Abstract
Introduction. Volume overload is a main factor in development of hypertension in hemodialysis patients. In order to demonstrate impact of ultrafiltration volume on blood pressure during 15-months period in a group of patients undergoing chronic hemodialysis therapy, we conducted this study. We hypothesized that ultrafiltration volume different affects the pre/postdialysis systolic pressure, diastolic pressure, mean arterial pressure (MAP), and pulse pressure (PP) values. Subjects and Methods. Study subjects were 23 anuric chronically hemodialyzed patients. The overall study time was 15 months, and 136 single hemodialysis treatments were analyzed. Results. Ultrafiltration was negatively correlated with predialysis systolic blood pressure (r = −0.169, p = 0.025), postdialysis systolic blood pressure (r = −0.292, p<0.001), postdialysis MAP (r = −0.186, p = 0.015), predialysis PP (r = −0.290, p<0.001), and postdialysis PP (r = −0.370, p<0.001). Ultrafiltration/dry body mass (UF/W) ratio was negatively correlated with predialysis PP (r = −0.222, p = 0.005), postdialysis PP (r = −0.340, p<0.001), and postdialysis systolic blood pressure (r = −0.243, p = 0.002). We found significant difference in postdialysis PP between dialyses with UF/W ratio ≤0.05 and dialyses with UF/W ratio >0.05 (63.49 ± 20.76 vs. 56.27 ± 16.33 mmHg, p = 0.033). Conclusion. The ultrafiltration volume strongly affects postdialysis PP values. Evaluation of elevated blood pressure treatment in patients undergoing chronic hemodialysis therapy must be considered in respect of postdialysis PP values, not just depending on pre/postdialysis systolic and diastolic pressure or MAP values.
Introduction
Cardiovascular disease is the leading cause of morbidity and mortality in end-stage renal disease.Citation[[1]] Hypertension is common and contributes to the high cardiovascular morbidity in patients undergoing chronic hemodialysis therapy.Citation[[2]]
The impact of ultrafiltration and volume control on hemodialysis blood pressure is a well-known fact.Citation[[3]] Although hypertension in the hemodialysis patient population is multifactorial, volume overload is a main factor in the development of hypertension in hemodialysis patients.Citation[[4]]
Definition of hypertension in patients undergoing chronic hemodialysis therapy is still under debate. Predialysis systolic and diastolic blood pressures are of particular importance. Interdialytic ambulatory blood pressure monitoring may help to determine if the patient is in fact hypertensive.Citation[[5]] The mean arterial pressure (MAP) usually serves as expression of blood pressure in hemodialysis patients, instead solely systolic or diastolic blood pressure.
Investigations in the general population have demonstrated pulse pressure (systolic minus diastolic blood pressure) as a measure reflecting the pulsatile nature of the cardiac cycle.Citation[[6]] Recent recognition revealed pulse pressure (PP) as an important correlate of mortality in patients receiving dialysis.Citation[[7]]
In order to demonstrate impact of ultrafiltration volume on pre/postdialysis blood pressure (systolic and diastolic pressure, MAP and PP values) during 15-months period in a group of patients undergoing chronic hemodialysis therapy, we conducted this study. We hypothesized that ultrafiltration volume differently affects the pre/postdialysis systolic pressure, diastolic pressure, MAP and PP values.
Patients and Methods
Study subjects were 23 anuric chronically hemodialyzed patients (11 males and 12 females) on three-time-per-week dialysis regime, with history of chronic hemodialysis treatment for at least one year (mean hemodialysis treatment period was 5.09 ± 3.59 years). Fifteen subjects suffered from chronic glomerulonephritis, five from chronic pyelonephritis, and three subjects had diabetic nephropathy. Three subjects had insulin-dependent diabetes mellitus. Twenty subjects were treated with erythropoietin (mean weekly dose was 3622 ± 1856 IU). Clinical characteristic of the subjects are demonstrated in .
Table 1. Clinical characteristic of the subjects (n = 23) on the study beginning
Overall study time was 15 months. Outcomes were measured on the study beginning and at three months intervals; six measurements per subject were totally conducted.
Antihypertensive drugs used were ACE inhibitors (three subjects), calcium antagonists (two subjects), and beta blockers (two subjects).
Primary outcome measures for every single observed hemodialysis (HD) were: systolic and diastolic blood pressure before and after hemodialysis treatment (mmHg), and ultrafiltration (L).
Secondary outcome measures were predialysis and postdialysis mean arterial pressure (MAP) calculated as (systolic + 2diastolic blood pressure)/3 (mmHg), predialysis and postdialysis pulse pressure (PP) computed as (systolic–diastolic blood pressure) (mmHg), and ultrafiltration volume/dry body mass (UF/W) ratio (L/kg). Differences between predialysis and postdialysis systolic, diastolic, MAP, and PP values for every single observed hemodialysis were also calculated (delta values).
The duration of single hemodialysis treatment was 3.5–5 h, mean heparinization dose was 4950 ± 1282.17 IU. Temperature of the dialysis bath was 35.5–36.6°C, and the dialysis bath consisted of: bicarbonate 32–35, sodium 138–145, potassium 2, and calcium 1.25–1.75 mmol/L. The subjects were dialyzed on low flux polysulphone dialyzers F6 and F8 (Fresenius, Bad Homburg, Germany). All subjects had Cimino fistula as blood access.
No subject dropped out from the study. During the study period, medication and dialysis conditions for the same subject were unchanged. If the prescribed conditions during dialysis treatment or medication were changed, that dialysis session was not included in the study. Two dialysis processes dropped out, so 136 dialyses were included in the investigation.
Blood pressure was measured with mercury column sphygmomanometer by trained clinicians, just before beginning of the hemodialysis treatment and just after the end of the same dialysis treatment in recumbent subject. Disappearance of bruits (Korotkoff phase V) identified diastolic blood pressure. Ultrafiltration was measured volumetrically on the dialysis machine.
The results were expressed as arithmetic mean ± standard deviation. Normality of distribution was tested with the Shapiro-Wilk's W test. Differences between two dialysis groups were calculated by Student's t tests for dependent and independent data, respectively. Correlations between variables were tested by Pearson correlation coefficients and linear regression analysis.
A p value of less than 0.05 was considered statistically significant.
Results
Data from 136 single dialysis treatments were processed. Differences between predialysis vs. postdialysis systolic blood pressure, diastolic blood pressure, mean arterial pressure (MAP), and pulse pressure (PP) with significances are demonstrated on the .
Table 2. Differences between predialysis vs. postdialyisis systolic blood pressure, diastolic blood pressure, mean arterial pressure, and pulse pressure with significances (p) (Student t test for dependent data)
Pearson's correlations between all blood pressure parameters and ultrafiltration (UF) and ultrafiltration/dry body mass ratio (UF/W) were performed.
We found that UF was negatively correlated with predialysis systolic blood pressure (r = −0.169, p = 0.025), postdialysis systolic blood pressure (r = −0.292, p<0.001), postdialysis MAP (r = −0.186, p = 0.015), predialysis PP (r = −0.290, p<0.001), postdialysis PP (r = −0.370, p<0.001), and positively correlated with delta MAP (r = 0.17, p = 0.045). We found that UF/W ratio was negatively correlated with predialysis PP (r = −0.222, p = 0.005), postdialysis PP (r = −0.340, p<0.001), and postdialysis systolic blood pressure (r = −0.243, p = 0.002). Linear regression plot between postdialysis pulse pressure (PP) and ultrafiltration volume per hemodialysis is depicted on the .
Figure 1. Linear regression plot between postdialysis pulse pressure (PP) and ultrafiltration per hemodialysis (HD).
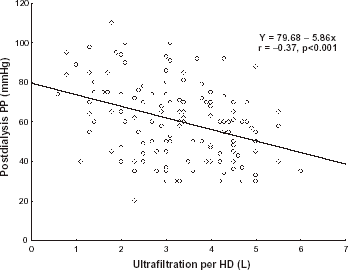
We also tested differences in blood pressure parameters (predialysis, postdialysis, and delta values of systolic pressure, diastolic pressure, MAP, and PP) between two groups of dialyses: dialyses with UF/W ratio ≤0.05 and dialyses with UF/W ratio >0.05. The value of UF/W ratio >0.05 means that total amount of ultrafiltration volume represents more than 5% of body mass. That percent defines high ultrafiltration rate hemodialysis.Citation[[8]] We found significant difference between dialyses with UF/W ratio ≤0.05 and dialyses with UF/W ratio >0.05 only in postdialysis PP (63.49 ± 20.76 vs. 56.27 ± 16.33 mmHg, p = 0.033, two-tailed).
Discussion
Arterial hypertension is frequent among chronically dialyzed patients. In dialysis patients the chronic fluid overload may represent a nonphysiologic condition, which brings both arterial hypertension and hemodynamic instability.Citation[[4]]
Our data confirmed our assumption of different influence of ultrafiltration volume on blood pressure parameters in dialysis patients. Changes in the ultrafiltration volume affected mostly systolic postdialysis blood pressure and consequently, pulse pressure (PP). We demonstrate the most significant influence of ultrafiltration volume on the postdialysis PP. Diastolic blood pressure was not affected with ultrafiltration volume, neither predialysis nor postdialysis.
Prakash et al.Citation[[9]] demonstrated positive correlation of volume overload with predialysis MAP. Ozkahya et al.Citation[[10]] displayed good blood pressure control (systolic and diastolic) with ultrafiltration, after cessation of antihypertensive drugs. On the contrary, Testa and PlouCitation[[11]] could not demonstrate influence of volume overload on systolic and diastolic blood pressure in patients undergoing chronic hemodialysis therapy. Luik et al.Citation[[12]] also concluded that interdialytic fluid load does not result in higher blood pressure (interdialytic blood pressure was measured with an ambulatory blood pressure monitor) in hemodialysis patients. Expansion of extracellular fluid volume is the major pathophysiologic mechanism for the development of hypertension in these patients; however, alterations in other humoral mechanisms (especially nitric oxide and endothelin 1 misbalance) also play a significant role.Citation[[13]], Citation[[14]]
Although various factors influence the pathogenesis of hypertension, volume overload is the most significant. The normalization of the patient hydration status is followed by a reduction in pressure values.Citation[[15]] Fluid removal is usually achieved by ultrafiltration to achieve a clinically derived value for “dry weight.”Citation[[16]] The achievement of an optimal dry weight is still one of the most difficult and important tasks of a dialysis clinic.Citation[[17]] Covic et al.Citation[[18]] concluded that 24-h blood pressure and blood pressure after dialysis are better related to total body water than blood pressure before dialysis, which was however weakly related to the acute volume overload, induced by interdialytic weight gain. In some hypertensive hemodialysis patients, blood pressure rises further during ultrafiltration. Cirit et al.Citation[[19]] concluded that the paradoxical blood pressure rise with ultrafiltration usually occurs in the presence of overhydration and cardiac dilatation and should be treated by intensified ultrafiltration. The explanation of this phenomenon remains speculative.
Finally, our findings support our assumption that amount of ultrafiltration volume affects different blood pressure clinical parameters. The ultrafiltration volume strongly affects postdialysis PP values. Treatment of arterial hypertension in dialyzed patients depends mostly on ultrafiltration volume removal and real dry weight achievement.Citation[[20]], Citation[[21]] Consequently, evaluation of elevated blood pressure treatment in patients undergoing chronic hemodialysis therapy must be considered in respect of postdialysis PP values, not just depending on pre/postdialysis systolic and diastolic pressure or MAP values. We recommend postdialysis PP values as a marker of ultrafiltration treatment and blood pressure control parameter.
References
- Locatelli F., Bommer J., London G.M., Martin-Malo A., Wanner C., Yaqoob M. Cardiovascular disease determinants in chronic renal failure: clinical approach and treatment. Nephrol. Dial. Transplant. 2001; 16: 459–468
- Rahman M., Smith M.C. Hypertension in hemodialysis patients. Curr. Hypertens. Rep. 2001; 3: 496–502
- Zucchelli P., Santoro A. Dry weight in hemodialysis: volemic control. Semin. Nephrol. 2001; 21: 286–290
- Fishbane S., Natke E., Maesaka J.K. Role of volume overload in dialysis-refractory hypertension. Am. J. Kidney Dis. 1996; 28: 257–261
- Horl M.P., Horl W.H. Hemodialysis-associated hypertension: pathophysiology and therapy. Am. J. Kidney Dis. 2002; 39: 227–244
- Franklin S.S., Sutton-Tyrrell K., Belle S.H., Weber M.A., Kuller L.H. The importance of pulsatile components of hypertension in predicting carotid stenosis in older adults. J. Hypertens. 1997; 15: 1143–1150
- Abdelfatah A.B., Motte G., Ducloux D., Chalopin J.M. Determinants of mean arterial pressure and pulse pressure in chronic haemodialysis patients. J. Hum. Hypertens. 2001; 15: 775–779
- Toz H., Ozerkan F., Unsal A., Soydas C., Mees E.J.D. Dilated uremic cardiomyopathy in a dialysis patient cured by persistent ultrafiltration. Am. J. Kidney Dis. 1998; 32: 664–668
- Prakash S., Reddan D., Heidenheim A.P., Kianfar C., Lindsay R.M. Central, peripheral, and other blood volume changes during hemodialysis. ASAIO J. 2002; 48: 379–382
- Ozkahya M., Toz H., Unsal A., Ozerkan F., Asci G., Gurgun C. Treatment of hypertension in dialysis patients by ultrafiltration: role of cardiac dilatation and time factor. Am. J. Kidney Dis. 1999; 34: 218–221
- Testa A., Plou A. Clinical determinants of interdialytic weight gain. J. Ren. Nutr. 2001; 11: 155–160
- Luik A.J., van Kuijk W.H., Spek J., de Heer F., van Bortel L.M., Schiffers P.M. Effects of hypervolemia on interdialytic hemodynamics and blood pressure control in hemodialysis patients. Am. J. Kidney Dis. 1997; 30: 466–474
- Erkan E., Devarajan P., Kaskel F. Role of nitric oxide, endothelin-1, and inflammatory cytokines in blood pressure regulation in hemodialysis patients. Am. J. Kidney Dis. 2002; 40: 76–81
- Raj D.S., Vincent B., Simpson K., Sato E., Jones K.L., Welbourne T.C. Hemodynamic changes during hemodialysis: role of nitric oxide and endothelin. Kidney Int. 2002; 61: 697–704
- Alvarez-Lara M.A., Martin-Malo A., Espinosa M., Rodriguez-Benot A., Aljama P. Blood pressure and body water distribution in chronic renal failure patients. Nephrol. Dial. Transplant. 2001; 16: 94–97
- Jaeger J.Q., Mehta R.L. Assessment of dry weight in hemodialysis: an overview. J. Am. Soc. Nephrol. 1999; 10: 392–403
- Perez-Garcia R., Lopez-Gomez J.M., Jofre R., Junco E., Valderrabano F. Haemodialysis dose, extracellular volume control and arterial hypertension. Nephrol. Dial. Transplant. 2001; 16: 98–101
- Covic A., Goldsmith D.J., Covic M. Reduced blood pressure diurnal variability as a risk factor for progressive left ventricular dilatation in hemodialysis patients. Am. J. Kidney Dis. 2000; 35: 617–623
- Cirit M., Akcicek F., Terzioglu E., Soydas C., Ok E., Ozbasli C.F. ‘Paradoxical’ rise in blood pressure during ultrafiltration in dialysis patients. Nephrol. Dial. Transplant. 1995; 10: 1417–1420
- Agarwal R., Lewis R.R. Prediction of hypertension in chronic hemodialysis patients. Kidney Int. 2001; 60: 1982–1989
- Mitra S., Chandna S.M., Farrington K. What is hypertension in chronic haemodialysis? The role of interdialytic blood pressure monitoring. Nephrol. Dial. Transplant. 1999; 14: 2915–2921