Abstract
Renal involvement may occur in cases with malignant disorders. Glomerular injury may be the first sign of these diseases and may predict the overall survival. In some cases, glomerular injury may be associated with tumor antigens, and in some others, viruses cause renal injury together with underlying malignant disease. Here we report four cases with malignant diseases and accompanying glomerular injury and review the literature.
Introduction
Various types of renal involvement may occur during the course of neoplastic diseases. Clinical findings, therapy, and prognosis are variable, and it depends on the reversibility of superimposed renal complications and underlying malignant disease. Studies of glomerular injury in patients with malignant disease have been published since the 1960s.Citation[1] Minimal change in disease state is seen generally in Hodgkin disease, while membranous glomerulonephritis is common with carcinomas.Citation[2&3] Large numbers of patients with cancer and glomerular disease have tended to minimize the frequency or importance of these associations.Citation[4-6] Herein, we report on four patients with glomerulopathy accompanying malignant disease.
Case Reports
Case 1
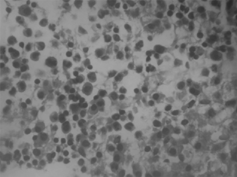
Seventy-one-year-old woman admitted to our hospital with renal failure and edema. Three weeks ago she was operated on for femoral neck prothesis for traumatic fracture. At that time, proteinuria and renal failure were detected. On physical examination, blood pressure and pulse were 120/80 mmHg and 88/min, respectively. She was unconscious, pale, and there was edema. Organomegaly was not found. Laboratory examination was as follows: hematocrit (Hct) 18.9%, hemoglobin (Hb) 6 g/dL, white blood cell (WBC) 6.6 × 109 L, platelet (plt) 280 × 109 L, glucose 93 mg/dL, blood urea nitrogen (BUN) 70 mg/dL, creatinine (Cr) 4 mg/dL, AST 60 IU, ALT 18 IU, lactic dehydrogenase (LDH) 1094 IU, total protein 5.1 g/dL, albumin 1.8 g/dL, calcium (Ca) 7 mg/dL, phosphorus (P) 6 mg/dL, and erythrocyte sedimentation rate (ESR) 98 mm/h. Peripheral blood smear showed ruloux formation. Daily proteinuria was 3 g. Protein electrophoresis revealed monoclonal gammopathy (gamma globulin % 66). Bone marrow aspiration showed morethan 60% atypical plasma cells (). ECG disclosed first-degree atrioventricular block and deleted R progression on V1-4. Kidney size was normal on abdominal ultrasonography. Amyloid deposition was found on abdominal fat aspiration ().
Case 2
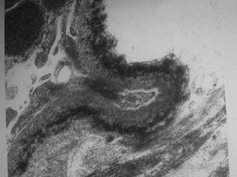
Sixty-year-old woman was hospitalized with dyspnea. She was hypertensive for 5 years. Autologous bone marrow transplantation was performed 4 years ago for light chain disease. Histopathological diagnosis of renal biopsy at that time was light chain deposition disease (). She had been treated due to acute pulmonary edema and heart failure in our hospital 2 months ago and at that time her BUN and creatinine were 42 mg/dL and 3.9 mg/dL, respectively. She was treated with angiotensin-converting enzyme inhibitor and nitrate. On last admission she was dyspneic, blood pressure 180/90 mmHg, pulse 110/min, breath 26/min. Aortic murmur and bibasilar crepitant rales, jugular venous distention, and pretibial edema were found at physical examination. Laboratory examination was as follows: hct 27%, Hb 9 g/dL, WBC 6.7 × 109, plt 224 × 109, glucose 73 mg/dL, BUN 73 mg/dL, creatinine 6.1 mg/dL, LDH 670, total protein 6.6 gr/dL, albumin 4.1 gr/dL, Ca 7.9 mg/dL. Cardiomegaly and left bundle block were found on telecardiography and electrocardiography, respectively. Ultrasonographic evaluation disclosed atrophic kidneys with increased resistive index. Renal function did not recover, and creatinine clearance was below 10 mL/min. Chronic hemodialysis program was begun.
Case 3
Sixty-year-old man was admitted to critical care unit for upper gastrointestinal bleeding and hepatic coma. Two months ago he was treated with sclerotherapy for bleeding esophageal varices. Hepatitis B surface antigen was positive, and he rejected percutaneous liver biopsy. On physical examination he was comatose, blood pressure 105/70 mmHg, pulse 120/min, and icterus and hepatomegaly were found. Sangstaken blackmore tube was inserted for bleeding esophageal varices (endoscopicexamination proven), and packed blood was transfused. Laboratory examination was as follows: hematocrit 19%, hemoglobin 6 g/dL, glucose 96 mg/dL, BUN 30 mg/dL, creatinine 1.3 mg/dL, AST 205 IU/L, ALT 320 IU/L, Ca-19.9: 3229 IU, AFP: 266 IU. Abdominal computerized tomography showed hepatic mass with portal venous invasion, peripancreatic lymphadenopathy, and peritoneal invasion. Hepatic coma progressed, and variceal bleeding continued. He died on fifth hospital day due to massive esophageal bleeding. Postmortem histopathological diagnosis was hepatocellular carcinoma, tumor necrosis, and glomerular immune complex deposition.
Case 4
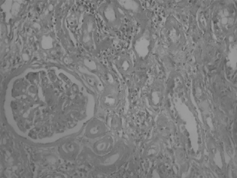
Forty-eight-year-old male was admitted to hospital because of edema. He was a heavy smoker (132 packet/year). On physical examination, blood pressure was 110/60 mmHg, pulse 96/min, temperature 36.7°C, and breath 28/min. He was dyspneic, pale, and edematous. There was evidence of left pleural effusion. Laboratory examinations were as follows: hemoglobin 9 g/dL, WBC count 9.5 × 109 L, platelet count 306 × 109 L, BUN 29 mg/dL, creatinine 1 mg/dL, Na 134 mEq/L, K 4.8 mEq/L, AST 25 IU/L, ALT 10 IU/L, alkaline phosphatase 244 IU/L, total protein 4.3 g/dL, serum albumin 1.7 g/dL, and daily proteinuria was 4.8 g/dL. HBsAg and anti-HCV were negative. Thoracal tomography disclosed a mass on the left upper and medial lobes and a left pleural effusion. On abdominal ultrasonography, liver echogenicity was heterogenous, and kidneys were enlarged. Cerebral and other organ metastases were not found. Percutaneous lung biopsy was reported as epidermoid carcinoma. Renal biopsy showed membranoproliferative glomerulonephritis (). Supportive therapy, including nasal oxygen, albumin infusion, diuretic therapy, antibiotic therapy, and intravenous fluid, was given. Only two doses of radiation therapy could be given, and he died on the 15th day of the hospital stay.
Discussion
In patients with malignant disease, renal failure develops due to many reasons but glomerular diseases are not common. However, sometimes the first presentationof malignant disease can be related to glomerular involvement, although no symptoms can be found in the case of glomerular involvement in patients with malignant disease. According to a large trial, the association of malignant disease and glomerular involvement is not important or common.Citation[6] We found four cases with malignant disease and glomerulopathy during the last year. The glomerular lesions commonly observed in association with neoplastic process are listed in .Citation[7]
Table 1. Major Glomerular Lesions Associated with Neoplastic Disease.
Membranous nephropathy is the most common lesion. Approximately 7–10% of patients found to have membranous nephropathy by renal biopsy (most often for the evaluation of nephrotic syndrome) will be found to have an underlying malignancy. Some epidemiologic studies suggest that the prevalence of malignancy is no higher in patients with membranous nephropathy than in aged-matched controls. Tumor neoantigens or antibodies have rarely been detected within the glomerular deposits, suggesting an immune pathogenesis. Less frequent lesions observed in patients with neoplasia include minimal change lesion, FSGS, proliferative GN, thrombotic glomerulopathy, and amyloidosis.Citation[7]
In multiple myeloma, renal lesions of excess immunoglobulin production have been typified as the "myeloma kidney." The prominent lesion in multiple myeloma (MM) is a tubular cast nephropathy with associated tubulointerstitial nephritis.Citation[8] Glomerular lesion seen in dysproteinemias is amyloidosis, which occurs in 5–20% of patients with multiple myeloma and Waldenström's macroglobulinemia.Citation[8&9] Other changes secondary to severe tubulointerstitial injury are nonamyloid glomerulopathy associated with deposition of light chains or apparent monoclonal immunoglobulins.Citation[10-12] Pathologic spectrum of nonamyloid glomerulopathy is heterogeneous with mesangiocapillary glomerulonephritis, proliferative glomerulonephritis, crescentic glomerulonephritis, and minimal change glomerulopathy.Citation[12-15] In patients with light-chain nephropathy, paraproteins serum or urine cannot be found, and 50% of these patients fail to show evidence of a lymphoplasmacytic disorder.Citation[12-14] The occurrence of light-chain deposition disease in multiple myeloma is close to 5%; however, myeloma is the underlying disease in two thirds of patients with light-chain deposition disease.Citation[16] It is not known why some patients with myeloma develop renal disease and others do not or why the spectrum of renal disease differs in multiple myeloma. It was hypothesized that physicochemical features of the individual light chains, including increased interaction and coprecipitation with Tamm–Horsfall protein in an acid environment to molecular charge is important. According to one hypothesis, Bence–Jones proteins with high isoelectric points are more susceptible to formation of myeloma casts.Citation[17] But this hypothesis has not been accepted by some authors. We did not know what charge of light chains was in our first two patients. In the first patient, we did not perform renal biopsy, but amyloid deposition was shown at subcutaneous biopsy. In addition to amyloid deposition, we would find additional renal histopathology. The diagnosis of renal biopsy of second patient is monoclonal light-chain deposition disease. We did not perform any physicochemical evaluation of deposited light-chain or circulating monoclonal immunoglobulin. Due to both renal and possible myocardial deposition of light chain, renal insufficiency and nephrotic syndrome and heart failure developed in this patient. At first diagnosis, 4 years prior, stem cell transplantation was performed and hematological improvement was noted, but other organ involvements were not regressed or prevented completely. It was reported that renal failure did not affect the quality of stem cell collections and did not affect engraftment. In the setting of renal failure, the role of autologous stem cell transplantation early in the disease course and benefits of tandem stem cell transplantation require further evaluation.Citation[18] In a report covering 34 patients with renal monoclonal immunoglobulin deposition disease, multivariate analysis showed that initial creatinine was the only predictor of kidney involvement and patient survival in pure monoclonal immunoglobulin deposition disease.Citation[19] Supportive therapy was done, but the first patient died on the 30th day of hospital stay.
In our third patient, clinical presentation did not suggest glomerular disease. In this patient with hepatocellular carcinoma related with hepatitis B virus, glomerular disease can be related with hepatitis B infection or hepatocellular carcinoma. The most common glomerular pathologies secondary to hepatitis B virus infection are membranous and membranoproliferative glomerulonephritis. Hepatitis B viral infection is a major cause of acute and chronic hepatitis, cirrhosis, and hepatocellular carcinoma.Citation[20] Extrahepatic manifestations of hepatitis B can cause significant morbidity and even mortality. HBV-MGN was first reported by Combes et al.Citation[21] in 1971 who had an HBsAg-positive patient with nephrotic syndrome and MGN. In this patient, HBsAg could be localized at the glomerular capillary wall by immunofluorescence. The association of the chronic carrier state of HBV with MGN is well established, especially in children, where the frequency of the HBsAg carrier state in MGN correlates with the underlying prevalence of HBsAg in the general population. The frequency of the HBsAg carriage in adult patients with MGN is significant. Male predominance in children with HBV-MGN is reported. History of HBV infection in this case was found to be uncommon. Liver function tests aremildly abnormal. The liver pathology in children with HBV-MGN usually shows chronic persistent hepatitis or minimal abnormalities, but chronic active hepatitis, cirrhosis, and fulminant hepatitis are uncommon. In contrast, the typical liver pathology in adults with HBV-MGN is chronic active hepatitis, but other forms of acute and chronic HBV-induced liver disease have been reported.Citation[22] Some years ago, we reported another patient with nephrotic syndrome due to MPGN- and HBV-related cirrhosis and acute delta hepatitis. Proteinuria and other renal findings were regressed spontaneously.Citation[23] In our patient, MPGN may be secondary to HBV or HBV + delta superinfection or delta hepatitis could be the causes of the exacerbation of glomerulonephritis. Because with decreasing liver enzyme, renal function improved, and proteinuria resolved in a short time. Immune complex deposits with early membranous and membranoproliferative changes have also been found in autopsy cases of patients with acute and chronic HBV hepatitis and cirrhosis despite the patients having had no clinical evidence of renal disease.Citation[24] In our patient, immune complex deposition was found in postmortem renal biopsy without evidence of clinical renal disease, and there was cirrhosis and hepatocellular carcinoma in liver biopsy.
HBsAg, HBeAg, and HBcAg have been shown to be localized by IF on the glomerular capillary wall in HBV-MGN, supporting a mechanism involving HBV antigen immune complexes localizing in the subepithelial space either as a result of passive trapping of circulating immune complexes or due to local immune complex formation at this site.Citation[25&26] Alternatively, the presence of HBV antigens in the glomerulus may be unrelated to the pathogenesis of HBV-MGN, and the HBV may cause the disease through other mechanisms, such as induction of autoantibodies to intrinsic glomerular antigens.
MPGN has also been reported in patients with chronic HBV infection. Several studies have reported that both adults and children with MPGN have a significantly higher carrier rate of HBsAg with the highest reported incidence of 66% in a study of children from Poland and 87.5% from a study of adult patients from South Korea. However, a study from Hong Kong did not confirm this association.Citation[26-28]
In chronic liver disease of any etiology, increased mesangial deposits of IgA can occur resulting in lesions similar to that of IgA nephropathy and Henoch–Schönlein purpura. Mixed cryoglobulinemia and immune complex nephritis may also be associated with mixed chronic liver disease due to HBV. Other types of glomerulonephritis rarely reported in HBV-infected patients include diffuse proliferative glomerulonephritis and crescentic glomerulonephritis.Citation[22] We did not find a similar case with hepatocellular carcinoma, HBV positivity, and glomerular immune deposition in literature.
Lung carcinoma and glomerulonephritis are not common. Glomerulonephritis secondary to carcinoma is commonly membranous glomerulonephritis. The association of membranoproliferative glomerulonephritis and lung carcinoma is rare and can be a component of paraneoplastic syndrome.Citation[29] Until now, a few cases of membranoproliferative glomerulonephritis and lung carcinoma have been reported. In a patient with membranous glomerulonephritis and lung carcinoma, nephrotic syndrome regressed after chemotherapy for small cell lung carcinoma. This regression persisted even when brain metastases developed, without simultaneous relapse of tumor outside the central nervous system.Citation[30]
In summary, we reported four cases with malignant disease and glomerular injury. In addition, a lot of causes of renal failure in malignant disease glomerular injury should be kept in mind. Sometimes the first presentation of malignant disease can be proteinuria or renal failure.
References
- Lee, J C.; Yamauchi, H.; Hopper, J. The association of cancer and the nephrotic syndrome. Ann. Intern. Med. 1966, 64, 41–51. [PUBMED], [INFOTRIEVE]
- Ozawa, T.; Pluss, R.; Lacher, J.; Boedecker, E.; Guggenheim, S.; Hammond, W.; McIntosh, R. Endogenous immune complex nephropathy associated with malignancy: I. Studies on the nature and immunopathogenic significance of glomerular bound antigen and antibody and circulating immune complexes. Q. J. Med. 1975, 44, 523. [PUBMED], [INFOTRIEVE]
- Kaptan, B S.; Kalssen, J.; Gault, M H. Glomerular injury in patients with neoplasia. Annu. Rev. Med. 1976, 27, 117. [CROSSREF]
- Gluck, M C.; Gallo, G.; Lowenstein, J.; Baldwin, D S. Membranous glomerulonephritis: evaluation of clinical and pathologic features. Ann. Intern. Med. 1973, 78, 1–12. [PUBMED], [INFOTRIEVE]
- Cameron, J S.; Turner, D R.; Ogg, C S.; Sharpstone, P.; Brown, C B. The nephrotic syndrome in adults with “minimal change” glomerular lesions. Q. J. Med. 1974, 43, 461–488. [PUBMED], [INFOTRIEVE]
- Heneghan, W.; Rao, T K.S.; Nicastri, A D.; Friedman, E A. Low incidence of malignancy associated with nephrotic syndrome in the elderly (abstract), Am. Soc. Nephrol. 13th Annual Mtg., Washington, DC, Nov., 1980; 20A.
- Glassock, R J. Other glomerular disorders. In Comprehensive Clinical Nephrology, 2nd Ed.; Johnson, R J., Feehally, J., Eds.; Elsevier Limited: Philadelphia, 2000, 406–407.
- Hill, G S. Multiple myeloma, amyloidosis. Waldenström's macroglobulinemia, cryoglobulinemias and benign monoclonal gammopathies. In Pathology of the Kidney, 3rd Ed.; Heptinstall, R H., Ed.; Little Brown: New York, 1983, 993–1067.
- Morel-Maroger, L.; Basch, A.; Danon, F.; Verroust, P.; Richet, G. Pathology of the kidney in Waldestrom's macroglobulinemia. N. Engl. J. Med. 1970, 283, 123–129. [PUBMED], [INFOTRIEVE]
- Ganeval, D.; Noel, L H.; Preud Homme, J L.; Droz, D.; Grunfeld, J P. Light-chain deposition disease: its relations with AL-type amyloidosis. Kidney Int. 1984, 26, 1–9. [PUBMED], [INFOTRIEVE]
- Seymour, A E.; Thompson, A J.; Smith, P S.; Woodroffe, A J.; Clarkson, A R. Kappa light chain glomerulosclerosis in multiple myeloma. Am. J. Pathol. 1980, 101, 557–580. [PUBMED], [INFOTRIEVE]
- Gallo, G R.; Feimer, H D.; Katz, L A.; Feldman, G M.; Correa, E B.; Chuba, J V.; Buxbaum, J N. Nodular glomerulopathy associated with non-amyloidotic Kappa light chain deposits and excess immunoglobulin light chain synthesis. Am. J. Pathol. 1980, 99, 621–644. [PUBMED], [INFOTRIEVE]
- Tubbs, R R.; Gephardt, G N.; McMahon, J T.; Hall, P M.; Valenzuela, R.; Vıdt, D G. Light chain nephropathy. Am. J. Med. 1981, 71, 263–269. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Ganeval, D.; Mignon, F.; Preudhomme, J L.; Noel, L H.; Morel-Maroger, L.; Droz, D.; Brouet, J C.; Mery, J P.; Grunfeld, J P. Visceral deposition of monoclonal light chains and immunoglobulins: a study of renal and immunopathologic abnormalities. Adv. Nephrol. 1982, 11, 25–63.
- Silva, F.; Mesa-Tejada, R.; Williams, G S.; Morel-Maroger, L.; Ramanarayanan, M.; Priani, C L. Light chain glomerulopathy (GN) as the first manifestation of plasma cell dyscrasia (abstract). Lab. Invest. 1980, 42, 151.
- Michopoulos, S.; Petrati, K.; Pertaki, C.; Dimopoulos, M A. Light chain deposition disease of the liver without involvement in a patient with multiple myeloma related to liver failure and rapid fatal outcome. Dig. Dis. Sci. 2002, 47, 730–734. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Clyne, D H.; Pesce, A J.; Thompson, R E. Nephrotoxicity of 2 Bence–Jones proteins in the rat: importance of protein isoelectric point. Kidney Int. 1979, 16, 345–352. [PUBMED], [INFOTRIEVE]
- Badros, A.; Barlogie, B.; Siegel, E.; Roberts, J.; Langmaid, C.; Zangari, M.; Desikan, R.; Shaver, M J.; Fassas, A.; McConneli, S.; Muwalla, F.; Barri, Y.; Anaissie, E.; Munshi, N.; Tricot, G. Results of autologous stem cell transplant in multiple myeloma patients with renal failure. Br. J. Hematol. 2001, 114, 822–829. [CROSSREF]
- Lin, J.; Markowitz, G S.; Valeri, A M.; Kambham, N.; Shermean, W H.; Apel, G B.; D'Agati, V D. Renal monoclonal immunoglobulin deposition disease: the disease spectrum. J. Am. Soc. Nephrol. 2001, 12, 182–192. [CSA]
- Sherlock, S. The natural history of hepatitis B. Postgrad. Med. J. 1987, 63, 7–11. [PUBMED], [INFOTRIEVE]
- Combes, B.; Shorey, J.; Barrera, A.; Stastny, P.; Eigenbrodt, E H.; Hull, A R.; Carter, N W. Glomerulonephritis with deposition of Australia antigen–antibody complexes in glomerular basement membrane. Lancet 1971, 2, 234–237. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Johnson, R J.; Couser, W G. Hepatitis B infection and renal disease: clinical, immunopathogenetic and therapeutic considerations. Kidney Int. 1990, 37, 663–676. [PUBMED], [INFOTRIEVE]
- Seyrek, N.; Paydas, S.; Kilic, H.; Karayaylali, I.; Sagliker, Y. Nephrotic syndrome accompanying hepatitis-B-related liver cirrhosis and delta hepatitis. Nephron 1996, 74, 227. [PUBMED], [INFOTRIEVE], [CSA]
- Morzycka, M.; Sulusarzyck, J. Kidney glomerular pathology in various form of acute and chronic hepatitis. Arch. Pathol. Lab. Med. 1979, 103, 38–41. [PUBMED], [INFOTRIEVE]
- Couser, W G.; Salant, D J. In situ immune complex formation and glomerular injury. Kidney Int. 1980, 17, 1–13. [PUBMED], [INFOTRIEVE]
- Couser, W G.; Abrass, C K. Pathogenesis of membranous nephropathy. Annu. Rev. Med. 1988, 39, 517–530. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Sham, M K.; Pun, K K.; Yeung, C K.; Ng, W L.; Chang, W K.; Chan, M K. Hepatitis B induced glomerulonephritis, fact or fiction? Aust. N.Z. J. Med. 1985, 15, 356–358. [PUBMED], [INFOTRIEVE]
- Lee, H S.; Choi, Y.; Yu, S H.; Koh, H I.; Kim, M J.; Ko, K W. A renal biopsy study of hepatitis B virus-associated nephropathy in Korea. Kidney Int. 1988, 34, 537–543. [PUBMED], [INFOTRIEVE]
- Usalan, C.; Emri, S. Membranoproliferative glomerulonephritis associated with small cell lung carcinoma. Int. Urol. Nephrol. 1998, 30, 209–219. [PUBMED], [INFOTRIEVE], [CSA]
- Boon, E S.; Vril, A A.; Nieuwhof, C.; van Noord, J A.; Zeppenfelt, E. Small cell cancer with paraneoplastic nephrotic syndrome. Eur. Respir. J. 1994, 7, 1192–1193. [PUBMED], [INFOTRIEVE]