Abstract
Objective. (1) To evaluate the impact of body composition and gender on serum leptin concentration in hemodialysis patients. (2) To study which marker of adiposity is most appropriate in Taiwanese hemodialysis patients without diabetes. (3) To compare the nutrition status between nonlean and lean subjects. Patients and Methods. Serum leptin concentrations were measured by radioimmunoassay collected in 88 hemodialysis patients without diabetes. Bioimpedance analysis was performed to determine percent fat mass (%FM), lean body mass (LM), and total body water (TBW). Body mass index (BMI) was calculated as weight/height2. Albumin and transferrin were measured by standard laboratory methods. Results. Serum leptin levels were more correlated with percent fat mass (r = 0.697; P<0.001) than with body fat mass (r = 0.672; P<0.001) or with BMI (r = 0.594; P<0.001) in the group as a whole and in each subgroup when analyzed separately by gender. The mean (±SD) serum leptin levels were 32.5 ± 34.3 ng mL−1 in women subjects and 13.6 ± 15.5 ng mL−1 in men subjects (P<0.001). Multiple regression analysis in all subjects revealed that serum leptin levels were independently affected by percent fat mass and gender. Adiposity corrected serum leptin, such as leptin/BMI, leptin/percent fat mass, and leptin/body fat mass was significantly different between sexes (P<0.001). The significantly higher serum leptin concentrations in women than in men were observed in obese subjects with BMI >25 kg/m2 (P<0.001) as well as nonobese subjects with BMI<25 kg/m2 (P<0.05). There were no differences in lean mass and albumin between nonlean and lean subjects. Conclusion. Gender and adiposity had impact on serum leptin levels in hemodialysis patients without diabetes. In terms of adiposity, serum leptin levels had stronger correlation with percent fat mass than with body fat mass (FM) or BMI in Taiwanese hemodialysis patients. Steady-state serum leptin levels could serve as valuable clinical markers for the body adiposity in stable hemodialysis patients without diabetes. Protein malnutrition markers and lean mass should be checked in lean subjects for the evaluation of the protein stores of hemodialysis patients.
Introduction
Leptin, the 14–16 kDa protein product of the ob gene, serves as an endocrine function to regulate food intake, energy expenditure, and body fat stores.Citation[[1]], Citation[[2]], Citation[[3]] Serum leptin concentrations are significantly correlated with measurements of adiposity, such as body fat mass, percent fat mass, body mass index (BMI) in patients without chronic renal failure,Citation[[4]], Citation[[5]] in patients with chronic renal failure,Citation[[6]] in patients on hemodialysis,Citation[[7]], Citation[[8]], Citation[[9]] and in hemodialysis patients with diabetes.Citation[[10]] Gender-based differences in serum leptin levels are demonstrated in patients without chronic renal failureCitation[[4]], Citation[[11]], Citation[[12]] in hemodialysis patients with diabetesCitation[[10]] and in patients on hemodialysis.Citation[[13]]
Reports regarding sexual dimorphism in serum leptin levels in hemodialysis patients without diabetes were rare. We performed a cross sectional study in a group of 88 hemodialysis patients without diabetes, with the aim of studying the impact of body composition and gender on serum leptin levels. In the current study, we demonstrated that serum leptin levels had closer association with percent fat mass than with BMI or body fat mass. Gender-based differences in serum leptin level were significantly proved in hemodialysis patients without diabetes even after adjustment of percent fat mass through the methods of multiple linear regression and the ratio of leptin to measurement of fatness. We demonstrated that there was no significant difference in albumin and lean mass between nonlean and lean subjects.
Patients and Methods
Subjects
Eighty-eight hemodialysis patients without diabetes were investigated: 47 females and 41 male receiving three sessions of 4 h dialysis per week, using bicarbonate-buffered dialysate and high flux dialyzes with a minimum Kt/V of 1.0. The main characteristics of study population were shown on the . The causes of chronic renal failure were chronic glomerulonephritis (n = 34), obstructive nephropathy (n = 4), autosomal dominant polycystic kidney disease (n = 2), chronic interstitial renal disease (n = 27), hypertensive nephrosclerosis (n = 19), and lupus nephritis (n = 2). All subjects were without active medical or surgical illness within the previous 6 months, and none were receiving corticosteroids that could result in body mass depletion.
Table 1. Mean±standard deviation for patient characteristics and body composition
Study Protocol
Total body water (TBW), lean body mass (LM), and body fat mass were determined by bioelectric impedance (BEI) analyzer on two separate occasions two weeks apart 30 min following a hemodialysis session.Citation[[14]] LM was assessed by BEI using Deurenberg equation.Citation[[15]] Immediately after dialysis on the same day, height and weight were measured while the patients were wearing only hospital gowns. Body mass index (BMI) was defined as dry body weight (DBW) in kilograms divided by the square of the height in meters. Percent fat mass was calculated by the following equation: percent fat mass = (DBW − LM)/DBW × 100%.Citation[[38]] Ideal body weight was derived from the following equations: (Height − 80 cm) × 70% for male, and (height − 70 cm) × 60% for female. Based on WHO definition, overweight was defined as a body mass index larger than 23 kg/m2, obesity as a body mass index larger than 25 kg/m2, and underweight as a body mass index smaller than 18.5 kg/m2 in Asians.Citation[[16]], Citation[[17]], Citation[[18]] In view of percent fat mass, lean subjects were defined as patients with percent fat mass of 23.2% or smaller, and obese subjects were of 37.4% or larger.Citation[[4]] The dry weight of each patient was individually assessed according to the bioelectric impedance (BEI), the cardiothoracic ratio, and clinical observations such as pretibial edema, facial edema, pulmonary edema, and jugular vein pressure, or decrease in blood pressure during the hemodialysis session.
Assays
In all subjects, venous blood samples were collected in the morning after an overnight fast of 12 h before the beginning of hemodialysis. The serum biochemical parameters were measured by standard laboratory methods. After centrifugation, serum samples were immediately stored at −20°C until leptin was measured. Serum leptin levels by radioimmunoassay (RIA) were stable on repeated freezing and thawing, up to 10 cycles, and also during incubation at 4°C and at room temperature up to 8 days. At 37°C, leptin levels started to decline steadily after 24 h and almost disappeared after 8 days. The serum leptin level was determined by means of a commercially available human leptin RIA kit (Linco Research Inc., St. Charles, Mo, USA). The leptin assay is completely homologous because the antihuman leptin antiserum was raised in rabbits immunized with highly purified recombinant human leptin; 125I—labeled human leptin was used as tracer; and human leptin was used as standard. The lower limit of detection of the assay was 0.5 ng/mL, and the intra-assay and inter-assay coefficients of variation were 3.4–9.2% and 5.0–9.0% respectively. Duplicate analyses were performed on all samples, and the average value was used for data analysis.
Statistical Analysis
Quantitative results were reported as the mean ± SD (standard deviation). Simple linear regression and multiple linear regression analyses were performed to analyze the data. Pearson's correlation coefficients were used to determine whether a regression coefficient was significantly different from zero. Unpaired t tests were used to compare differences in clinical variables between sexes, and between nonlean and lean subjects. All probability (p) values are two tailed. A value of P<0.05 was taken to indicate statistical significance.
Results
Descriptive characteristics of the study patients were shown in . Because there was significant difference in body fat content between females and males, we adjusted the confounding factors that are measurements of adiposity. The ratio of leptin to measurement of body fatness, such as BMI, FM, and %FM, were significantly higher in females than in males (). Serum leptin correlated with body weight (r = 0.508; P<0.001), BMI (r = 0.679; P<0.001), body fat mass (r = 0.707; P<0.001), percent fat mass (r = 0.620; P<0.001) in male subjects. Also, serum leptin correlated with body weight (r = 0.614; P<0.001), BMI (r = 0.679; P<0.001), body fat mass (r = 0.707; P<0.001), percent fat mass (r = 0.620; P<0.001) in female subjects. Serum leptin concentrations were more strongly correlated with the percent fat mass (r = 0.697; P<0.001) than with BMI (r = 0.594; P<0.001) or with body fat mass (r = 0.672; P<0.001) in total subjects. Because the relationship between the serum leptin levels and body fatness was not linear, leptin was depicted as the natural logarithm of serum leptin. The log of serum leptin values also had stronger relationships with percent fat mass than with body mass index (BMI) or body fat mass (A–C). Serum leptin levels did not show significant correlation with age, length on hemodialysis of the patients, LM, TBW. summarized multiple regression analysis of factors independently affecting serum leptin levels. For all subjects (top), percent fat mass considered alone accounted for 45.2% of the variance in serum leptin values (see column labeled R2) and was the dominant predicting variable (P<0.001). Furthermore, adding gender to percent fat mass increased the explained variance to 55.3%, and gender was a significant determinant independent of percent fat mass (P<0.05). When analyzed separately in both men and women in multiple regression models, percent fat mass accounted for 52.1% (female) and 49.9% (male) of the variance in serum leptin values. The mean value of serum leptin levels between female and male (32.5 ± 34.3 vs. 13.6 ± 15.5) were significantly different (). The reported gender dichotomy in serum leptin concentrations also was preserved even analyzed separately by BMI (). In this figure, serum leptin level was 2.59 times (103.73/40.08) in obese females than that in obese male subjects (P<0.001). And the serum leptin level was 2.24 times (23.96/10.72) in thin females than that in thin male subjects (P<0.05). showed that nonlean subjects (female or male) had significantly higher DBW, BMI, FM, %FM, leptin, and transferrin than lean subjects. No significant differences were observed in LM, albumin, TBW, IBW, height, time on dialysis, and age between nonlean and lean subjects (female or male).
Figure 1. (A) The relationship (r2 = 0.8244; P<0.001) between percent body fat and the log of serum leptin in 88 hemodialysis patients without diabetes; (B) the relationship (r2 = 0.8172; P<0.001) between body fat mass and the log of serum leptin in the same patients; (C) the relationship (r2 = 0.5016; P<0.001) between body mass index and the log of serum leptin in the same patients.
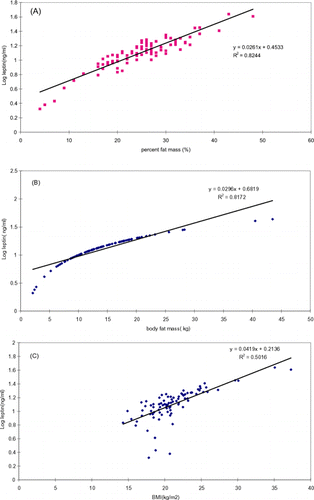
Figure 2. Gender-based difference in serum leptin. Leptin levels are shown for 79 subjects (42 females and 37 males) with BMI below 25 (P<0.05) and for 9 subjects (5 females and 4 males) with BMI of 25 or more (P<0.001). Difference between men and women were analyzed by student's two-tailed unpaired t-test.
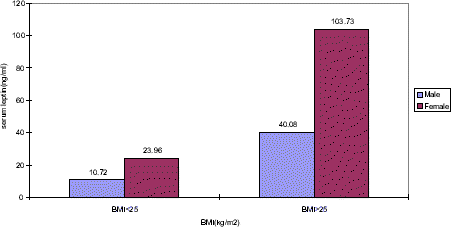
Table 2. Multiple regression analysis of factors affecting serum leptin levels and mathematical models for serum leptin
Table 3. Selected clinical parameters in lean and nonlean subjects of both genders (mean±standard deviation)
Discussion
Leptin, a 167-amino acid hormone, functions as a lipostat mechanism mainly through modulation of satiety signals and resting energy expenditure.Citation[[1]], Citation[[3]] Leptin receptor is coded by diabetic (db) locus, which belongs to class I cytokines receptor family.Citation[[2]], Citation[[3]] Leptin crosses the blood brain barrier via a saturable transport system and actions on the leptin receptor in the brain through mainly two signaling pathways, namely neuropeptide Y system and melanocortin-4 system.Citation[[1]], Citation[[19]]
In addition to overall adiposity and gender, other important factors operating to modulate the release of leptin and serum leptin concentrations include insulin concentration,Citation[[13]], Citation[[20]], Citation[[21]] insulin growth factor −1,Citation[[22]] renal function,Citation[[21]], Citation[[22]], Citation[[23]], Citation[[24]] age,Citation[[7]] corticoid,Citation[[25]], Citation[[26]] androgen,Citation[[27]] estradiol,Citation[[26]] short term fasting,Citation[[4]] dietary cholesterol,Citation[[4]] glucosamine,Citation[[28]] mean fat cell weight,Citation[[29]] steady state plasma glucose,Citation[[30]] skeletal muscle mass,Citation[[31]] defects of leptin in human obesity,Citation[[2]] modalities of dialysis therapy,Citation[[7]], Citation[[22]] types of artificial kidney,Citation[[9]], Citation[[32]] recombinant human erythropoietin therapy.Citation[[33]], Citation[[34]], Citation[[35]] Recent studies showed that the serum leptin levels are significantly higher in chronic hemodialysis patients as compared with normal healthy subjects with similar body fatness.Citation[[7]], Citation[[8]], Citation[[13]]
The main results of the present study indicates that (1) serum leptin levels were correlated with measures of adiposity, such as percent fat mass, body fat mass, and BMI; (2) serum leptin concentrations were more strongly related to percent fat mass than to body fat mass or BMI, and this difference became especially clear after logarithmic transformation of leptin values (); (3) serum leptin concentrations were higher in women than in men even after corrected for markers of adiposity, such as BMI, body fat mass and percent fat mass ( and ); (4) gender- based differences in serum leptin levels were significantly noted even after adjustment for adiposity (); (5) There was no difference in lean body mass and albumin between lean and nonlean subjects ().
We found that serum leptin concentrations varied more closely with percent fat mass (r = 0.697; P<0.001) than with BMI (r = 0.594; P<0.001), or body fat mass (r = 0.672; P<0.001). Although there were marginal differences, the relationship between body fatness and serum leptin levels in Taiwanese hemodialysis patients was best explained by a model, in which serum leptin level is determined primarily by the relative degree of adiposity (percent fat mass) rather than the body fat mass. In this way, a small increase in body fat mass in a slender individual will result in large change in percent fat mass. On the contrary, a large change in body fat mass in a very obese individual will result in only a small change in the parameter. Most of our study subjects (55.7%) were below the ideal body weight (49/88). Lean subjects (percent fat mass <23.2%) in the present study represented about 40% of the total subjects, and obese subjects (percent fat mass >37.4%) represented 3% (3/88) of the total subjects. Overweight subjects (BMI >23 kg/m2) was about 21.6% of total subjects (19/88); underweight subjects (BMI <18.5 kg/m2) was about 22.7% of total subjects (20/88); and normal-weight subjects was about 55.7% of total subjects (49/88). Our study subjects had a very low mean body mass index (21.1 ± 3.8). Furthermore, recent reports showed that subjects with smaller body build have higher percent fat mass at the same level of BMI.Citation[[36]], Citation[[37]] Thus, percent fat mass is a more sensitive marker of adiposity than BMI or body fat mass in Taiwanese hemodialysis patients without diabetes. For accuracy, we emphasized that percent fat mass should be determined by the same method.Citation[[38]]
In the current study, there were many evidences of gender-based differences in serum leptin concentrations. The mean leptin level in women (uncorrected for body fatness; 32.4 ± 34.3 vs. 13.6 ± 15.5) was about 2.4 times that in male (P<0.001). The adiposity corrected serum leptin concentrations, namely leptin/body fat mass, leptin/BMI, leptin/percent fat mass, were significantly higher in female than in men (). The results of multiple regression analysis revealed the gender differences in serum leptin levels endured after the influence of total body fat content was accounted for (). Likewise, the gender-based difference was similar in obese individuals with BMI of 25 or more and nonobese individuals with BMI below 25 ().
Recent reports confirmed that the gender-based difference in serum leptin levels could be explained by at least two different mechanisms: a larger adipose tissue mass and a higher production rate of leptin per unit mass of adipose tissue in women than in men.Citation[[39]] Several researchers showed that amount of subcutaneous fat depot from female, measured by sum of skinfold thickness, was greater than that from male.Citation[[12]], Citation[[40]] In the current study, female subjects had significantly higher mean value of percent fat mass than male subjects (). Adiposity corrected serum leptin levels, such as leptin/BMI, leptin/body fat mass, and leptin/percent fat mass, were significantly larger in females than in males (). The above data suggested that at all level of measures of adiposity, such as BMI, body fat mass, and percent fat mass, serum leptin concentrations were higher in women than in men.
In the present study, the rank order of leptin/body fat mass was postmenopausal females (2.04 ± 1.52 ng/mL/kg)>premenopausal females (1.77 ± 0.762 ng/mL/kg)> males (0.884 ± 0.655 ng/mL/kg). The leptin/body fat mass ratio of men was significantly lower than those of postmenopausal and premenopausal women. This finding is consistent with the role of androgen in the gender-based difference. Recent reports showed that there is inverse correlation between leptin concentrations and androgen in men.Citation[[41]], Citation[[42]], Citation[[43]] Wabitsch et al. demonstrated that in vitro human adipocyte tissue organ culture system; androgen had a suppressive effect on leptin production or expression in adipocytes.Citation[[43]] Recent reports identified that androgen receptor is found on adipocytes.Citation[[44]] These suggest a direct effect of androgen at the protein and mRNA level. In human experiments, Elbers et al. demonstrated that exogenous administration of cross sex hormones in male to female and female to male transsexuals induced a reversal of the sexual dimorphism in serum leptin levels over 4 months independent of the amount of body fat.Citation[[45]] In a separate study with hypogonadal men receiving androgen replacement, Jockenhovel et al. demonstrated that serum leptin levels and mRNA expression are significantly depressed.Citation[[46]]
Couillard et al. showed that there is a significant gender-based difference in mean fat cell weight (FCW) as women have higher mean adipose cell weight values compared with men. Furthermore, this scholar also demonstrated that mean fat cell weight is significantly associated with body fatness in both gender, and mean fat cell weight is more closely correlated to serum leptin concentration than the estimated fat cell number. This phenomenon may partly explain the gender difference in serum leptin levels.Citation[[29]]
In our three obese hemodialysis patients (percent fat mass >37.4%), serum leptin levels were not low, and they were as follows: 50.12, 136.2, 170 ng/mL. This suggested that most obese people were resistant to the endogenous production of leptin,Citation[[5]] rather than due to leptin deficiency.Citation[[3]]
From a biological point of view, land dwelling mammalian species store large quantities of energy-dense fuel in the form of adipose tissues triglyceride, which permits survival during prolonged period of food scarcity.Citation[[1]] In a variety of chronic and acute illnesses, protein depletion may occur and result in reduced immunity and muscle wasting when stored energy fuel is deficient. Because the stored fat can be used in face of hard times, such as infection, surgical complication, and brief period of fasting, it is wise to suggest chronic hemodialysis patients to maintain their dry body weight at about 110–115% of ideal body weight.Citation[[47]]
The results of the present study indicate the following conclusions. (1) Serum leptin levels in 88 chronic hemodialysis patients without diabetes were significantly correlated to gender after adjustment for percent fat mass; (2) under steady-state conditions, the serum leptin concentration could serve as a good marker for body fat content even separated by gender. Serial steady-state serum leptin concentrations may be added to the list of nutritional assessment of body fat content; (3) for patients with low leptin, markers of protein malnutrition, such as albumin, LM, should be checked to help evaluate the protein stores of chronic hemodialysis patientsCitation[[48]]; and (4) percent fat mass was the best predictors of leptin in Taiwanese hemodialysis patients.
References
- Friedman J.M. Leptin, leptin receptors, and the control of body weight. Nutr. Rev. 1998; 56(2)S38–S46
- Caro J.F., Sinha M.K., Kolaczynski J.W., Zhang P.L., Considine R.V. Leptin—the tale of an obesity gene. Diabetes 1996; 45: 455–1462
- Sharma K., Considine R.V. The Ob protein (leptin) and the kidney. Kidney Int. 1998; 53: 1483–1487
- Ostlund R.E., Jr., Yang J.W., Klein S., Gingerich R. Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J. Clin. Endocrinol. Metab. 1996; 81: 3909–3913
- Considine R.V., Sinha M.K., Heiman M.L., Kriaugiunas A., Stephens T.W., Nyce M.R., Ohannesian J.P., Marco C.C., McKee L.J., Bauer T.L., Caro J.F. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. New Engl. J. Med. 1996; 334: 292–295
- Heimburger O., Lonnqvist F., Danielsson A., Nordenstrom J., Stenvinkel P. Serum immunoreactive leptin concentration and its relation to the body fat content in chronic renal failure. J. Am. Soc. Nephrol. 1997; 8: 1423–1430
- Johansen K.L., Mulligan K., Tai V., Schambelan M. Leptin, body composition, and indices of malnutrition in patients on dialysis. J. Am. Soc. Nephrol. 1998; 9: 1080–1084
- Merabet E., Dagogo-Jack S., Coyne D.W., Klein S., Santiago J.V., Hmiel S.P., Landt M. Increased plasma leptin concentration in end-stage renal disease. J. Clin. Endocrinol. Metab. 1997; 82: 847–850
- Nakazono H., Nagake Y., Ichikawa H., Makino H. Serum leptin concentrations in patients on hemodialysis. Nephron 1998; 80: 35–40
- Haluzik M., Sulkova S., Svobodova J., Bedarova V., Bodlakova B., Markova M., Turkova G., Jiskra J., Haas T. Serum leptin levels in diabetic patients on hemodialysis: the relationship to parameters of diabetes metabolic control. Endocr. Res. 2000; 26(2)303–317
- Rosenbaum M., Nicolson M., Hirsch J., Heymsfield S.B., Gallagher D., Chu F., Leibel R.L. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J. Clin. Endocrinol. Metab. 1996; 81: 3424–3427
- Roemmich J.N., Clark P.A., Berr S.S., Mai V., Mantzoros C.S., Flier J.S., Weltman A., Rogol A.D. Gender differences in leptin levels during puberty are related to the subcutaneous fat depot and sex steroids. Am. Physiol. Soc. 1998; E543–E551
- Nishizawa Y., Shoji T., Tanaka S., Yamashita M., Morita A., Emoto M., Tabata T., Inoue T., Morii H. Plasma leptin level and its relationship with body composition in hemodialysis patients. Am. J. Kidney Dis. 1998; 31(4)655–661
- Chertow G.M., Lowrie E.G., Wilmore D.W., Gonzalez J., Lew N.L., Ling J., Leboff M.S., Gottlieb M.N., Huang W., Zebrowski B., College J., Lazarus J.M. Nutritional assessment with bioelectrical impedance analysis in maintenance hemodialysis patients. J. Am. Soc. Nephrol. 1995; 6: 75–81
- de Fijter W.M., de Fijter C.W.H., Oe P.L., ter Wee P.M., Donker A.J.M. Assessment of total body water and lean body mass from anthropometry, Watson formula, creatinine kinetics, and body electrical impedance compared with antipyrine kinetics in peritoneal dialysis patients. Nephrol. Dial. Transplant. 1997; 12: 151–156
- Wang J., Thornton J.C., Russell M., Burastero S., Heymsfield S., Pierson R.N., Jr. Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. Am. J. Clin. Nutr. 1994; 60: 23–28
- He M., Tan K.C.B., Li E.T.S., Kung A.W.C. Body fat determination by dual energy X-ray absorptionmetry and its relation to body mass index and waist circumference in Hong Kong Chinese. Int. J. Obesity 2001; 25: 748–752
- Steering Committee of the WHO Western Pacific Region, IASO & IOTF. The Asia-Pacific perspective: redefining obesity and its treatment, Australia, 2000
- Dallongeville J., Fruchart J.-C., Auwerx J. Leptin, a pleiotropic hormone: physiology, pharmacology, and strategies for discovery of leptin modulators. J. Med. Chem. 1998; 41(27)5337–5352
- Kolaczynski J.W., Nyce M.R., Considine R.V., Boden G., Nolan J.J., Henry R., Mudaliar S.R., Olefsky J., Caro J.F. Acute and chronic effect of insulin on leptin production in humans. Diabetes 1996; 45: 699–701
- Shoji T., Nishizawa Y., Emoto M., Maekawa K., Hiura Y., Tanaka S., Kawagishi T., Okuno Y., Morii H. Renal funtion and insulin resistance as determinants of plasma leptin levels in patients with NIDDM. Diabetologia 1997; 40: 676–679
- Fontan M.P., Carmona-Rodriguez A., Cordido F., Garcia-Buela J. Hyperleptinemia in uremic patients undergoing conservative management, peritoneal dialysis, and hemodialysis: a comparative analysis. Am. J. Kidney Dis. 1999; 34(5)824–831
- Meyer C., Robson D., Rackovsky N., Nadkarni V., Gerich J. Role of the kidney in human leptin metabolism. Am. J. Physiol. 1997; 273: E903–E907, (Endcrinol. Metal 36)
- Jensen M.D., Moller N., Nair K.S., Eisenberg P., Landt M., Klein S. Regional leptin kinetics in humans. Am. J. Clin. Nutr. 1999; 69: 18–21
- Williams L.B., Fawcett R.L., Waechter A.S., Zhang P., Kogon B.E., Jones R., Inman M., Huse J., Considine R.V. Leptin production in adipocytes from morbidly obese subjects: stimulation by dexamethasone, inhibition with troglitazone, and influence of gender. J. Clin. Endocrinol. Metab. 2000; 85: 2678–2684
- Casabiell X., Pineiro V., Peino R., Lage M., Camina J., Gallego R., Vallejo L.G., Dieguez C., Casanueva F.F. Gender differences in both spontaneous and stimulated leptin secretion by human omental adipose tissue in vitro: dexamethasone and estradial stimulate leptin release in women, but not in men. J. Clin. Endocrinol. Metab. 1998; 83: 2149–2155
- Wabitsch M., Blum W.F., Muche R., Braun M., Hube F., Rascher W., Heinze E., Teller W., Hauner H. Contribution of androgens to the gender difference in leptin production in obese children and adolescents. J. Clin. Invest. 1997; 100: 808–813
- Considine R.V., Cooksey R.C., Williams L.B., Fawcett R.L., Zhang P., Ambrosius W.T., Whitfield R.M., Jones R., Inman M., Huse J., Mcclain D.A. Hexosamine regulate leptin production in human subcutaneous adipocytes. J. Clin. Endocinol. Metab. 2000; 85: 3551–3556
- Couillard C., Mauriege P., Imbeault P., Prud'homme D., Nadeau A., Tremblay A., Bouchard C., Despres J.-P. Hyperleptinemia in more closely associated with adipose cell hypertrophy than with adipose tissue hyperplasia. Int. J. Obesity 2000; 24: 782–788
- Sheu W.H.-H., Lee W.J., Chen Y.-T. Gender differences in relation to leptin concentration and insulin sensitivity in nondiabetic Chinese subjects. Int. J. Obesity 1999; 23: 754–759
- Fernandez-Real J.-M., Vayreda M., Casamitjana R., Gonzalez-Huix F., Ricart W. The fat-free mass compartment influences serum leptin in men. Eur. J. Endocrinol. 2000; 142: 25–29
- Wiesholzer M., Harm F., Hauser A.-C., Pribasnig A., Balcke P. Inappropriately high plasma leptin levels in obese haemodialysis patients can be reduced by high flux haemodialysis and haemodiafiltration. Clin. Sci. 1998; 94: 431–435
- Kokot F., Wiecek A., Mesjasz J., Adamczak M., Spiechowicz U. Influence of long-term recombinant human erythropoietin (rHuEpo) therapy on plasma leptin and neuropeptide Y concentration in haemodialysed uraemic patients. Nephrol. Dial. Transplant. 1998; 13: 1200–1205
- Malyszko J., Zbroch E., Wolczynski S., Malyszko J.S., Hryszko T., Mysliwiec M. Leptin and serum erythropoietin in hemodialyzed and peritoneally dialyzed uremic patients during rHuEPO therapy. Am. J. Nephrol. 2000; 20: 180–186
- Kokot F., Samardzija G., Klimek D., Wiecek A. Intravenous single dose of erythropoietin (rHuEPO) does not influence plasma leptin concentration in hemodialyzed patients with chronic renal failure (CRF). Clin. Nephrol. 1999; 51(6)389–391
- Deurenberg P., Yap M.D., Wang J., Lin F.P., Schmidt G. The impact of body build on the relationship between body mass index and percent body fat. Int. J. Obesity 1999; 23: 537–542
- Deurenberg P., Yap M., van Staveren W.A. Body mass index and percent body fat: a metaanalysis among different ethnic groups. Int. J. Obesity 1998; 22: 1164–1171
- Stall S., Ginsberg N.S., DeVita M.V., Zabetakis P.M., Lynn R.I., Gleim G.W., Wang J., Pierson R.N., Jr., Michelis M.F. Percentage body fat determination in hemodialysis and peritoneal dialysis patients: a comparison. J. Renal. Nutri. 1998; 8(3)132–136
- Hellstrom L., Wahrenberg H., Hruska K., Reynisdottir S., Arner P. Mechanisms behind gender differences in circulating leptin levels. J. Intern. Med. 2000; 247: 457–462
- Fruehwald-Schultes B., Peters A., Kern W., Beyer J., Pfutzner A. Influence of sex differences in subcutaneous fat mass on serum leptin concentrations. Diabetes Care 1998; 21(7)1204–1205
- Blum W.F., Englaro P., Hanitsch S., Juul A., Hertel N.T., Muller J., Skakkebaek N.E., Heiman M.L., Birkett M., Attanasio A.M., Kiess W. Plasma leptin levels in healthy children and adolescents: dependence on body mass index, body fat mass, gender, pubertal stage, and testosterone. J. Clin. Endocrinol. Metab. 1997; 82: 2904–2910
- Erfurth E.M., Ahren B. The negative association between serum free testosterone and leptin is dependent on insulin-like growth factor—binding protein 1 in healthy young and middle aged men. Clin. Endocrinol. 2000; 52: 493–498
- Wabitsch M., Blum W.F., Muche R., Braun M., Hube F., Rascher W., Heinze E., Teller W., Hauner H. Contribution of androgens to the gender difference in leptin production in obese children and adolescents. J. Clin. Invest. 1997; 100: 808–813
- Pedersen S.B., Fuglsig S., Sjogren P., Richelsen B. Identification of steroid receptors in human adipose tissue. Eur. J. Clin. Invest. 1996; 26: 1051–1056
- Elbers J.M.H., Asscheman H., Seidell J.C., Frolich M., Meinders A.E., Gooren L.J.G. Reversal of the sex difference in serum leptin levels upon cross-sex hormone administration in transsexuals. J. Clin. Endocrinol. Metab. 1997; 82: 3267–3270
- Jockenhovel F., Blum W.F., Vogel E., Englaro P., Muller-Wieland D., Reinwein D., Rascher W., Krone W. Testosterone substitution normalizes elevated serum leptin levels in hypogonadal men. J. Clin. Endocrinol. Metab. 1997; 82: 2510–2513
- Blumenkrantz M.J., Kopple J.D., Gutman R.A., Chan Y.K., Barbour G.L., Roberts C., Shen F.H., Gandhi V.C., Tucker T., Curtis F.K., Coburn J.W. Methods for assessing nutritional status of patients with renal failure. Am. J. Clin. Nutri. 1980; 33: 1567–1585
- Lazarus J.M. Nutrition in hemodialysis patients. Am. J. Kidney Dis. 1993; 21(1)99–105