Abstract
Pentoxifylline (PTX) has been reported to inhibit TNF-α production and prevent several types of acute renal failure. This study was undertaken to determine the effect of PTX on the cisplatin-induced acute renal failure in rabbits. Rabbits received a single injection of cisplatin (5 mg/kg, i.p.) with or without PTX pretreatment (30 mg/kg, i.v.). Alterations in renal function, apoptotic cell death, and TNF-α mRNA expression were measured at 24 or 48 h after cisplatin injection. Cisplatin caused an increase in BUN and serum creatinine levels, a reduction in GFR, and an increase in fractional Na+ excretion. Such changes were significantly attenuated by PTX pretreatment (30 mg/kg, i.p.) 30 min before and 24 h after cisplatin injection. Morphological evaluation showed that cisplatin injection induced diffuse proximal tubular necrosis and the effect was reduced by PTX pretreatment. Cisplatin induced apoptotic cell death in renal cortex and the effect was significantly prevented by PTX. Treatment of opossum kidney cells with cisplatin resulted in cell death, which was significantly prevented by PTX. The increase in lipid peroxidation and the decrease in renal blood flow induced by cisplatin were not affected by PTX. The expression of TNF-α mRNA was increased after cisplatin injection and the effect was inhibited by PTX pretreatment. These results suggest that cisplatin-induced acute renal failure in rabbits is associated with an induction of TNF-α-mediated apoptosis, and that PTX may exert a protective effect against cisplatin nephrotoxicity by inhibiting TNF-α production.
Introduction
Cisplatin (cis-diamminedichloroplatinum II) is an important agent in the management of a variety of tumors, including cancers of the ovary, testis, bladder, head and neck, lung, cervix, and endometrium.Citation[[1]] However, its clinical usefulness is limited by the development of nephrotoxicity, a side effect that may be produced in various animal species.Citation[[2]], Citation[[3]] Although cisplatin has been employed as a model drug to induce experimentally nephrotoxicant-induced acute renal failure, the precise mechanism by which cisplatin induces renal failure remains unclear.
Tumor necrosis factor-α (TNF-α) is a cytokine with pleiotropic actions that participates in inflammation and immunity. Previous studies have demonstrated that the levels of TNF-α or its mRNA expression are increased in acute nephrosis induced by aminonucleosideCitation[[4]] and adriamycin,Citation[[5]] in glomerulonephritis,Citation[[6]], Citation[[7]] in diabetic nephropathy,Citation[[8]] and in endotoxin-induced acute renal failure.Citation[[9]] In vitro studies have shown that TNF-α production is increased in cultured rat and mouse mesangial cells exposed to adriamycin or puromycinCitation[[5]] and in LLC-PK1 cells subjected to simulated ischemia.Citation[[10]]
Pentoxifylline (PTX) is a methylxanthine derivative with multiple hemorheologic properties that has favorable effects on microcirculatory blood flow, but the precise mechanisms of its pharmacological action are not understood. Clinically, PTX has been used to treat intermittent claudication.Citation[[11]] In addition, PTX has been known to decrease serum TNF-α levelCitation[[8]], Citation[[12]] and TNF-α mRNA expression.Citation[[13]] PTX has been reported to exert protective effect against acute renal failure induced by mercury, glycerol, and cyclosporine.Citation[[14]], Citation[[15]] Whether it has a similar effect on the cisplatin-induced acute renal failure has not been extensively investigated.
This study was therefore undertaken to examine if TNF-α is involved in cisplatin-induced acute renal failure and PTX exerts the protective effect against the renal failure. The results suggested that PTX might prevent cisplatin nephrotoxicity by inhibiting TNF-α production.
Materials and Methods
In Vivo Nephrotoxicity Experiments
New Zealand White rabbits weighing 1.5–2.5 kg were housed in metabolic cages to collect urine. The animals were allowed 2 days to acclimate to the cages, and followed by a 24 h basal period, during which urine and blood samples were collected. Animals were divided into two groups: a cisplatin group and a cisplatin plus PTX group. Cisplatin group received a single intraperitoneal dose of cisplatin (5 mg/kg body wt.). Cisplatin plus PTX group received PTX (30 mg/kg, i.v.) 30 min before and 24 h after the cisplatin injection. Individual 24 h urine samples were collected for 24 and 48 h after the cisplatin injection and blood samples were taken from ear artery.
Analysis and Calculation
Urine and blood samples were analyzed for creatinine, blood urea nitrogen (BUN) (COBAS MIRA PLUS, Roche, Swiss), and Na+ (Automatic Electrolyte AMA, NOVA Biochemical, USA). Glomerular filtration rate (GFR) was estimated from the endogenous creatinine clearance.
Renal blood flow was measured in a renal artery of normal and cisplatin-treated animals (48 h after cisplatin injection) with a flowmeter (Transonic System Inc., NY).
Lipid Peroxidation Measurement
Lipid peroxidation was estimated by measuring the renal cortical content of malondialdehyde (MDA) according to the method of Ref.Citation[[16]] Renal cortical tissues were homogenized in ice-cold 1.15% KCl (5% wt/vol). A 0.5 mL aliquot of homogenate was mixed with 3 mL of 1% phosphoric acid and 1 mL of 0.6% thiobarbituric acid. The mixture was heated for 45 min on a boiling water bath. After addition of 4 mL of n-butanol the contents were vigorously vortexed and centrifuged at 2000 g for 20 min. The absorbance of the upper, organic layer was measured at 535 and 520 nm with a diode array spectrophotometer (Hewlett Packard 8452A, Germany), and compared with freshly prepared malondialdehyde tetraethylacetal standards. MDA values were expressed as pmoles per mg protein. Protein was measured by the method of Bradford.Citation[[17]]
Morphological Studies
Morphological evaluation of kidneys was performed 48 h after cisplatin injection. Kidneys were removed after renal artery perfusion with freshly prepared 1/2 Karnovsky solution. Following perfusion fixation, the samples were immersed in fixative for 4 h and processed for embedding in paraffin. Paraffin sections were stained with hematoxylin-eosin, examined, and photographed.
Measurement of Apoptotic Cells
Apoptotic cells were assessed with the terminal deoxynucleotidyl transferase (TdT) mediated fluorescein-dUTP nick-end labeling (TUNEL) technique. The kidney tissues were fixed in 4% neutral-buffered formalin solution (pH 7.4), dehydrated in graded alcohol, and embedded in paraffin. Thick sections (4 µM) were treated with 10 µg/mL proteinase K solution. Apoptosis in each section was quantified using an in situ Apoptosis Detection Kit, horseradish peroxidase (POD) (Boehringer Mannheim, Germany). After TUNEL staining, the cells were stained again with hematoxylin and analyzed under a light microscope (Olympus BX50, Japan). The number of TUNEL-positive cells was assessed in five randomly selected fields.
RNA Isolation and RT-PCR
Kidneys were isolated 48 h after cisplatin injection. Animals treated with lipopolysaccharide were used as a positive control. Kidneys were isolated 12 h after lipopolysaccharide injection (1 mg/kg, i.v.) with or without PTX pretreatment. Total RNA was isolated from rabbit kidney cortex, and reverse-transcribed with Moloney murine leukemia virus transcriptase (Promega) and oligo-dT primers. PCR was carried out using primers for β-actin and TNF-α in the following profile: 94°C 1 min, 52°C 1 min, 72°C 1 min for 30 cycles. PCR primers were designed from the rat cDNA sequences, as shown below:
Measurement of Cell Viability in Opossum Kidney (OK) Cells
OK cells were obtained from the American Type Culture Collection (Rockville, MD) and maintained by serial passages in 75-cm2 culture flasks (Costar, Cambridge, MA). The cells were grown in Dulbecco's modified Eagle medium/Ham's F12 (DMEM/F12, Sigma Chemical Co.) containing 10% fetal bovine serum at 37°C in 95% air/5% CO2 incubator. When the cultures reached confluence, subculture was prepared using a 0.02% EDTA-0.05% trypsin solution. The cells were grown on 24-well tissue culture plates in DMEM/F12 medium containing 10% fetal bovine serum. All experiments started 3–4 days after plating when a confluent monolayer culture was achieved. Cells were treated with 0.2 mM cisplatin for 24 h in Hank's balanced salt solution (HBSS) (Sigma Co. USA) containing 115 mM NaCl, 5 Mm KCl, 25 Mm NaHCO3, 2 mM NaH2PO4, 1 Mm MgSO4, 1 Mm CaCl2, and 5 mM glucose (pH 7.4) with or without 0.2 mM PTX.
Cell viability was estimated by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) reduction assay. After washing the cells, 500 µL of a medium containing 0.5 mg/mL of MTT in HBSS was added to each well. The cells were incubated for 60 min after which the overlying medium was removed and 110 µL of DMSO were added for 5 min. Absorbance at 550 nm was read for each well using a microplate reader (Molecular Devices Corp., Menlo Park, CA, USA). Data were expressed as a percentage of the control value, which was obtained in the absence of cisplatin.
Statistical Analysis
The data are expressed as means ± SEM and the difference between two groups was evaluated using Student's t-test. A probability level of 0.05 was used to establish significance.
Results
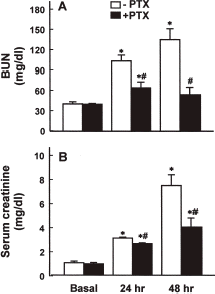
Effect of PTX Pretreatment on Renal Function in Rabbits with Cisplatin-Induced Acute Renal Failure
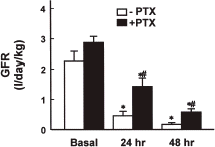
depicts changes in BUN and serum creatinine levels in the cisplatin and cisplatin plus PTX groups. The BUN level increased significantly 24 h after cisplatin injection and it increased further after 48 h. Similarly, the serum creatinine level 24 and 48 h after cisplatin injection was more than 3- and 7-fold greater than that observed in the basal period. The PTX pretreatment significantly attenuated the increase in BUN and serum creatinine by cisplatin injection. The PTX pretreatment also partially prevented the reduction in GFR caused by cisplatin injection (). The fractional Na+ excretion 48 h after cisplatin injection was approximately 20-fold of the basal value (10.54 ± 2.87 vs. 0.54 ± 0.06% in the basal period), which was significantly reduced by PTX (1.69 ± 0.64 vs. 0.56 ± 0.13% in the basal period).
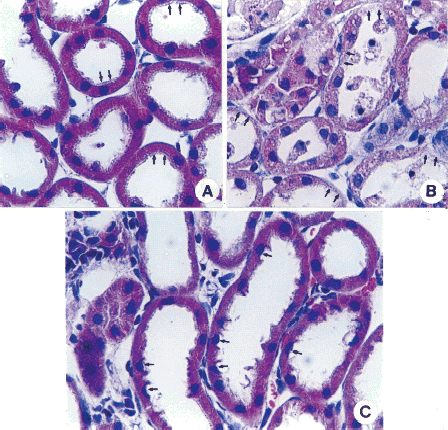
Effect of PTX on Morphological Changes
Morphological evaluation was done 48 h after cisplatin injection. A shows proximal tubules of the normal kidney with normal cytoplasm and nucleus, and regular appearance of brush border (arrows). In the kidney of cisplatin treated animals, diffuse tubular necrosis was evident in the proximal tubules (arrows) (B). The morphology of proximal tubular cells of PTX-pretreated animals was similar to that of the normal kidney, although in some cells supranuclear cytoplasm was lost (arrows) and the brush-borders was irregularly arranged (C).
Figure 3. Effect of pentoxifylline (PTX) pretreatment on renal cortical morphological changes in cisplatin-injected rabbits. The animal was sacrificed 48 h after cisplatin injection. Normal rabbits did not receive any drug. Samples were stained with hematoxylin-eosin. In normal kidneys (A), proximal tubules show regular appearance in brush border (arrows) with normal cytoplasm and nucleus. In the animal treated with cisplatin, diffuse tubular necrosis was present in proximal tubules (arrows) (Fig. B). The morphology of proximal tubular cells in the PTX-pretreated animals was similar to the normal kidney, although cells with the loss of supranuclear cytoplasm were present (arrows) and brush-borders were irregularly arranged (Fig. C). Magnitude × 132. (Go to www.dekker.com to view this figure in color.)
Effect of PTX on Apoptotic Cell Death
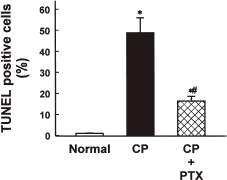
To quantify the degree of apoptosis in the kidney of cisplatin-injected rabbits, TUNEL assay was performed 48 h after cisplatin injection. As shown in , the number of apoptotic nuclei (brown color) was drastically increased by cisplatin injection (B), and the effect was significantly reduced by PTX pretreatment (C). The relative number of apoptotic cells was approximately 45% in animals treated with cisplatin alone and approximately 15% in animals treated with cisplatin after PTX pretreatment ().
Figure 4. Effect of pentoxifylline (PTX) pretreatment on apoptosis in the renal cortex in cisplatin-injected rabbits. Animals were sacrificed 48 h after cisplatin injection. Normal rabbits did not receive any drug. Apoptotic cells were evaluated by terminal deoxynucleotidyl transferase (TdT) mediated fluorescein-dUTP nick-end labeling (TUNEL) staining in the kidneys of normal (A), cisplatin-treated (B), and cisplatin + PTX-treated animals (C). (Go to www.dekker.com to view this figure in color.)
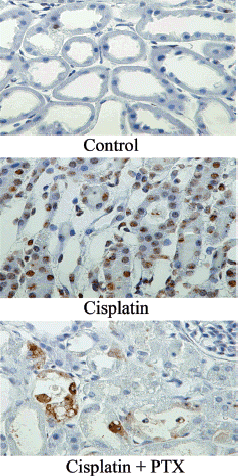
Figure 5. Quantitation of apoptotic cells in renal cortical tissues of normal, cisplatin (CP)-treated, and CP + pentoxifylline (PTX)-treated rabbits. Normal rabbits did not receive any drug. Data are means ± SEM of three animals in each group. *p<0.05 compared with the normal; #p<0.05 compared with CP alone.
Effect of PTX on Cisplatin-Induced Renal Cell Death In Vitro
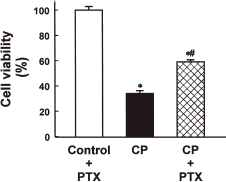
In order to examine if PTX exerts a protective effect against cisplatin nephrotoxicity in the in vitro system, OK cells, an established renal proximal epithelial cell line, were treated with 0.2 mM cisplatin for 24 h in the presence or absence of 0.2 mM PTX. As shown in , cisplatin caused a significant loss of OK cell viability and the effect was significantly prevented by PTX.
Figure 6. Effect of pentoxifylline (PTX) on the viability of normal and cisplatin (CP)-treated opossum kidney (OK) cells. Cells were treated with 0.2 mM CP for 24 h in the presence or absence of 0.2 mM pentoxifylline (PTX) and the viability was measured by MTT assay. Data are means ± SEM of five experiments. *p<0.05 compared with the control; #p<0.05 compared with CP alone.
Effect of PTX on Lipid Peroxidation and Renal Blood Flow
Since PTX inhibits the generation of reactive oxygen speciesCitation[[18]] and scavenges hydroxyl radicals,Citation[[19]] it was suspected that the protective effect of PTX against the cisplatin-induced acute renal failure is associated with an inhibition of lipid peroxidation. To test this possibility, the effect of PTX on the lipid peroxidation was examined in kidneys of cisplatin-treated animals. Cisplatin injection caused an increase in lipid peroxidation (337.04 ± 47.68 vs. 272.76 ± 27.42 pmol MDA/mg protein in normal), and the increase was not altered by PTX pretreatment (352.04 ± 21.97 pmol MDA/mg protein).
Since PTX is known to stimulate the biosynthesis of renal vasodilator prostaglandins,Citation[[20]], Citation[[21]] one may suspect that the protective effect of PTX might be attributed to a restoration of renal blood flow. However, the cisplatin-induced reduction in renal blood flow was not altered by PTX pretreatment. The renal blood flow 48 h after cisplatin injection with and without PTX pretreatment was 6.67 ± 0.50 and 7.30 ± 1.87 mL/min, respectively, which was approximately 34 and 37% of the normal (19.91 ± 1.34 mL/min), respectively.
Effect of PTX on TNF-α mRNA
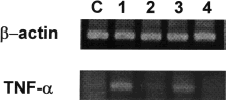
In the final series of experiments we determined the effect of PTX on TNF-α mRNA expression in cisplatin-treated animals. As shown in , the expression of TNF-α mRNA was increased by cisplatin injection and this was prevented by PTX pretreatment. As expected, lipopolysaccharide used as a positive control increased the expression of TNF-α mRNA, which was inhibited by PTX.
Figure 7. TNF-α mRNA expression in renal cortical tissues of rabbits treated with cisplatin, cisplatin + pentoxifylline (PTX), lipopolysaccharide (LPS), and LPS + PTX. Total RNAs were isolated from the kidney cortex of animals treated with cisplatin or lipopolysaccharide with or without PTX pretreatment. C, normal control; lane 1, cisplatin; lane 2, cisplatin plus PTX; lane 3, lipopolysaccharide; lane 4, lipopolysaccharide plus PTX.
Discussion
The present study demonstrated that PTX pretreatment provides a protective effect against cisplatin-induced acute renal failure. The increase in BUN and serum creatinine levels and the reduction in GFR induced by the cisplatin injection were significantly prevented by PTX pretreatment ( and ). The proximal tubular necrosis induced by cisplatin could also be largely prevented by PTX (). These results are partly consistent with those reported by Luke et al.Citation[[22]] observed that PTX produced a significant improvement in GFR in rats given 5 and 10 mg/kg of cisplatin, although it did not attenuate the morphological impairments.
The mechanisms by which PTX provided these effects are not entirely clear, but the effect on the proximal tubule may be associated, at least in part, with the prevention of apoptosis. Cisplatin injection resulted in a marked apoptosis, which was significantly suppressed by PTX pretreatment ( and ). In vitro studies employing mouse primary cultured proximal tubule cells have shown that a lower concentration of cisplatin results in apoptosis over several days, while a higher concentration results in necrosis within a few hours.Citation[[23]] In cisplatin-exposed animals apparent renal functional alterations are observed after 24 h of cisplatin exposure.Citation[[24]], Citation[[25]] In renal epithelial cells exposed to 0.2 mM cisplatin for 24 h, conditions in which apoptosis is induced,Citation[[26]] the cell viability was markedly impaired, and this was partly prevented by PTX (). These findings suggest that PTX may protect the proximal tubular structure and function in cisplatin-treated animals by suppressing the cisplatin-induced apoptosis. This conclusion is consistent with the notion that attenuation of cisplatin nephrotoxicity is closely associated with a reduction in apoptotic cell death.Citation[[27]]
Several studies have shown that reactive oxygen species are involved in the pathogenesis of cisplatin nephrotoxicity.Citation[[28]], Citation[[29]], Citation[[30]] Although PTX is known to inhibit the generation of leukocyte-derived reactive oxygen species in humans subjected to exerciseCitation[[18]] and to scavenge hydroxyl radicals in vitro,Citation[[19]] its effect on cisplatin nephrotoxicity in the present study may not be associated with its antioxidant action. Lipid peroxidation was elevated in renal tissues of the cisplatin-treated animals, reflecting an increased free radical production, and the effect was not altered by PTX pretreatment.
PTX stimulates the production of vasodilatory prostaglandinsCitation[[20]], Citation[[21]] and interacts with adenosine receptors,Citation[[31]] which have been known to increase the renal vascular resistanceCitation[[32]] and cisplatin causes a significant reduction in renal blood flow.Citation[[33]], Citation[[34]] Thus, the protective effect of PTX against the cisplatin-induced fall in GFR could be due to a restoration of renal blood flow. However, the present study showed that renal blood flow was markedly reduced following cisplatin treatment regardless of PTX pretreatment.
TNF-α, a potent proinflammatory cytokine capable of upregulating its own expression as well as the expression of other genes pivotal to the inflammatory response,Citation[[35]] induces apoptosis in renal tubular epithelial cells.Citation[[36]] Since cisplatin induces TNF-α production in various cell typesCitation[[37]], Citation[[38]], Citation[[39]] and PTX inhibits the production of TNF-α in animals and humansCitation[[12]], Citation[[40]] and suppresses TNF-α mRNA levels in vitro,Citation[[13]] we suspected that the effect of PTX on the cisplatin-induced renal failure is mediated by the change in TNF-α production. Indeed, in the present study TNF-α mRNA levels in the renal tissue was increased by cisplatin injection, and this was prevented by PTX pretreatment (). It is, therefore, likely that the prevention of cisplatin-induced apoptosis in PTX-pretreated animals in the present study was due to a suppression of TNF-α production.
In summary, the results of the present study indicated that PTX could prevent the renal cell injuries induced by cisplatin treatment in vivo and in vitro. This effect of PTX may be partly attributed to its ability to suppress apoptosis, but not to its abilities to inhibit lipid peroxidation or to stimulate vasodilator synthesis. PTX suppressed the TNF-α mRNA expression induced by cisplatin, implying that TNF-α plays an important role in the pathogenesis of cisplatin nephrotoxicity.
Acknowledgment
This work was supported by grant R01-2002-000-00460-0 (2002) from the Basic Research Program of the Korean Science & Engineering foundation.
References
- Inselmann G., Blomer A., Kottny W., Nellessen U., Hanel H., Heidemann H.T. Modification of cisplatin-induced renal p-aminohippurate uptake alteration and lipid peroxidation by thiols, Ginkgo biloba extract, deferoxamine and torbafylline. Nephron 1995; 70: 425–429
- Garnick M.B., Mayer R.J., Abelson H.T. Acute renal failure associated with cancer treatment. Acute Renal Failure, B.M. Brenner, J.M. Lazarus. Churchill Livingtone, New York 1988; 621–657
- Madias N.E., Harrington J.T. Platinum nephrotoxicity. Am. J. Med. 1978; 65: 307–314
- Diamond J.R., Pesek I. Glomerular tumor necrosis factor and interleukin 1 during acute aminonucleoside nephrosis. An immunohistochemical study. Lab. Invest. 1991; 64: 21–28
- Egido J., Gomez C.M., Ortiz A., Bustos C., Alonso J., Gomez G.C., Gomez G.D., Lopez A.M., Plaza J., Gonzalez E. Role of tumor necrosis factor-alpha in the pathogenesis of glomerular diseases. Kidney Int. 1993; 39: S59–S64
- Tipping P.G., Leong T.W., Holdsworth S.R. Tumor necrosis factor production by glomerular macrophages in anti-glomerular basement membrane glomerulonephritis in rabbits. Lab. Invest. 1991; 65: 272–279
- Mulligan M.S., Johnson K.J., Todd R.F., Issekutz T.B., Miyasaka M., Tamatani T., Smith C.W., Anderson D.C., Ward P.A. Requirements for leukocyte adhesion molecules in nephrotoxic nephritis. J. Clin. Invest. 1993; 91: 577–587
- Navarro J.F., Mora C., Rivero A., Gallego E., Chahin J., Macia M., Mendez M.L., Garcia J. Urinary protein excretion and serum tumor necrosis factor in diabetic patients with advanced renal failure: effects of pentoxifylline administration. Am. J. Kidney Dis. 1999; 33: 458–463
- Baud L., Ardaillou R. Tumor necrosis factor alpha in glomerular injury. Kidney Int. Suppl. 1994; 45: S32–S36
- Meldrum K.K., Meldrum D.R., Hile K.L., Yerkes E.B., Ayala A., Cain M.P., Rink R.C., Casale A.J., Kaefer M.A. p38 MAPK mediates renal tubular cell TNF-alpha production and TNF-alpha-dependent apoptosis during simulated ischemia. Am. J. Physiol. Cell Physiol. 2001; 281: C563–C570
- Gillings D.B. Pentoxifylline and intermittent claudication: review of clinical trials and cost-effectiveness analyses. J. Cardiovasc. Pharmacol. 1995; 25(suppl. 2)544–550
- Staudinger T., Presterl E., Graninger W., Locker G.J., Knapp S., Laczika K., Klappacher G., Stoiser B., Wagner A., Tesinsky P., Kordova H., Frass M. Influence of pentoxifylline on cytokine levels and inflammatory parameters in septic shock. Intensive Care Med. 1996; 22: 888–893
- Strieter R.M., Remick D.G., Ward P.A., Spengler R.N., Lynch J.P., Larrick J., Kunkel S.L. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem. Biophys. Res. Commun. 1998; 155: 1230–1236
- Brunner L.J., Vadiei K., Iyer L.V., Luke D.R. Prevention of cyclosporine-induced nephrotoxicity with pentoxifylline. Ren. Fail. 1989; 11: 97–104
- Vadiei K., Brunner L.J., Luke D.R. Effects of pentoxifylline in experimental acute renal failure. Kidney Int. 1989; 36: 466–470
- Uchiyama M., Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978; 86: 271–278
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976; 72: 248–254
- Ciuffetti G., Mercuri M., Ott C., Lombardini R., Paltriccia R., Lupattelli G., Santambrogio L., Mannarino E. Use of pentoxifylline as an inhibitor of free radical generation in peripheral vascular disease. Results of a double-blind placebo-controlled study. Eur. J. Clin. Pharmacol. 1991; 41: 511–515
- Franzini E., Sellak H., Hakim J., Pasquier C. Oxidative damage to lysozyme by the hydroxyl radical: comparative effects of scavengers. Biochim. Biophys. Acta. 1993; 1203: 11–17
- Sinzinger H. Pentoxifylline enhances formation of prostacyclin from rat vascular and renal tissue. Prostaglandins Leukot. Med. 1983; 12: 217–226
- Ward A., Clissold S.P. Pentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs 1987; 34: 50–97
- Luke D.R., Vadiei K., Lopez-Berestein G. Role of vascular congestion in cisplatin-induced acute renal failure in the rat. Nephrol. Dial. Transplant. 1992; 7: 1–7
- Lieberthal W., Triaca V., Levine J. Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: apoptosis vs. necrosis. Am. J. Physiol. 1996; 270: F700–F708
- Goldstein R.S., Noordewier B., Bond J.T., Hook J.B., Mayor G.H. cis-Dichlorodiammineplatinum nephrotoxicity: time course and dose response of renal functional impairment. Toxicol. Appl. Pharmacol. 1981; 60: 163–175
- Kim Y.K., Byun H.S., Kim Y.H., Woo J.S., Lee S.H. Effect of cisplatin on renal function in rabbits: mechanism of reduced glucose reabsorption. Toxicol. Appl. Pharmacol. 1995; 130: 19–26
- Lee R.H., Song J.M., Park M.Y., Kang S.K., Kim Y.K., Jung J.S. Cisplatin-induced apoptosis by translocation of endogenous Bax in mouse collecting duct cells. Biochem. Pharmacol. 2001; 62: 1013–1023
- Zhou H., Miyaji T., Kato A., Fujigaki Y., Sano K., Hishida A. Attenuation of cisplatin-induced acute renal failure is associated with less apoptotic cell death. J. Lab. Clin. Med. 1999; 134: 649–658
- Hannemann J., Baumann K. Cisplatin-induced lipid peroxidation and decrease of gluconeogenesis in rat kidney cortex: different effects of antioxidants and radical scavengers. Toxicology 1988; 51: 119–132
- Balde G.S., van den Hamer C.J., Los G., Vermeulen N.P., de Goeij J.J., McVie J.G. Selenium-induced protection against cis-diaminedichloroplatinum (II) nephrotoxicity in mice and rats. Cancer Res. 1989; 49: 3020–3023
- Basinger M.A., Jones M.M., Holscher M.A. l-Methionine antagonism of cis-platinum nephrotoxicity. Toxicol. Appl. Pharmacol. 1990; 103: 1–15
- Vadiei K., Brunner L.J., Luke D.R. Effects of pentoxifylline in experimental acute renal failure. Kidney Int. 1989; 36: 466–470
- Jackson E.K. Renal actions of purines. Purinergic Approaches in Experimental Therapeutics, K.A. Jackson, M.F. Jarvis. John Wiley & Sons, Inc., New York 1997; 217–250
- Agarwal A., Balla J., Alam J., Croatt A.J., Nath K.A. Induction of heme oxygenase in toxic renal injury: a protective role in cisplatin nephrotoxicity in the rat. Kidney Int. 1995; 48: 1298–1307
- Jeong J.C., Hwang W.M., Yoon C.H., Kim Y.K. Salviae radix extract prevents cisplatin-induced acute renal failure in rabbits. Nephron 2001; 88: 241–246
- Vilcek J., Lee T.H. Tumor necrosis factor. New insights into the molecular mechanisms of its multiple actions. J. Biol. Chem. 1991; 266: 7313–7316
- Peralta Soler A., Mullin J.M., Knudsen K.A., Marano C.W. Tissue remodeling during tumor necrosis factor-induced apoptosis in LLC-PK1 renal epithelial cells. Am. J. Physiol. 1996; 270: F869–F879
- Palma J.P., Aggarwal S.K. Cisplatin and carboplatin-mediated activation of murine peritoneal macrophages in vitro: production of interleukin-1 alpha and tumor necrosis factor-alpha. Anticancer Drugs 1995; 6: 311–316
- Okamoto M., Kasetani H., Kaji R., Goda H., Ohe G., Yoshida H., Sato M. cis-Diaminedichloroplatinum and 5-fluorouracil are potent inducers of the cytokines and natural killer cell activity in vivo and in vitro. Cancer Immunol. Immunother. 1998; 47: 233–241
- Riesbeck K. Cisplatin at clinically relevant concentrations enhances interleukin-2 synthesis by human primary blood lymphocytes. Anticancer Drugs 1999; 10: 219–227
- Loftis L.L., Meals E.A., English B.K. Differential effects of pentoxifylline and interleukin-10 on production of tumor necrosis factor and inducible nitric oxide synthase by murine macrophages. J. Infect. Dis. 1997; 175: 1008–1011