Abstract
Atherosclerosis is by far the leading cause of mortality and morbidity in patients with end stage renal disease undergoing chronic hemodialysis (HD). Vascular endothelial cell adhesion molecules like the intercellular adhesion molecule-1 (ICAM-1) and the vascular cell adhesion molecule-1 (VCAM-1) are involved in the pathogenesis of atherosclerosis. Their soluble forms (sICAM-1, sVCAM-1) are considered potential serum markers of endothelial activation and atherosclerosis. The aim of this study was to clarify the influence of the HD procedure on the levels of sICAM-1 and sVCAM-1 in patients with end stage renal disease. We evaluated 35 clinically stable patients (18 males, 17 females, mean age 61 ± 12) on chronic HD treatment. Diabetes mellitus coexisted in eight patients and arterial hypertension in 23 patients. Blood was drawn before, every hour during, and after a single HD session in each patient. Low-flux cuprophane dialyzers (GFS 12®, Gambro, Lund, Sweden) were used in 22 and high-flux polysulfone dialyzers (Hemoflow F 60S®, Fresenius, Oberursel, Germany) in 13 cases. At 30 min into the HD session (n = 31, 20 low-flux HD, 11 high-flux HD) blood was drawn simultaneously from the entrance and the exit line of the dialyzer. From all these samples, serum concentrations of sICAM-1 and sVCAM-1 were determined by commercially available enzyme immunoassays (ELISA, R&D Systems, Minneapolis, USA). Results were corrected according to hemoconcentration, where appropriate. Plasma levels of sVCAM-1 were elevated in patients with end stage renal disease before the beginning of the dialysis session when compared to healthy controls (1449 ± 497 ng/mL vs. 691 ± 118 ng/mL). On the contrary, such an elevation was not found in the case of sICAM-1 (231 ± 58.5 ng/mL vs. 236.4 ± 96.8 ng/mL in healthy controls). These levels remained stable in all measurements throughout the dialysis procedure. Furthermore, serum sICAM-1 and sVCAM-1 levels remained unaltered after the passage of the dialyzer. The levels of sICAM-1 and sVCAM-1 were not influenced by the existence of diabetes mellitus, hypertension, or by the utilization of biocompatible, high flux dialyzers. Our study confirms that in chronic HD patients serum levels for sVCAM-1 are elevated. The levels of adhesion molecules are not affected by the HD procedure. These findings probably can be attributed to a decreased renal clearance or catabolism of sICAM-1 and sVCAM-1 and to the different sources of the two molecules. Neither coexisting diabetes mellitus nor arterial hypertension influences the circulating levels of these adhesion molecules. The functional role of sVCAM-1 and sICAM-1, the exact renal contribution to their metabolism, and their role as markers of atherosclerosis in chronic renal disease need further evaluation.
INTRODUCTION
Atherosclerosis is by far the leading cause of mortality and morbidity in patients undergoing hemodialysis (HD).Citation[1] It is believed that uremia and end stage renal disease is an atherogenic condition and, although the exact mechanisms are not well understood, inflammation has been incriminated.Citation[2]
There is extensive evidence that supports the immunologic cause of atherosclerosis and many immune system cells are present in atherosclerotic plaques.Citation[3] Before they invade the vascular wall, leucocytes have toadhere first and bind afterwards to the vascular endothelium. This process, which is necessary for leucocytes to cross the endothelial barrier under the influence of chemokines, is accomplished by an interaction between leucocyte membrane integrins and their specific endothelial cell ligands.Citation[4] The major endothelial receptors are the intercellular adhesion molecule-1 (ICAM-1) and the vascular cell adhesion molecule-1 (VCAM-1). Both are 90–110 kD proteins and belong to the immunoglobulin-like adhesion molecules. Almost exclusively endothelial cells express VCAM-1, while ICAM-1 is also expressed by monocytes, activated lymphocytes, fibroblasts, and epithelial cells.Citation[4] Under normal conditions, the vascular endothelium expresses very little or no VCAM-1 and ICAM-1. The expression of VCAM-1 can be induced by cytokine stimulation, especially by TNFa, IFNγ, IL-1β, and IL-4.Citation[5&6] Oxidized LDLCitation[7] and dyslipidemiaCitation[8] can upregulate VCAM-1, as well. The ICAM-1 production can be induced by cytokine stimulation, mainly by TNFa,Citation[9] and by hemodynamic stress.Citation[10]
Both VCAM-1 and ICAM-1 are over-expressed on endothelial cells overlaying areas of atherogenesis. Furthermore, serum levels of the soluble VCAM-1 (sVCAM-1) correlate with the observed cellular expression in the human aorta.Citation[11] It has been also shown that both sVCAM-1 and sICAM-1 are released from cytokine activated–endothelial cells by proteolytic cleavage of the extracellular portion.Citation[12] These observations have led to a growing interest in these molecules as potential markers for atherosclerosis. Indeed, the relation between sVCAM-1 and/or sICAM-1, and various forms of atherosclerotic disease, like coronary heart disease and carotid atherosclerosis was determined in previous clinical studies.Citation[13-15] An interrelation between atherogenic risk factors such as diabetes mellitus,Citation[16] hypertension,Citation[16&17] and dyslipidemiaCitation[18] and the sICAM-1 and sVCAM-1 levels was also confirmed.
These observations have led many researchers to study the levels of sICAM and sVCAM in hemodialyzed patients, a patient group with a very high incidence of atherosclerotic disease.Citation[19-23] The levels of s-VCAM-1 in dialysis patients were found to be elevated in many of these studies, whereas the results for s-ICAM-1 are controversial. Findings about the influence of the HD procedure on sICAM-1 and sVCAM-1 were also contradictory.Citation[19-22] The aim of the study presented here is to clarify the influence of HD procedure on sICAM-1 and sVCAM-1 levels in patients on dialysis.
PATIENTS AND METHODS
Thirty-five clinically stable patients (18 males, 17 females, mean age 61 ± 12 years) with end stage renal disease on hemodialysis were evaluated. At the same time, 10 healthy individuals, not taking any medication (serum creatinine and creatinine clearance within normal range, urine sediment without abnormalities, no proteinuria, no acute infectious disease, two males, eight females, mean age 25.3 ± 3.7 years) served as controls for the measurement of serum concentrations of the adhesion molecules. All patients gave informed consent to participate in the study and the local ethical committee approved the study protocol. None of the subjects studied had clinical or laboratory evidence of active infections, malignancies, liver disease, or any inflammatory conditions; they were not taking any antibiotics or immunosuppressive medications. The clinical and laboratory features of the patients included in the present study are described in .
Table 1. Clinical and laboratory features of patients with end stage renal disease on hemodialysis (n = 35).
All patients underwent regular hemodialysis for 4 hours (3 times/week) with hollow fiber dialyzers and bicarbonate buffer. Low-flux cuprophane dialyzers (GFS 12®, Gambro, Lund, Sweden) were used in 22 patients and high-flux polysulfone dialyzers (Hemoflow F 60S®, Fresenius, Oberursel, Germany) in 13 patients. Continuous i.v. infusion of unfractionated heparin (Liquemin®, Roche, Basel, Schwitzerland) was applied for anticoagulation. Recombinant human erythropoietin (rHuEPO 40 U/kg) was administered subcutaneously (s.c.) after completion of the hemodialysis session 3 times/week in 16 patients ().
Blood samples were drawn before the beginning, every hour during, and after the end of HD, 30 min after the beginning of HD the ultrafiltration was stopped, and in 31 of the 33 sessions (20 low-flux HD, 11 high-flux HD) blood was drawn simultaneously from the entrance and the exit line of the dialyzer in order to determine the dialyzer clearance for the adhesion molecules. Posttreatment blood samples were drawn using the “slow flow/stop pump technique,” which minimizes sample dilution with recirculated blood.Citation[24&25] In all samples serum sICAM-1 and sVCAM-1 concentrations were determined by enzyme immunoassays (ELISA) using commercially available kits (R&D Systems, Minneapolis, USA).
Results of sICAM-1 and sVCAM-1 measurements were corrected according to hemoconcentration at the timeof blood collection (t). The percent change of plasma volume reduction (%) was used as the correction factor (Fc). If hematocrit values at the time t (Ht) as well as before the beginning of the hemodialysis session (H0) are measured the correction factor Fc can be easily derived from the following equation described by van Beaumont:Citation[26]
Patients with elevated blood pressure (>140/80 mmHg) and those receiving antihypertensive medication for previously diagnosed arterial hypertension were considered to be hypertensives. Blood pressure (BP) was measured through a standard sphygmomanometer prior to blood sampling.
Data were expressed always as a mean value (x) and standard deviation (SD) or if specific denoted as mean value (x) and standard error of mean (SEM). The probability of error for comparison of the measured values was calculated using the Wilcoxon signed rank test for paired data and the Mann–Whitney U test for unpaired data. The interdependence of the different variables was checked by means of a Spearman correlation analysis. The P-values < 0.05 were regarded as significant.
RESULTS
Hemodialysis with an ultrafiltration of 2474 ± 800 mL was performed. Elevated plasma levels of sVCAM-1 (1449 ± 497 ng/mL vs. 691 ± 118 ng/mL in healthy controls) were found in our patients before the start of the dialysis session, whereas the levels of sICAM-1 (231 ± 58.5 ng/mL vs. 236.4 ± 96.8 ng/mL) were found to be similar to those of healthy controls. These levels remained stable in all hourly measurements throughout the dialysis procedure (Tables and ). The levels of both sICAM-1 and sVCAM-1 measured at 30 minutes into HD remained unaltered after the passage of the dialyzer ( and ).
Table 2. The time course of plasma sVCAM-1 (pg/mL) during hemodialysis treatment in patients with end stage renal disease (n = 35).
Table 3. The time course of plasma sICAM-1 (pg/mL) during hemodialysis treatment in patients with end stage renal disease (n = 35).
Figure 1. Serum levels of sVCAM-1 measured at 30 minutes into HD before and after the passage of the dialyzer. Values are presented as mean ± SD. There was no statistically significance difference between sVCAM-1 levels before and after the passage of the dialyzer.
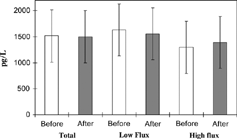
Figure 2. Serum levels of sICAM-1 measured at 30 minutes into HD before and after the passage of the dialyzer. Values are presented as mean ± SD. There was no statistically significance difference between sICAM-1 levels before and after the passage of the dialyzer.
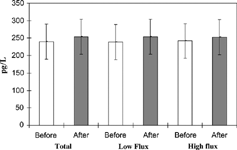
We analyzed the results and compared the levels of the adhesion molecules in various groups of patients, i.e., diabetics and nondiabetics, hypertensives and normotensives, and finally those dialyzed with biocompatible, high-flux dialyzers and those treated with conventional, low-flux dialyzers. No statistically significant differences were found among all these cases (Tables and ).
Furthermore, we found no correlation between sICAM-1 and sVCAM-1 levels on the one hand and age, duration of dialysis treatment, arterial blood pressure, heart rate, and predialysis levels of urea and creatinine on the other.
DISCUSSION
In agreement with most previous studiesCitation[19-23] the results presented here confirmed the elevated levels of sVCAM-1 in the serum of patients undergoing chronic hemodialysis (HD). On the contrary, serum levels of sICAM-1 were not elevated, in accordance with the findings of Mrowka et al.Citation[23] The levels of sICAM-1 and sVCAM-1 did not differ among the various patient subgroups and were equal in diabetics and non-diabetics, hypertensives and normotensives, and patients dialyzed with biocompatible (high-flux) and bioincompatible (low-flux) membranes. Increased levels of sVCAM-1 werepreviously shown to be independent of the compatibility properties of the dialyzer.Citation[19], Citation[21-23] The lack of difference between diabetic and nondiabetic HD patients is in agreement with the findings of a previous study in HD patients.Citation[19] In the case of diabetic patients with normal renal function, previous studies have shown controversial results.Citation[27&28] Furthermore, in patients with hypertension, but no renal impairment, elevated levels of adhesion molecules were detected by other investigators.Citation[17] It seems, therefore, reasonable to assume that sVCAM-1 uremia provokes an elevation in levels overrides caused by diabetes, hypertension, and bioincompatible membranes.
The elevated levels of sVCAM-1 can probably be attributed to the decreased renal clearance or catabolism of this molecule in chronic renal failure (CRF). This assumption can be based on a previous study in patients with CRF (predialysis stage) who showed a positive relation between the levels of sVCAM-1 with serum creatinine.Citation[21] However, it also seems probable that increased expression of VCAM-1 can be due to an activation of the vascular endothelium induced by either uremia or the HD treatment, a condition well documented in many studies.Citation[29-31] The VCAM-1 has a limited distribution and, with the exception of tumor cells, is mainly expressed by activated endothelial cells.Citation[4] Therefore, it may be considered as a sensitive marker of endothelial dysfunction.Citation[15]
As long as ICAM-1 is concerned, a flow cytometry study indicated decreased levels of ICAM-1 on the monocytes of patients with CRF either before or after the initiation of HD.Citation[32] In another study by Konstantopoulos et al. it was shown that VCAM-1, but not ICAM-1, functions as an important adhesive mechanism under conditions of flow.Citation[33] Activated endothelial cells are not the only important source of ICAM-1; it is also expressed by leucocytes, fibroblasts, and epithelial cells,Citation[3&4] a finding that raises the possibility of other functional roles for ICAM-1. In two more studies it was speculated that decreased monocyte ICAM-1 expression is responsible for the established impairment of T-cell dependent–immune responseCitation[34&35] in hemodialyzed patients, as the interaction between ICAM-1 on the monocyte and LFA-1 on the T-cell is necessary for an effective antigen presentation. The importance of ICAM-1 in antigen presentation is obvious in the leukocyte adhesion deficiency syndrome.Citation[36]
During the HD procedure no alteration of the sICAM-1 and sVCAM-1 levels was detected in comparison to predialysis values, neither in the total patient group nor in each group separately, i.e., diabetics and nondiabetics, hypertensives and normotensives, patients dialyzed with biocompatible and bioincompatible membranes. In previous studies decreased,Citation[19] stable,Citation[20] or increasedCitation[22] levels of sICAM-1 and sVCAM-1 were found after HD in comparison to predialysis values. In our opinion, these contradictory findings may have two causes: The unavailability of standard commercial ELISA kits in the early studies and the fact that in many studies the results were not corrected for the hemoconcentration caused by ultrafiltration during the HD session, or were corrected inaccurately.
In this study all results were corrected according to van Beaumont's equation,Citation[26] which accurately overcomes the influence of hemoconcentration on the concentration of a substance in the serum, taking into account the discrepancy in proportional changes between hematocrit and plasma volume. When the results of this study were corrected with simpler and inaccurate equations using hemoglobulin concentrations, the sICAM-1 and sVCAM-1 levels were found to be increased after HD (data not shown).
In our study the concentrations of sICAM-1 and sVCAM-1 were the same when measured before and after the passage of the dialyzer, whatever the type of the dialyzer (biocompatible high-flux or bioincompatible low-flux). Therefore, there is no important clearance of these molecules during the HD procedure that could result in alterations of their levels.
The finding that HD procedure does not affect the concentrations of sICAM-1 and sVCAM-1 may at first glance seem to contradict those of immune activation and cytokine secretion after the contact between leucocytes and HD membrane.Citation[37] Among the cytokines, TNFa and IL-1 are shown to be elevated in HD patients and it would be expected that these cytokines should activate the vascular endothelium resulting in elevation of sICAM-1Citation[9] and sVCAM-1Citation[5&6] levels during the HD session.
This discrepancy, confirmed by other studies as well,Citation[20] might be explained by the possibility that the preceding endothelium activation induced by uremia itself reaches a level that makes further expression of the adhesion molecules impossible. This hypothesis is in accordance with a study in which adhesion molecules were found to be increased in predialysis patients, as well as in patients undergoing HD or continuous ambulatory peritoneal dialysis (CAPD) without statistically significant differences.Citation[21] It must be mentioned that many cytokine levels, like the TNFa and IL-1β, are also found to be elevated in predialysis patients.Citation[38] It is likely that the endothelium capacity for activation and consequent increase in sICAM-1 and sVCAM-1 is limited, and this is in accordance with the observation that although essential hypertension and diabetes mellitus provoke equal increases in the levels of these molecules, when the two conditions coexist no further elevation is provoked.Citation[16]
CONCLUSION
In conclusion, this study confirms the elevated levels of sVCAM-1 in hemodialysis patients and also indicates that the circulating levels of adhesion molecules are not influenced by the HD procedure, as well as the type of the dialyzer (biocompatible, high-flux dialyzers, and conventional, low-flux dialyzers). Neither coexisting diabetes mellitus nor arterial hypertension influenced the levels of sICAM-1 and sVCAM-1 as their levels were the same in all subgroups, i.e., diabetics and nondiabetics, hypertensives and normotensives, and patients hemodialyzed with biocompatible and bioincompatible membranes. These findings, as well as the exact source of sVCAM-1 and sICAM-1, the renal contribution to the clearance of these molecules, and finally their functional role in uremia need further evaluation.
REFERENCES
- Levey, A.; Beto, J.; Coronado, B E.; Eknoyan, G.; Foley, R N.; Kasiske, B L.; Klag, M J.; Mailloux, L U.; Manske, C L.; Meyer, K B.; Parfrey, P S.; Pfeffer, M A.; Wenger, N K.; Wilson, P W.; Wright, J T., Jr. Controlling the epidemic of cardiovascular disease in chronic renal failure, What do we know? What do we need to learn? Where do we go from here? Am. J. Kidney Dis. 1998, 32, 853–906. [PUBMED], [INFOTRIEVE]
- Walter, R.; Mischak, H.; Haller, H. Hemodialysis, atherosclerosis and inflammation-identifying molecular mechanisms of chronic vascular diseases in ESRD patients Nephrol. Dial. Transplant. 2002, 17 (Suppl. 3), 24–29. [PUBMED], [INFOTRIEVE]
- Hansson, G K. Immune mechanism in atherosclerosis Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1876–1890. [PUBMED], [INFOTRIEVE]
- Johnson-Leger, C.; Aurrand-Lions, M.; Imhof, B A. The parting of the endothelium, miracle, or simply a junctional affair? J. Cell. Sci. 2000, 113, 921–933. [PUBMED], [INFOTRIEVE]
- Masinovsky, B.; Urdal, D.; Gallatin, W M. IL-4 acts synergistically with IL-1b to promote lymphocyte adhesion by induction of vascular cell adhesion molecule-1 J. Immunol. 1990, 145, 2886–2895. [PUBMED], [INFOTRIEVE]
- Thornhill, M H.; Wellicome, S M.; Mahiouz, D L.; Lanchbury, J S.; Kyan-Aung, U.; Haskard, D O. Tumor necrosis factor combines with IL-4 or interferon-gamma to selectively enhanced epithelial cell adhesiveness for T-cells. The contribution of vascular cell adhesion molecule-1 dependent and independent mechanisms J. Immunol. 1991, 146, 592–598. [PUBMED], [INFOTRIEVE]
- Frostegard, J.; Haegerstrand, A.; Gidlund, M.; Nilsson, J. Biologically modified LDL increases the adhesive properties of endothelial cells Atherosclerosis 1991, 90, 119–126. [PUBMED], [INFOTRIEVE]
- Cybulsky, M I.; Gimbrone, M A. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherosclerosis Science 1991, 251, 788–791. [PUBMED], [INFOTRIEVE]
- Morandini, R.; Boeynaems, J M.; Duhant, X.; Jacquemotte, F.; Kinnaert, E.; Ghanem, G. SODs are involved in the regulation of ICAM expression in human melanoma and endothelial cells Cell. Mol. Biol. 1999, 45, 1053–1063. [PUBMED], [INFOTRIEVE], [CSA]
- Walpola, P L.; Gotlieb, A I.; Cybulsky, M I.; Langille, B L. Expression of ICAM-1 and VCAM-1 and monocyte adherence in arteries exposed to altered shear stress Arterioscler. Thromb. Vasc. Biol. 1995, 15, 2–10. [PUBMED], [INFOTRIEVE], [CSA]
- Nakai, K.; Itoh, C.; Kawazoe, K.; Miura, Y.; Sotoyanagi, H.; Hotta, K.; Itoh, T.; Kamata, J.; Hiramori, K. Concentration of soluble vascular cell adhesion molecule-1 (s VCAM-1) correlated with expression of VCAM-1 mRNA in the human atherosclerotic aorta Coron. Artery Dis. 1995, 6, 497–502. [PUBMED], [INFOTRIEVE], [CSA]
- Pigott, R.; Dillon, L P.; Hemingway, I H.; Gearing, A J. Soluble forms of E-selectin, ICAM-1 and VCAM-1 are present in the supernatants of cytokine activated cultured endothelial cells Biochem. Biophys. Res. Commun. 1992, 187, 584–589. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Hwang, S J.; Ballantyne, C M.; Sharrett, A R.; Smith, L C.; Davis, C E.; Gotto, A M., Jr., Boerwinkle, E. Circulating adhesion molecules VCAM-1, ICAM-1, and E-Selectin in carotid atherosclerosis and incident coronary heart disease cases. [The Atherosclerosis In Communities (ARIC) Study] Circulation 1997, 96, 4219–4225. [PUBMED], [INFOTRIEVE], [CSA]
- Van der Meer, I M.; de Maat, M P.; Bots, M L.; Breteler, M M.; Meijer, J.; Kiliaan, A J.; Hofman, A.; Witteman, J C. Inflammatory mediators and cell adhesion molecules as indicators of severity of atherosclerosis (The Rotterdam Study) Arterioscler. Thromb. Vasc. Biol. 2002, 22, 838–842. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Huo, Y.; Haferi-Moghamad, A.; Ley, K. Role of vascular cell adhesion molecule-1 and fibronectin connecting segment-1 in monocyte rolling and adhesion on early atherosclerotic lesions Circ. Res. 2000, 87, 153–159. [PUBMED], [INFOTRIEVE], [CSA]
- Rizzoni, D.; Muiesan, M L.; Porteri, E.; Castellano, M.; Salvetti, M.; Monteduro, C.; De Ciuceis, C.; Boari, G.; Valentini, U.; Cimino, A.; Sleiman, I.; Agabiti-Rosei, E. Circulating adhesion molecules and carotid artery structural changes in patients withnon insulin dependent diabetes mellitus J. Hum. Hypertens. 2003, 17, 463–470. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- DeSouza, C A.; Dengel, D R.; Macko, R F.; Cox, K.; Seals, D R. Elevated levels of circulated cell adhesion molecules in uncomplicated essential hypertension Am. J. Hypertens. 1997, 10, 1335–1341. [PUBMED], [INFOTRIEVE], [CSA]
- Hackman, A.; Abe, Y.; Insull, W., Jr., Pownall, H.; Smith, L.; Dunn, K.; Gotto, A M., Jr., Ballantyne, C M. Levels of soluble adhesion molecules in patients with dyslipidemia Circulation 1996, 93, 1334–1338. [PUBMED], [INFOTRIEVE], [CSA]
- Rabb, H.; Calderon, E.; Bittle, P A.; Ramirez, G. Alteration in soluble intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in hemodialysis patients Am. J. Kidney Dis. 1996, 27, 239–243. [PUBMED], [INFOTRIEVE], [CSA]
- Thylen, P.; Fernvik, E.; Lundahl, J.; Lins, L E.; Jacobson, S H. Monocyte and granulocyte CD11b/cd18, CD62L expression and sICAM-1 concentration in the intradialytic period Nephron 1996, 74, 275–282. [PUBMED], [INFOTRIEVE], [CSA]
- Bonomini, M.; Reale, M.; Santarelli, P.; Stuard, S.; Settefrati, N.; Albertazzi, A. Serum levels of soluble adhesion molecules in chronic renal failure and dialysis patients Nephron 1998, 79, 399–407. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Papayianni, A.; Alexopoulos, E.; Giamalis, P.; Gionanlis, L.; Belechri, A M.; Koukoudis, P.; Memmos, D. Circulating levels of ICAM-1, VCAM-1 and MCP-1 are increased in hemodialysis patients, association with inflammation, dyslipidemia and vascular events Nephrol. Dial. Transplant. 2002, 17, 435–441. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Mrowka, C.; Heintz, B.; Sieberth, H G. Is dialysis membrane type responsible for increased circulating adhesion molecules during chronic hemodialysis? Clin. Nephrol. 1999, 52 (5), 312–321. [PUBMED], [INFOTRIEVE], [CSA]
- Pflederer, B R.; Torrey, C.; Priester-Coary, A.; Lau, A H.; Daugirdas, J T. Estimating equilibrated Kt/V from an intradialytic sample: effects of access and cardiopulmonary recirculations Kidney Int. 1995, 48, 832–837. [PUBMED], [INFOTRIEVE]
- Daugirdas, J T.; Burke, M S.; Balter, P.; Priester-Coary, A.; Majka, T. Screening for extreme postdialysis urea rebound using the Smye method: patients with access recirculation identified when a slow flow method is not used to draw the postdialysis blood Am. J. Kidney Dis. 1996, 28, 727–731. [PUBMED], [INFOTRIEVE], [CSA]
- Van Beaumont, W. Evaluation of hemoconcentration from hematocrit measurements J. Appl. Phys. 1972, 32 (5), 712–713.
- Boulbou, M S.; Gourgoulianis, K I.; Petinaki, E A.; Klisiaris, V K.; Maniatis, A N.; Molyvdas, P A. Pulmonary function and circulating adhesion molecules in patients with diabetes mellitus Can. Respir. J. 2003, 10, 259–264. [PUBMED], [INFOTRIEVE], [CSA]
- Elhadd, T A.; Kennedy, G.; Robb, R.; McLaren, M.; Jung, R T.; Belch, J J. Elevated soluble adhesion molecules E-selectin and intercellular cell adhesion molecule-1 in type-2 diabetic patients with and without asymptomatic peripheral arterial disease Int. Angiol. 2004, 23, 128–133. [PUBMED], [INFOTRIEVE]
- Stefanidis, I.; Wurth, P.; Mertens, P R.; Ikonomov, V.; Philippidis, G.; Golphinopoulos, S.; Makropoulos, V.; Liakopoulos, V.; Mann, H.; Heintz, B. Plasma endothelin-1 in hemodialysis treatment—The influence of hypertension J. Cardiovasc. Pharmacol. 2004, 44, S43–S48. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Warrens, A N.; Cassidy, M J.; Takahashi, K.; Ghatei, M A.; Bloom, S R. Endothelin in renal failure Nephrol. Dial. Transplant. 1990, 5, 418–422. [PUBMED], [INFOTRIEVE]
- Deray, G.; Carayon, A.; Maistre, G.; Benhmida, M.; Masson, F.; Barthelemy, C.; Petitclerc, T.; Jacobs, C. Endothelin in chronic renal failure Nephrol. Dial. Transplant. 1992, 7, 300–305. [PUBMED], [INFOTRIEVE]
- Meier, P.; von Fliedner, V.; Markert, M.; van Melle, G.; Deppisch, R.; Wauters, J P. One year immunological evaluation of chronic hemodialysis end stage renal disease patients Blood Purif. 2000, 18, 128–137. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Konstantopoulos, K.; Kukreti, S.; Smith, S W.; McIntyre, L V. Endothelial P-selectin and VCAM-1 each can function as primary adhesive mechanisms for T cells under conditions of flow J. Leukoc. Biol. 1997, 61, 179–187. [PUBMED], [INFOTRIEVE], [CSA]
- Beaman, M.; Michael, J.; MacLennan, I C.; Adu, D. T-cell-independent and T-cell-dependent antibody responses in patients with chronic renal failure Nephrol. Dial. Transplant. 1989, 4, 216–221. [PUBMED], [INFOTRIEVE]
- Eleftheriadis, T.; Papazisis, K.; Kortsaris, A.; Vayonas, G.; Voyatzi, S.; Vargemezis, V. Impaired T-cell proliferation and zeta chain phosphorylation after stimulation with staphylococcal enterotoxin-B in hemodialysis patients Nephron Clin. Pract. 2004, 96, c15–c20. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Springer, T A. Adhesion receptors of the immune system Nature 1990, 346, 425–434. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Luger, A.; Kovarik, J.; Stummvoll, H K.; Urbanska, A.; Luger, T A. Blood membrane interaction in hemodialysis leads to increased cytokine production Kidney Int. 1987, 32 (1), 83–88.
- Pereira, B J.; Shapiro, L.; King, A J.; Falagas, M E.; Strom, J A.; Dinarello, C A. Plasma levels of IL-1b, TNFa and their specific inhibitors in undialyzed chronic renal failure, CAPD and hemodialysis patients Kidney Int. 1994, 45, 890–896. [PUBMED], [INFOTRIEVE]