Abstract
Background. Accurate estimation of the Total Antioxidant Status (TAS) in the myoglobinuric acute renal failure (ARF) is necessary because its pathogenesis is believed to be mediated, at least in part, by the development of oxidative stress resulting from the generation of oxygen free radicals and reduced antioxidant defense system. The purpose of this study is to examine the TAS 24 and 72 h after glycerol injection in a model of myoglobinuric-ARF. Experimental Design. The study was conduced in 28 Sprague-Dawley rats. In group 1 (n = 7) rats were placed into individual metabolic cages and deprived of water during 24 h, afterwards an intramuscular injection of glycerol was administrated (50% vol/vol in sterile saline) 10 mg/100 g of body weight and 24 h later blood samples were collected for biochemical measurements (urea, creatinine, creatine-kinase, and TAS levels). In group 2 (n = 7), rats followed the same conditions than group 1 ones but blood samples were collected 72 h after glycerol injection. In groups 3 (n = 7) and 4 (n = 7) rats didn't receive glycerol injection, and blood samples were collected within 24 and 72 h respectively after they were placed into metabolic cages. Results. In groups 1 and 2 we observed a renal function decrease, with higher serum levels of urea and creatinine in group 2 (urea levels: 269 ± 16 mg/dL vs. 586 ± 147 mg/dL; p < 0.001. Creatinine levels: 2.8 ± 0.2 mg/dL vs. 5.8 ± 0.7 mg/dL; p<0.001). TAS levels in groups 2, 3, and 4 were similar, but in group 1 was significantly lower (group 1: 0.81 ± 0.2 mmol/L; group 2: 1.3 ± 0.1 mmol/L; group 3: 1.2 ± 0.3 mmol/L, and group 4: 1.2 ± 0.2 mmol/L; p<0.005). Conclusion. In the model of glycerol induced myoglobinuric-ARF we observed a decrease of serum TAS level within 24 h with spontaneous recuperation 72 h after.
Introduction
Rhabdomyolysis-induced acute renal failure (ARF) constitutes a social problem and not just a medical disease. War, terrorism, trauma, earthquake, and drug abuse remain the leading causes of this syndrome. Experimental myoglobinuric-ARF can be induced by glycerol injection in rats.Citation[[1]] Many hypothetical pathogenic mechanisms had been reported: (1). vasoconstriction, (2). reduction of glomerular filtrate due to tubular obstruction by myoglobin casts formation, and (3). Hem cytotoxicity on tubular cells cause of the persistence of myoglobinuric casts into the proximal tubule where iron is released from myoglobin and promotes free radicals formation and lipid peroxidation with tubular-cell necrosis.Citation[[2]] In this way, over the last years, there are increasing interest to elucidate the role of oxidative stress due to the formation of excessive amounts of free radicals (superoxide and hydroxyl radicals) and other oxygen metabolites (hydrogen peroxide and hypochlorous acid) in tubular cells after glycerol injection in the development of ARF.Citation[[3]], Citation[[4]], Citation[[5]], Citation[[6]] Abbul-Ezz et al.Citation[[7]] and YeungCitation[[8]] have demonstrated early depletion of intracellular antioxidants after glycerol administration, mainly glutathione, the most important intracellular antioxidant. On the other hand, different experimental studies have shown that hydroxyl radical scavengers and iron chelators provide a marked functional and histological renal protection in the glycerol ARF model.Citation[[2]], Citation[[3]], Citation[[7]], Citation[[9]] However, although laboratory observations indicate that oxidative stress is increased in myoglobinuric-ARF,Citation[[3]] total antioxidant status (TAS) and its evolution in this animal model has not been evaluated previously to our knowledge. The purpose of the present study was to examine serum TAS level and its evolution in the model of myoglobinuric-ARF.
Patients and Methods
Experiments were completed in 28 Sprague-Dawley rats weighing 200–300 g. Before inducing ARF, the animals were placed into individual metabolic cages for 24 h before any manipulation for their adaptation to metabolic cages; urine was collected daily. The rats were maintained on standard rat chow and were deprived of water, but not food, for 24 h. The animals were randomly assigned into four groups: Group 1 (n = 7), the rats were slightly sedated with diazepam before the administration of glycerol (50% vol/vol in sterile saline) 10 mg/100 g of body weight, half of the dose in each hind limb muscle, and then allowed free access to water; 24 h after glycerol injection, under anesthesia, blood samples were collected by cardiac puncture and then they were sacrificed through intracardiac pentobarbital sodium administration. In group 2 (n = 7), the rats stayed in similar conditions of group 1 ones, but their blood samples were collected 72 h after glycerol injection and being sacrificed thereafter. In group 3 (n = 7), the rats didn't receive glycerol, blood samples were collected by cardiac puncture and then they were sacrificed 24 h after being placed in individual metabolic cages. In group 4 (n = 7), the rats didn't receive glycerol, blood samples were collected and were sacrificed 72 h after they were placed in individual metabolic cages. Blood samples were immediately centrifugated and urea, creatinine, creatine kinase, and TAS levels were measured.
TAS was determined by ABTS• +commercialized kit (ABTS®. RANDOX United Kingdom) a well-established technique as described by Miller and Rice-Evans.Citation[[10]], Citation[[11]] Generation of the ABTS [2,2′-azinobis-3(-ethylbenzothialozine-6-sulfonic acid)] radical cation forms the basis of one of the spectrophotometric methods that have been applied for the measurement of TAS. ABTS• + assay is based on the activation of metmyoglobin with hydrogen peroxide in the presence of ABTS to produce the ABTS• + radical cation through interaction with the ferryl myoglobin radical in the presence or absence of antioxidants.Citation[[10]], Citation[[12]]
This reaction by ABTS• + has a relatively stable blue-green color and is measured at 600 nm. Antioxidants in the added sample cause suppression of this color production by a proportional manner to their concentration. For quantitative in vitro determination of TAS we used the Aeroset System (Abbot Cientifica SA) and Randox Reagents. Due to lability of chromogen and substrate, the reagents must be reconstituted the same day of the assay.
Statistical Analysis
Data are presented as mean values with the standard error of mean as the index of dispersion. Significance of the experimental results among animals was evaluated using the Student's t-test. For comparisons involving more than two groups ANOVA was applied. Pearson's correlation coefficient was used to express the correlation between variables. The differences were considered statistically significant when the p value was less than 0.05.
Results
The results of diuresis, renal function tests, and creatine-kinase serum level in the four groups are shown in . The creatine-kinase serum levels of groups 1 and 2 was significantly higher than those of the control groups 3 and 4 (118,849 ± 17,568 U/L in group 1, 1279 ± 1011 U/L in group 2, 77 ± 16 U/L in group 3, and 80 ± 15 U/L in group 4) (p<0.0001), and the serum creatine-kinase levels of the group 2 rats were significantly lower than those of the group 1 (p<0.001) (). The urinary flow showed significant reduction in groups 1 and 2 when compared with groups 3 and 4 (control groups) (group 1: 4 ± 2 mL/24 h, group 2: 5 ± 2 mL/24 h, group 3: 13 ± 3 mL/24 h and group 4: 12 ± 4 mL/24 h) (p<0.001). As expected, in groups 1 and 2 we observed an increase in serum urea and creatinine levels, with a higher serum urea in group 2 (586 ± 147 mg/dL vs. 269 ± 16 mg/dL in group 1; p<0.001), and higher serum creatinine levels in group 2 (5.8 ± 0.7 mg/dL vs. 2.8 ± 0.2 mg/dL in group 1; p<0.001) (). TAS in group 1 was lower than those ones of groups 2, 3, and 4 (group 1: 0.81 ± 0.12 mmol/L; group 2: 1.3 ± 0.1 mmol/L; group 3: 1.2 ± 0.3 mmol/L, and group 4: 1.2 ± 0.2 mmol/L, p < 0.005) (). We have not observed a correlation between TAS and serum creatinine levels (group 1: r = 0.106; group 2: r = −0.078; group 3: r = −0.188; group 4: r = −0.183).
Figure 1. The administration of glycerol injection in groups 1 and 2 induced rhabdomyolysis with severe increase of serum creatine-kinase (CK). The levels of CK in the group 2 were significantly lower than those of the group 1 (p<0.001). The CK in the groups 1 and 2 were significantly higher than those of the groups control (3 and 4) (p<0.0001).
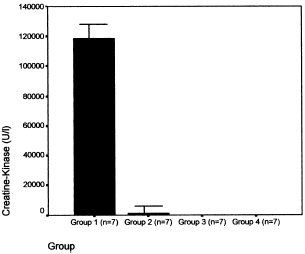
Figure 2. Serum urea (top panel) and serum creatinine (bottom panel) values in four groups. In groups 1 and 2 the glycerol injection induced severe acute renal failure (ARF) serum urea in groups 1 and 2 were higher than in groups control: 3 and 4 (p<0.0001), and urea were higher in group 2 than in group 1 (p<0,001). Serum creatinine in groups 1 and 2 were higher than in groups control: 3 and 4 (p<0.0001), and creatinine were higher in group 2 than in group 1 (p<0.001).
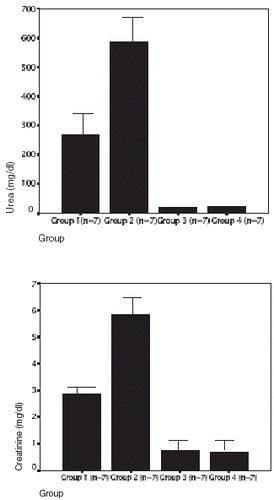
Figure 3. Total antioxidant status levels in group 1 was lower than in groups 2, 3, and 4 (p<0.005).
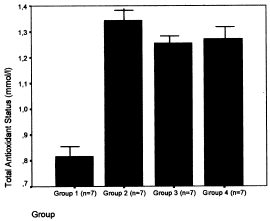
Table 1. General animal and laboratory data
Discussion
The natural defensive mechanisms implicated in the control of oxidative stress are constituted by many antioxidant components (glutathione peroxidase, superoxide dismutase, ferritin, hem oxygenase, ceruloplasmin, albumin, ascorbic acid, and calciferol among others). The antioxidant status could be estimated by measuring the status of each antioxidant or by measuring the general antioxidant capacity or TAS in serum.Citation[[13]] In the present study we observed a dramatic decrement of serum TAS level 24 h after glycerol injection but 72 h later these TAS levels were higher than in control rats although without statistically significant difference. Decrease of TAS level after 24 h is attributable to consumption of antioxidants employed in neutralize excessive free radical production in early stages of renal injury. These findings support previous observations demonstrating an increased production of free radicals within the first hours after glycerol-injection.Citation[[14]] On the other hand, we observed similar TAS levels 72 h after glycerol injection compared with control rats, suggesting a brief action of free radicals with rapid cessation in their production and posterior recuperation of antioxidant status. This recuperation could be attributed to an adaptive reaction to oxidative stress, although it doesn't seem to protect against progression to renal failure. Different models of renal injury such as glycerol, heavy metal, gentamicin, and some models of ischemia, can induce resistance to subsequent renal injury.Citation[[15]], Citation[[16]], Citation[[17]], Citation[[18]], Citation[[19]], Citation[[20]], Citation[[21]] Thus, previous reports by Anteby et al.,Citation[[15]] who convincingly demonstrated that rats previously uninephrectomized develop a lesser degree of renal failure after glycerol injection, and Backenroth et al.,Citation[[16]] who found that following the development of myoglobinuric-ARF, the kidney acquires resistance to further mercuric chloride nephrotoxicity 4 days and 2 weeks after glycerol administration; authors conclude that the degree of renal tubular damage correlates with the renal protection observed with iterative insults. To explain this observations, different mechanisms of protection have been considered in the literature, including changes in blood flow in various organs such as the kidney, heart, or liver with renin depletion,Citation[[17]] solute diuresis in remnant nephrons, loss of glomerular contractile response, changes in cell metabolism, decrease in sensitivity to toxic substances in new cells formed after the initial renal injury, or whether there is an induction of enzymes that neutralize toxins formed following the first renal injury with induction of antioxidant response.Citation[[17]], Citation[[18]], Citation[[22]], Citation[[23]], Citation[[24]], Citation[[25]] We wonder if the spontaneous recuperation of TAS 72 h after glycerol injection could contribute to the protection observed to subsequent renal injury.
The practical application of our results suggest that antioxidant treatment used in renal damage induced by glycerol injection only would be useful at early stages of the renal injury when a decrease of TAS levels develops, but not afterwards with TAS level normalization. In this way, in those studies which results show a protective effect of antioxidants on the myoglobinuric-ARF, antioxidant is administrated before or immediately after the glycerol injection,Citation[[2]], Citation[[3]], Citation[[7]], Citation[[9]], Citation[[14]], Citation[[26]], Citation[[27]], Citation[[28]] when the TAS level is decreased.
In conclusion, our results indicate that in this model of myoglobinuric-ARF, there is a decrease in TAS level with posterior spontaneous recuperation.
Acknowledgment
We thank to Dra. María Melero for translating the original manuscript into English. This work was supported by grants from Junta of Comunidades of Castilla-La Mancha. Consejeria of Sanidad. Centro Regional Salud Pública (98195).
References
- Ayer G., Grandchamp A., Wyler T., Truniger B. Intrarenal hemodynamic on glycerol induced myohemoglobinuric acute renal failure in the rat. Circ. Res. 1971; 29: 128–135
- Paller M.S. Hemoglobin and myoglobin induced acute renal failure: role of iron in nephrotoxicity. Am. J. Physiol. 1988; 255(Renal Fluid Electrolyte Physiol. 24)F539–F544
- Shah S.V., Walker P.D. Evidence suggesting a role for hydroxyl radical in glycerol-induced acute renal failure. Am. J. Physiol. 1988; 255(Renal Fluid Electrolyte Physiol. 24)F438–F443
- Guidet B., Shah S.V. Enhanced in vivo H2O2 generation by rat kidney in glycerol-induced renal failure. Am. J. Physiol. 1989; 257(Renal Fluid Electrolyte Physiol. 26)F440–F445
- Zager R.A., Foerder C.A. Effects of inorganic iron and myoglobin on in vitro proximal tubular lipid peroxidation and cytotoxicity. J. Clin. Invest. 1992; 89: 989–995
- Shah S.V., Walker P.D. Reactive oxygen metabolites in toxic acute renal failure. Ren. Fail. 1992; 14: 363–370
- Abbul-Ezz S.R., Walker P.D., Shah S. Role of glutathione in an model of myoglobinuric acute renal failure. Proc. Natl. Acad. Sci. 1991; 88: 9833–9837
- Yeung J.H. Effects of glycerol-induced acute renal failure on tissue glutathione and glutathione-dependent enzymes in the rat. Methods Find Exp. Clin. Pharmacol. 1991; 13: 23–28
- Zager R.A. Combined mannitol and deferoxamine therapy of myohemoglobinuric renal injury and oxidant tubular stress. Mechanistic and therapeutic implications. J. Clin. Invest. 1992; 90: 711–719
- Miller N.J., Rice-Evans C., Davies M.J., Gopinathan V., Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonatos. Clin. Sci. (Lond.) 1993; 84: 407–412
- Rice-Evans C., Miller N.J. Total antioxidant status in plasma and body fluids. Methods Enzymol. 1994; 234: 279–293
- Miller N.J., Rice-Evans C.A. Spectrophotometric determination of antioxidant activity. Redox Report 1996; 2: 161–171
- Rice-Evans C.A. Measurement of total antioxidant activity as a marker of antioxidant status in vivo: procedures and limitations. Free Radic. Res. 2000; 33(supl.)S59–S66
- Ishizuka S., Nagashima Y., Numata M., Yano T., Hagiwara K., Ozasa H., Sone M., Nihei H., Horikawa S. Regulation and immunohistochemical analysis of stress protein hem oxygenase-1 in rat kidney with myoglobinuric acute renal failure. Biochem. Biophys. Res. Commun. 1997; 240: 93–98
- Anteby E.Y., Wald H., Popovtzer M.M. Glycerol-induced acute renal failure in the rat: the protective effect of unilateral nephrectomy. Nephrol. Dial. Transplant. 1990; 5: 118–122
- Backenroth R., Schuger L., Wald H., Popovtzer M.M. Glycerol-induced acute renal failure attenuates subsequent HgCl2-associated nephrotoxicity: correlation of renal function and morphology. Ren. Fail. 1998; 20: 15–26
- Honda N., Hishida A., Ikuma K., Yonemura K. Acquired resistance to acute renal failure. Kidney Int. 1987; 31: 1233–1238
- Oken D.E., Mende C.W., Taraba I., Flamenbaum W. Resistance to acute renal failure afforded by prior renal failure: examination of the role of renal renin content. Nephron 1975; 15: 131–142
- Zager R.A., Baltes L.A., Sharma H.M., Jukowitz M.S. Responses of the ischemic acute renal failure kidney to additional ischemic events. Kidney Int. 1984; 26: 689–700
- Wilkes B.M., Hollenberg N.K. Protection against acute renal failure by prior acute renal failure: differences between myohemoglobinuric and ischemic models. Nephron 1987; 47: 220–226
- Hayes J.M., Boonshaft B., Maher J.F., O'Connell J.M.B., Schreiner G.E. Resistance to glycerol induced hemoglobinuric acute renal failure. Nephron 1970; 7: 155–164
- Ikuma K., Honda N., Hishida A., Nagese M. Loss of glomerular response to vasoconstrictor agents in rabbits recovering from ARF. Kidney Int. 1986; 30: 836–841
- Nath K.A., Balla G., Vercelloti G.M., Balla J., Jacob H.S., Levitt M.D. Rosemberg ME: induction of heme oxygenase is a rapid protective response in rhabdomyolysis in the rat. J. Clin. Invest. 1992; 90: 267–270
- Wilkes B.M., Caldicott W.J.H., Schulman G., Hollenberg N.K. Loss of the glomerular contractile response to angiotensin in rats following myohemoglobinuric acute renal failure. Circ. Res. 1981; 49: 1190–1195
- Zager R.A. Heme protein-induced tubular cytoresistance: expression at the plasma membrane level. Kidney Int. 1995; 47: 1336–1345
- Salhudeen A.K., Wang C., Bigler S.A., Dai Z., Tachikawa H. Synergistic renal protection by combining alkaline-diuresis with lipid peroxidation inhibitors in rhabdomyolysis: possible interaction between oxidant and nonoxidant mechanisms. Nephrol. Dial. Transplant. 1996; 11: 635–642
- Avramovic V., Vlahovic P., Mihailovic D., Stefanovic V. Protective effect of Bioflavonoid Proanthocyanidin-BPI in glycerol-induced acute renal failure in the rat: renal stereological study. Ren. Fail. 1999; 21: 627–634
- Stefanovic V., Savic V., Vlahovic P., Cvetkovic T., Najman S., Mitic-Zlatkovic M. Reversal of experimental myoglobinuric acute renal failure with bioflavonoids from seeds of grape. Ren. Fail. 2000; 22: 255–266