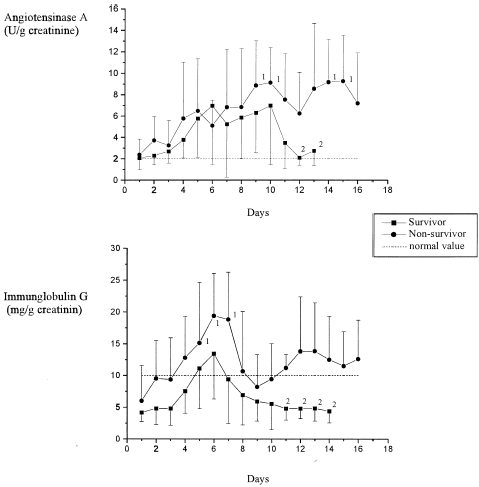
In 90% of the examined patients normal elimination rates were found at admission to the ICU for the glomerular markers IgG and ATA (). With IgG, the maximum elimination rate in both groups was between the 5th and 7th days. These values had already normalized in the survivors several days before discharge from the ICU; amongst the deceased patients the excretion rates remained pathological. Unlike IgG, the maximum elimination rate of ATA was seen on the 9th day for both groups. Here as well, elimination rates normalized only amongst the survivors, while in the deceased they remained pathological.
DISCUSSION
Acute renal failure is a potentially fatal complication amongst polytraumatized patients. The large proportion of deceased patients (10 of 30 patients, 33%) we found was within reported ranges of mortality for operated intensive-care patients.Citation[[1]], Citation[[2]], Citation[[10]] The severity of the illness was also illustrated by the APACHE-II scores (). This score was developed as a means to evaluate the severity of disease and in this way represents a satisfactory parameter for comparing the groups. At admission to the ICU, the APACHE-II score as well as the hemodynamic parameters AP and CVP were comparable for both groups.
The standard parameters for investigating renal function in clinical routine are diuresis and the serum concentrations of creatinine and urea. Diuresis revealed no significant group differences during the stay in the ICU – even when diuretics were applied.Citation[[11]] The still adequate diuresis and the fact that urea concentrations did not increase excessively rendered a hemofiltration unnecessary during the study period.
While serum creatinine concentrations were divergent for both groups at the late stage of treatment, no significant differences could actually be found due to the large inter-individual deviations. This result allows us to conclude that the glomerular filtration rate was not severely reduced.
Plasma urea concentrations varied significantly over time and differed significantly between the two groups from the 6th and 7th days of treatment. Here it was not the change in kidney function, but rather the metabolic state and the activation of the renin–angiotensin–aldosterone system which played the major roles in determining the post-aggression phase (systemic inflammatory response syndrome, SIRS) involving the increased accumulation of urea.Citation[[12]], Citation[[13]], Citation[[14]]
The sharp increase in the excretion rates of α1-MG and NAG already at the start of ICU treatment betrayed the early appearance of tubular damage. Such results confirm the early increases in urinary levels of this parameter described elsewhere in the literature.Citation[[15]], Citation[[16]] Here, the trauma itself plays a major role with fluid losses and hypotonic phases. The raised excretion rates following on from the 5th to 7th days of treatment must have been due to intensive care measures such as controlled ventilation with PEEP, fluid substitution, or the administration of blood derivatives, colloids or potentially toxic medications.Citation[[17]], Citation[[18]] This applied particularly to those patients who later passed away and who also revealed glomerular damage as shown by the increased excretion rate of IgG and ATA. The high molecular weight protein IgG is a physiological marker of glomerular integrity, and ATA is a glycoprotein, which is particularly prevalent in the glomerular endothelium.Citation[[9]] The maximum IgG elimination occurred simultaneously with the tubular damage, while the maximum ATA elimination occurred some 4 days later. This result shows that the raised permeability towards large molecules such as IgG (molecular weight 150 kD) occurs earlier than the actual damage to the cellular structures (endothelium).Citation[[19]], Citation[[20]], Citation[[21]]
While serum creatinine and creatinine clearance only reach pathological levels when there is an organ loss of at least 50% of the GFR, little can be said regarding the appearance of individual pathological protein and enzyme fractions in the urine of operated intensive-care patients.Citation[[22]] Numerous investigations have confirmed the informative value of enzymes and proteins for specific situations such as kidney transplant rejection, drug over-dosing or intoxications.Citation[[21]], Citation[[23]], Citation[[24]], Citation[[25]], Citation[[26]] However, only a few reports have appeared regarding the etiology of renal dysfunction in operated intensive-care patients and the frequency distribution of its possible cause.Citation[[27]]
According to the literature, 80% of all cases of acute renal failure are caused by circulation-induced forms. However, a fully developed shock syndrome does not precede every case of renal failure. Vasoconstriction due to central involvement can already lead to ischemic kidney damage, while fully blown shock can actually be prevented by this. Here, the postoperative and posttraumatic statuses are worthy of special mention. The patients we examined were probably afflicted by circulation-induced renal dysfunction because the complete spectrum of renal structural damage was simultaneously found.
Thus, an increase in tubular markers in urine (α1-MG, NAG) was demonstrated at the same time as an increase in IgG and before a rise in ATA, but a clearly earlier and larger increase in those parameters was observed before any changes were seen regarding the plasma concentrations of creatinine and urea.Citation[[28]], Citation[[29]]
In conclusion it can be stated that the routine clinical parameters plasma creatinine and urea can contribute significantly to the diagnosis of renal dysfunctions when applied in combination but most patients with tubular and beginning glomerular damages would not be detected.
REFERENCES
- Byrick R.J., Rose D.K. Pathophysiology and Prevention of Acute Renal Failure: The Role of the Anesthetist. Can. J. Anesth. 1990; 37: 457–467
- Kellen M., Aronson S., Roizen M., Barnard J., Thisted R. Predictive and Diagnostic Tests of Renal Failure: A Review. Anesth. Analg. 1994; 78: 134–142
- Hannan E.L., Farrell L.S., Gorthy S.F. Predictors of Mortality in Adult Patients with Blunt Injuries in New York State: a Comparison of the Trauma and Injury Severity Score (TRISS) and the International Classification of Disease, Ninth Revision-Based Injury Severity Score (ICISS). J. Trauma 1999; 47: 8–14
- Bouillon B., Lefering R., Vorweg M., Tiling T., Neugebauer E., Troidl H. Trauma Score Systems: Cologne Validation Study. J. Trauma 1997; 4: 652–658
- Luk S.S., Jacobs L., Ciraulo D.L., Cortes V., Sable A., Cowell V.L. Outcome Assessment of Physiologic and Clinical Predictors of Survival in Patients After Traumatic Injury with a Trauma Score Less than 5. J. Trauma 1999; 1: 122–128
- Dati F., Lammers M. Immunochemical Methods for Determination of Urinary Proteins in Kidney Disease. Journal of the Federation of Clinical Chemistry 1989; 1: 68–77
- Sharif M.N., Kaushal R.D., Iyer P., Wilson T.W. Diltiazem Potentiates Angiotensin II-Mediated Renal Prostacyclin Synthesis. J. Cardiovasc. Pharmacol. 1992; 20: 638–642
- Simane Z.J. N-Acetyl-β-d-Glucosaminidase. Urinary Enzymes in Clinical and Experimental Medicine, K. Jung, H. Mattenheimer, U. Burchardt. Springer, Berlin 1992; 118–124
- Scherberich J.E. Aminopeptidase A (Angiotensinase A). Urinary Enzymes in Clinincal and Experimental Medicine, K. Jung, H. Mattenheimer, U. Burchardt. Springer, Berlin 1992; 116–124
- Sural S., Sharma R.K., Singhal M. Etiology, Prognosis, and Outcome of Post-Operative Acute Renal Failure. Ren. Fail. 2000; 1: 87–97
- Dixon B.S., Anderson R.J. Nonoliguric Acute Renal Failure. Am. J. Kidney Dis. 1985; 6: 71–80
- Reha W.C., Mangano F.A., Zeman R.K., Pahira J.J. Rhabdomyolysis: Need for High Index of Suspicion. Urology 1989; 34: 292–296
- Kehlet H. The Stress Response to Anesthesia and Surgery: Release Mechanisms and Modifying Factors. Clin. Anesthesiol 1984; 2: 315–339
- Hartl W.H., Jauch K.W. Post-Aggression Metabolism: Attempt at a Status Determination. Infusionsther Transfusionsmed 1994; 2: 30–40
- Rebel W., Bertsch T., Bode G. Enzymuria as an Indicator of Renal Pathomorphology. Urinary Enzymes, K. Jung, H. Mattenheimer, U. Burchardt. Springer, Berlin 1992; 43–72
- McAuley F.T., Simpson J.G., Thomson A.W., Whiting P.H. The Predictive Value of Enzymuria in Cyclosporin A-Induced Renal Toxicity in the Rat. Toxicol. Lett. 1986; 32: 163–169
- Dehne M.G., Mühling J., Sablotzki A., Sucke N., Dehne K.L., Hempelmann G. HES Does Not Directly Affect Renal Function in Patients Without Prior Renal Impairment. Journal of Clinical Anesthesia 2001; 13: 103–111
- Ferraris V.A., Propp M.E. Outcome in Critical Care Patients: a Multivariate Study. Crit. Care Med. 1992; 20: 967–976
- Scherberich J.E., Wolf G., Albers C., Nowack A., Stuckhardt C., Schoeppe W. Glomerular and Tubular Membrane Antigens Reflecting Cellular Adaptation in Human Renal Failure. Kidney Int. Suppl. 1989; 27: S38–S51
- Wolf G., Thaiss F., Scherberich J.E., Schoeppe W., Stahl R.A. Glomerular Angiotensinase A in the Rat: Increase of Enzyme Activity Following Renal Ablation. Kidney Int. 1990; 38: 862–868
- Scherberich J.E., Fischer A., Rautschka E., Kollath J., Riemann H. Nephrotoxicity of High and Low Osmolar Contrast Media: Case Control Studies Following Digital Subtraction Angiography in Potential Risk Patients. Fortschr Geb Röntgenstrahlen Nuklearmed Ergänzungsbd 1989; 128: 91–94
- Miller T.R., Anderson R.J., Linas S.L. Urinary Diagnostic Indices in Acute Renal Failure: A Prospective Study. Ann. Intern. Med. 1978; 89: 47–50
- Diener U., Knoll E., Ratge D., Langer B., Wisser H. Urinary Excretion of Alanine-Aminopeptidase and N-acetyl-beta-d-Glucosaminidase During Sequential Combination Chemotherapy. J. Clin. Chem. Clin. Biochem. 1982; 20: 615–619
- Pergande M., Jung K., Precht S., Fels L.M., Herbort C., Stolte H. Changed Excretion of Urinary Proteins and Enzymes by Chronic Exposure to Lead. Nephrol. Dial. Transplant 1994; 9: 613–618
- Jung K., Diego J., Strobelt V. Diagnostic Significance of Urinary Enzymes in Detecting Acute Rejection Crises in Renal Transplant Recipients Depending on Expression of Results Illustrated Through the Example of Alanine Aminopeptidase. Clin. Biochem. 1985; 18: 257–260
- Donadio C., Puccini R., Lucchesi A., Giordani R., Rizzo G. Urinary Excretion of Proteins and Tubular Enzymes in Renal Transplant Patients. Ren. Fail. 1998; 20: 707–715
- Wilkes B.M., Mailloux L.U. Acute Renal Failure: Pathogenesis and Prevention. Am. J. Med. 1986; 80: 1129–1136
- Itoh Y., Enomoto H., Takagi K., Kawai T. Clinical Usefulness of Serum Alpha 1-Microglobulin as a Sensitive Indicator for Renal Insufficiency. Nephron 1983; 33: 69–70
- Dehne M.G., Mühling J., Papke G., Nopens H., Kuntzsch U., Hempelmann G. Unrecognized Renal Damage in Critically Ill Patients. Ren. Fail. 1999; 21: 695–706