Abstract
Carbon nanotubes (CNTs) are a unique class of nanomaterials that can be imagined as rolled graphene sheets. The inner hollow of a CNT provides an extremely small, one-dimensional space for storage of materials. In the last decade, enormous effort has been spent to produce filled CNTs that combine the properties of both the host CNT and the guest filling material. CNTs filled with various inorganic materials such as metals, alloys, semiconductors and insulators have been obtained using different synthesis approaches including capillary filling and chemical vapor deposition. Recently, several potential applications have emerged for these materials, such as the measurement of temperature at the nanoscale, nano-spot welding, and the storage and delivery of extremely small quantities of materials. A clear distinction between this class of materials and other nanostructures is the existence of an enormous interfacial area between the CNT and the filling matter. Theoretical investigations have shown that the lattice mismatch and strong exchange interaction of CNTs with the guest material across the interface should result in reordering of the guest crystal structure and passivation of the surface dangling bonds and thus yielding new and interesting physical properties. Despite preliminary successes, there remain many challenges in realizing applications of CNTs filled with inorganic materials, such as a comprehensive understanding of their growth and physical properties and control of their structural parameters. In this article, we overview research on filled CNT nanomaterials with special emphasis on recent progress and key achievements. We also discuss the future scope and the key challenges emerging out of a decade of intensive research on these fascinating materials.
Introduction
The discovery of new materials with novel functionalities is the key to the sustained development of new devices and for improving device performances. During the last decade, intensive research efforts have been devoted to creating a large number of exotic materials, particularly those with sizes in the nanometer range. Thus, zero-, one- and two-dimensional nanostructures, such as nanoparticles, nanorods, nanowires, nanotubes, graphene and so forth have been discovered. With their small sizes, these materials are superior to their bulk counterparts for many applications owing to their higher surface-to-volume ratio, size-dependent electronic properties and their potential for device miniaturization. They are therefore expected to revolutionize areas such as electronic devices, computation, power generation, catalysis, medicine and drug delivery.
In this review, we focus on some of the most important developments that have recently taken place for a specific class of nanomaterials: carbon nanotubes (CNTs) filled with different classes of inorganic materials. CNTs were discovered in 1991 by Iijima [Citation1]. Subsequently, extensive studies of these materials have led to the discovery of numerous synthesis strategies and novel properties [Citation2–4]. Usually regarded as a single-layer graphene sheet rolled up into a seamless cylinder, a CNT can be metallic or semiconducting depending on the way the graphene is rolls up. Fundamental studies show that CNTs have remarkable room-temperature properties, such as extremely high current-carrying capacity and mechanical strength [Citation4, Citation5]. The tubular empty space inside a CNT provides an interesting scope for hosting a variety of materials. Thus, CNTs have been filled with fullerenes, various solvents, metals, alloys, ionic and non-ionic compounds and molecules and even biomolecules, creating a new family of nanocomposites that combine the functionality of different materials into one. The first examples of this class were reported back in 1993 [Citation6]. Subsequent developments have been summarized in a few comprehensive review articles [Citation7, Citation8].
In this article, we highlight some of the major developments in the area of inorganically filled CNTs, particularly emphasizing the key results from the recent past. We first discuss the various synthesis strategies and then focus on recent developments in characterization and applications. We conclude with a discussion of the various challenges that need to be overcome in order to successfully fulfill the potential of these systems. The described examples are representative of the various classes of encapsulated inorganic materials.
Synthesis strategies
Many reports have been published on filled CNTs over the last two decades. The initial studies were carried out in 1993 on systems which demonstrated the successful encapsulation of metals such as Pb [Citation6] or binary oxides such as Pb3O4 [Citation9]. In the following years, increasing effort was directed to the application of multi-walled CNTs (MWCNTs) as templates for one-dimensional nanowires. This culminated in the discovery of several filling methods and the encapsulation of a broad range of substances that included biomolecules and carbides [Citation10, Citation11]. Additionally, single-walled CNTs (SWCNT) was also explored with the first report of metal halide fillings published in 1998 [Citation12].
Overall, several conceptually different approaches have been developed for the synthesis filled CNTs. The filling materials can broadly be classified into the following categories: (i) metals and alloys, (ii) ionic compounds and (iii) covalent compounds. Depending on the nature of the filling material, a synthesis technique can be devised. For example, metals catalyze CNT synthesis, and therefore it is possible to tailor the synthesis conditions to fill the CNTs with the same metal. It is also possible to infiltrate a CNT with solutions of metal salts; subsequent reduction of the filled metal salts yields metal-filled CNTs. For soluble materials, the filling strategy involves filling CNTs with solutions and subsequent removal of the solvent molecules. Recently, there were also reports on coating semiconducting nanowires with carbon layers. This can be achieved, for instance, inside a transmission electron microscope (TEM) [Citation13, Citation14]. In this method, the outer shells are assembled a posteriori, i.e. after production and manipulation of the nanowires. The carbon fully envelops a nanowire having a large aspect ratio, meaning that the produced core-shell structure can be considered as a filled CNT. Thus, filling strategies can usually be divided into a single-step formation of filled CNTs or a multi-step process in which the CNT is synthesized first and then it is filled with another material or vice versa.
CNTs filled with metals and alloys
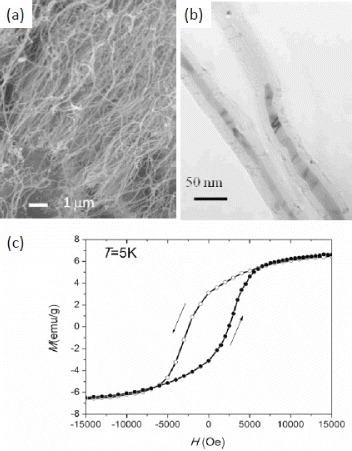
Metals catalyze the formation of CNTs and therefore it is often possible to tailor the synthesis conditions to encapsulate the metal catalyst inside the CNT. There are several reports on the synthesis of CNTs filled with Fe, Co, Ni, Sn, Ga and In [Citation15–29]. In order to prepare them, high-temperature chemical vapor deposition (CVD) techniques have usually been employed [Citation30, Citation31]. These techniques always yield MWCNTs with inner diameters ranging from 20 to 100 nm and outer diameter up to 200 nm. Figure shows a typical sample of CNTs filled with Fe (Fe@CNTs where @ means encapsulated in). The sample was prepared by pyrolysis of C60 and high purity ferrocene ((C5H5)2Fe) in a 1:1 ratio under an argon flow of 20–40 sccm. The temperature of the C60/ferrocene mixture was 350 °C and Fe-filled CNTs were deposited at a higher temperature of 1100 °C. Interestingly, this type of filling is expected to result in magnetic coercivities greater than those of the bulk phase owing to the anisotropic spatial confinement and high interface area. Figure (c) shows a plot of magnetization versus magnetic field for the Fe@CNTs. The ferromagnetic hysteresis loop has a coercive field of 2500 Oe and a saturation magnetization of 7 emu g-1. Detailed studies have pointed out that many interesting phenomena might occur in these structures owing to the complex existence of mixed phases coexisting at a nanometer length scale. Ruskov et al showed that in such encapsulated CNTs, the top of the Fe core consisted of γ-Fe, α-Fe and Fe3C occurring sequentially along the nanowire axis [Citation32, Citation33]. In another study, Marco et al showed the concentric distribution of these three phases, where the innermost α-Fe core is surrounded by a γ-Fe layer and then by a Fe3C layer near the CNT interface [Citation34]. Cu nanowires encapsulated in CNT can be obtained by doping Cu catalyst with alkali metals during the CVD synthesis of CNTs. These have been used for spot wielding with the attogram precision through Cu deposition [Citation19].
Figure 1 (a) Overview and (b) high-magnification TEM images of the Fe-filled CNTs. (c) Magnetization versus magnetic field strength at 5 K. Arrows indicate the direction of the field sweep. (Reproduced with permission from [Citation30] © 2010 IOP Publishing Ltd.)
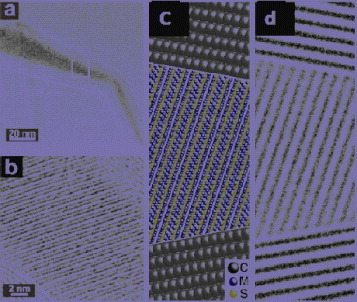
In addition to various metals, filling of CNTs with alloys has been accomplished recently thus making available a wide range of controllable and useful physical properties. For example, using electrodeposition and a porous anodic alumina template, permalloy (an alloy of Ni and Fe) nanocrystals have been filled inside one-end open CNTs with a uniform diameter of 40 nm and a length of 900 nm [Citation35]. Interestingly, even isolated permalloy nanoparticles inside CNTs displayed prominent magnetic anisotropy with an easy magnetization direction along the CNT axis. In addition, elongated nanowire-like Fe–Ni alloy nanostructures were obtained using chlorobenzene as the carbon precursor [Citation36]. In 2003, one of the authors identified a quaternary alloy encapsulated within tubular carbon shells [Citation37]. To this day, cobalt pentlandite remains the most chemically complex compound material to fill carbon nanotubes (figure ). This system was a by-product of the arc-discharge method used to produce double-walled CNTs (DWCNT) [Citation38]. The catalyst employed in the process contained Fe, Ni, Co and S, which under the extreme and non-equilibrium arc conditions led to the one-step synthesis of the pentlandite-filled nanotubes.
Figure 2 (a) Overall view of a cobalt pentlandite filled MWCNT. (b) Detail of the region boxed in (a). (c) Model of a CNT filled with cobalt pentlandite. (d) TEM image simulation based on the model in (c). (Reproduced with permission from [Citation37] © 2003 RSC Publishing.)
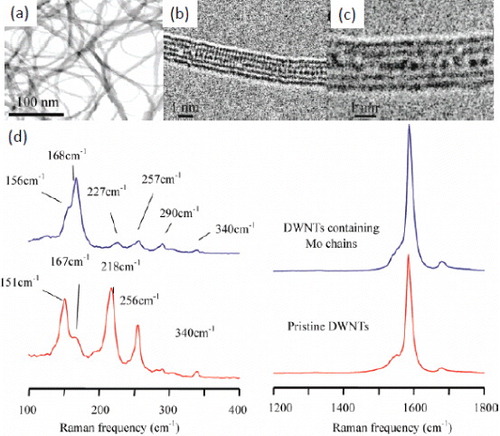
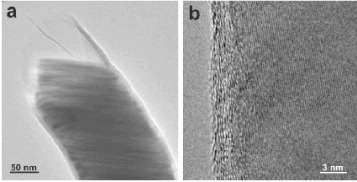
A large number of theoretical studies have recently predicted that thin metal nanowires encapsulated in the SWCNTs or DWCNTs might be interesting for both fundamental studies and technological applications. Therefore one of the most important challenges in the nanomaterials synthesis is the production of atomically thin metallic nanowires [Citation39, Citation40]. However, there were very few reports on the synthesis of such structures inside SWCNTs or DWCNTs. A notable initial success was the synthesis of Ag nanowires within SWCNTs by a liquid-phase method, wherein molten silver halide was first infiltrated into SWCNT by capillary action and then reduced by exposing to electromagnetic or electron irradiation. The filling fraction was up to 50% and the diameter of the Ag nanowires was as small as 3 nm [Citation41]. Recently, Dresselhaus and co-workers reported a unique and controllable synthesis of individual atomic molybdenum chains, with the diameter between 0.6 and 0.8 nm, inside DWCNTs [Citation42]. They found that these individual atomic chains form spontaneously within the hollow core of tubes in the absence of any reducing agent. For this purpose, clean, purified DWCNTs were subjected to a two-step procedure. At first, oxidation in air was carried out at 550 °C to remove amorphous carbon and open the ends of the DWCNTs. Subsequently, the air-oxidized tubes and hexaammonium heptamolybdate tetrahydrate were chemically treated in hydrochloric acid solution at 100 °C and then oxidized in air at 500 °C. The interatomic Mo distances were slightly larger than the bulk values and varied between 0.32 and 0.38 nm. Figures (a)–(c) show low- and high-resolution TEM images of the nanowires and the characteristic Raman spectra are shown in figure (d). Notably, the radial breathing modes between 200 and 270 cm-1, corresponding to the inner DWCNT shells, are suppressed by the inclusion of Mo chains. The above method can be extended to obtain encapsulated Pt nanowires. These atomic-scale nanowires are robust to oxidation, and therefore it should be possible to investigate the physical and chemical properties of metallic nanowires at the atomic scale limit.
Figure 3 (a) TEM images of encapsulated DWCNTs. (b, c) Typical HRTEM images of DWCNTs including long molybdenum chains. (d) Typical Raman spectra acquired from the sample under 633 nm excitation. (Reproduced with permission from [Citation42] © 2008 American Chemical Society.)
CNTs filled with ionic compounds
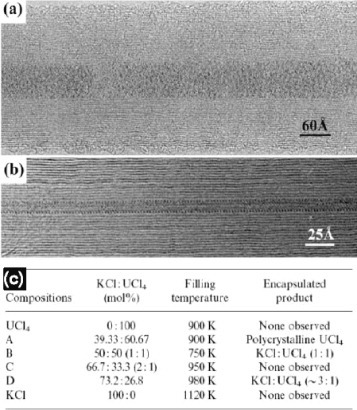
Ionic inorganic compounds usually have low melting points and are soluble in various polar solvents. Therefore strategies for filling CNTs with these materials are markedly different from those for metals. One of the first reports on such compounds was authored by Sloan et al [Citation43], who incorporated eutectic and non-eutectic mixtures of UCl4 and KCl into MWCNTs using capillary action. Interestingly, the structural ordering of the final encapsulated materials was highly dependent on the initial ratio of uranium and potassium halides. As a result, fillings with structural diversity were obtained spanning from glass to long uniform crystals (figure ).
Figure 4 (a) MWCNT filled with an amorphous 1:1 mixture of KCl/UCl4. (b) MWCNT filled with a long crystalline wire of eutectic KCl/UCl4 mixture. (c) Table showing some of the attempted compositions and obtained products. (Reproduced with permission from [Citation43] © 1998 Elsevier B.V.)
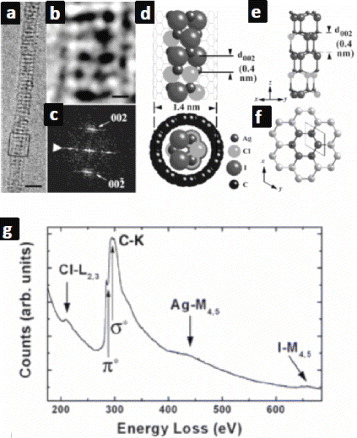
The same researchers reported two other ternary systems based on silver halides and SWCNTs. The first one concerned the encapsulation of AgCl–AgBr mixtures using a high-yield molten salt method [Citation39]. Detailed structural and chemical analyses of this compound were challenging owing to its strong sensitivity to electron beams. As a result of the high-energy particle bombardment (with 200–400 keV kinetic energy), halides turn into metallic silver inside the nanotubes. A closely related system, based on an eutectic AgCl–AgI mixture, was also investigated [Citation44]. Interestingly, these materials were relatively robust and it was possible to study the composition and crystal structure of the material inside the nanotubes (figure ). This is particularly noteworthy because the metastable AgCl1-xIx solid solution was the first clear evidence for the formation of a ternary crystalline phase inside an SWCNT. Other one-dimensional metal halide (KI, CsI) single crystals were also encapsulated into CNTs [Citation45]. Owing to the quantum-confined size of the filling materials and the high interfacial area between the filling and the CNT, interesting structure–property correlations can be expected. For example, very recently Chen et al reported the existence of noticeable charge transfer between AgCl nanowires and the inner shell of DWCNT [Citation46].
Figure 5 (a–c) TEM images of an SWCNT filled with AgCl1-xIx. (d–f) Model of the encapsulated structure. (g) Electron energy-loss spectrum of alloy-filled CNTs. (Reproduced with permission from [Citation44] © 2008 American Chemical Society.)
CNTs filled with covalent compounds
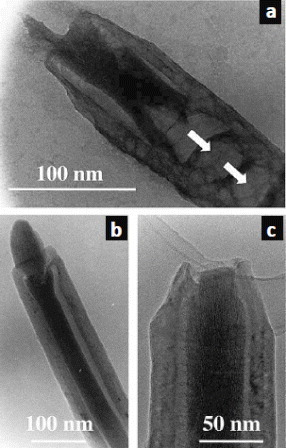
The inclusion of useful semiconducting or insulating nanostructures into CNTs is the most challenging among the filling materials, as these materials have high melting points and do not easily dissolve in solvents. Therefore, even though Pb3O4-filled CNTs were reported nearly two decades ago [Citation9], the syntheses and investigations of similar nanostructures are unfortunately rather limited. In 2004, Keller et al reported the inclusion of magnetic CoFe2O4 nanowires into CNTs [Citation47, Citation48]. The synthesis was achieved under relatively mild conditions (100 °C and atmospheric pressure) and had a high filling yield (∼70%, figure ). Furthermore, it was found that the CoFe2O4@MWCNTs had high coercivity making them potentially useful for high-density data storage.
Figure 6 (a–c) Various examples of MWCNTs filled with CoFe2O4. The arrows in (a) point to solid caps that impair the filling progression. (Reproduced with permission from [Citation47] © 2004 Elsevier B.V.)
Controlled carbon nanotube sheathing on ultrafine InP nanowires was realized through a simple vapor–solid process [Citation49]. The synthesized InP–C nanocables were composed of crystalline InP core with a diameter of 13–15 nm and carbon sheathing layers with a thickness of few nanometers. The growth of the carbon layers takes place via a layer-by-layer process and the number of parallel layers can be controlled from several to more than 10 by adjusting the deposition time. Figure shows typical characteristics of the nanocables. The photoluminescence spectrum of these nanostructures shows a large blue shift as compared with that of the bulk sample, which is attributed to the quantum confinement effect. More recently, our group also demonstrated that CNTs can serve as nanoreactors for the high-yield fabrication of high-quality, single-crystalline Mg3N2 nanowires [Citation50]. The Mg3N2 nanowires were homogeneously sheathed with thin graphitic tubular layers that effectively prevent their decomposition, even when the samples are soaked in water. Owing to fast decomposition of Mg3N2 in humid atmosphere, the synthesis of such nanowires had previously been a challenge. The most recent addition to the small collection of encapsulated ternary compounds was reported by us [Citation51–53]. Ga-doped ZnS, or more precisely Zn0.92Ga0.08S, was deposited inside CNTs in a one-step, high-temperature process. The stacking of the external C layers (on average <10) was akin to that of pyrolytic CNTs (i.e. with turbostratic-type disorders), whereas the inner core, a long crystalline nanowire, had a wurtzite-type structure. The reaction yield was extremely high, with more than 99% CNTs entirely filled (figure ). Such yields are very rare for CNTs filled with compound materials. In fact, considering their morphological and structural characteristics, the Zn0.92Ga0.08S@MWCNTs are more like the systems obtained in situ, using a TEM [Citation14] than those resulting from conventional CNT filling methods.
Figure 7 (a) SEM and (b) TEM images of ultrafine InP nanowires sheathed by CNTs. (c) Electron diffraction pattern along the [001] zone axis. HRTEM image of (d) the nanocable, (e) the carbon layers and (f) energy-dispersive spectrum (EDS) of the nanocable. (Reproduced with permission from [Citation49] © 2004 AIP.)
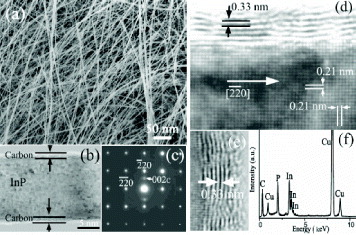
Figure 8 (a) Open end of a Zn0.92Ga0.08S@MWCNT. (b) Detail of the carbon shell and crystalline core [Citation51].
Thus, a number of approaches have been adopted to obtain filled CNTs based on the nature of the filling material. The synthesis temperatures varies from room temperature to as high as 1400 °C, while the specialized synthesis inside a TEM requires a low-pressure ambience. In table , we list the key synthesis strategies leading to the successful filling of CNTs with inorganic compounds. These materials exhibit many fascinating properties and potential applications, as discussed in the next section.
Table 1 Various types of inorganically filled CNTs. AAO stands for anodized aluminum oxide.
Properties and prospects of filled CNTs
The filling of an MWCNT results in a high-aspect-ratio wire that is protected against oxidation by the graphitic shield. The filled nanowire, unlike the bare ones, has a very small open surface area, but shares a high interfacial area with the carbon coat. This leads to unusual physical properties. For example, the regular growth direction of certain nanowires such as ZnS, changes from [0001] to [10-10] inside the CNTs [Citation53]. This allows the piezoelectric effect to be suppressed along the main axis of these materials. In addition, a combination of different functionalities can also be observed paving the way for novel applications, as predicted by a large number of recent theoretical investigations.
The magnetism of Co@CNTs was theoretically studied by Yang et al, showing a strong interaction with Co–C distance of about 2.2 Å [Citation54]. The Co nanowires have the magnetic moments of about 1.35 μB/atom irrespective of the chirality of the CNTs, which is larger than that of hcp bulk Co, but 33% lower than that of a freestanding Co nanowire. Their spin polarizations are higher than that of bulk Co owing to hybridization between the C p and the Co d orbitals. Jo et al investigated the electronic and magnetic properties of Fe, Co and Ni nanowires loosely wrapped in CNTs, such as those reported in the literature, using spin-polarized ab initio calculations and showed that a gap between the CNT shell and the nanowire can further enhance the magnetic moment [Citation55, Citation56]. Similarly, Kasai et al found that the magnetic moment of SWCNTs filled with 3d transition metals decreases with increasing atomic radius or decreasing SWCNT diameter. Specifically, the magnetic moment increases with the decreasing bonding strength between the metal atoms and carbon atoms [Citation57–59]. In the case of Pd nanowires inside SWCNT, the magnetism depends on the growth direction. Besides, the Pd–Pd bond length increases inside CNT in order to stabilize the CNT shell [Citation20]. The theoretical studies usually pertain to the SWCNTs and metallic nanowires where the diameter of the CNT is restricted to just a few nanometers, unlike experimentally obtained products.
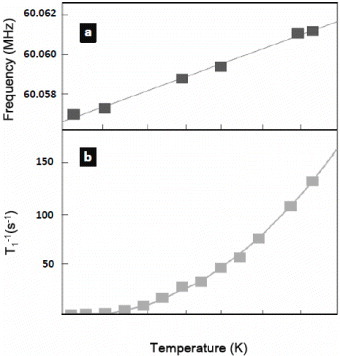
Many unique applications can be envisioned using these functional materials [Citation60–64]. One example is the fabrication of nanothermometers consisting of liquid Ga filled CNTs, wherein our group showed that the liquid Ga column inside the CNT expands and compresses reversibly within a wide temperature range of about 500 °C [Citation23]. This process requires calibration and the temperature measurement is tedious, as the Ga expansion has to be observed in situ and in real time. More recently, however, a reliable method of measuring the temperature without a calibration process was demonstrated. The method relies on the fact that the contraction of the Ga column after its initial expansion leaves a trail of thin gallium oxide layer on the CNT wall that can be easily identified a posteriori [Citation65]. Recently, a contactless nanothermometer has been developed for measurement of temperature fluctuations inside a biological system [Citation66]. It is based on CNTs filled with CuI, for which there is a strong relation between the temperature and the nuclear magnetic resonance (NMR) properties. Using this system, the accuracy of temperature measurement can be as high as 0.1 °C. Herein, CNT plays a major role, as it shields the toxic CuI from being dissolved in cells. Figure shows the temperature dependence of the 127I NMR signal from the filled CuI phase. Because CNTs can now be successfully transferred into human cell, this demonstration reveals great potential for therapeutic and diagnostic usage of these functional materials.
Figure 9 Temperature dependence of (a) NMR frequency and (b) nuclear spin lattice relaxation rate of 127I from CuI@CNT. The symbols represent experimental data and the solid lines are a fit. (Reproduced with permission from [Citation68] © 2008 Future Medicine Ltd.)
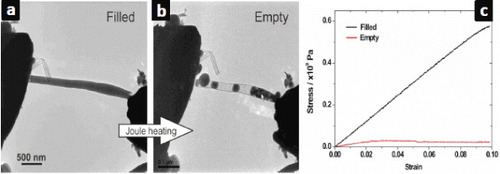
Investigations on the mechanical and electrical transport properties of these nanostructures have also been progressing recently. For instance, we have recently shown that the electrical conductance of Cu-filled CNTs can be manipulated so that the systems behave as rheostats [Citation67]. Changes to the electrical behavior of filled CNTs may also enable the design of efficient nanodelivery devices [Citation19]. In contrast to the electrical studies and controlled manipulation of fillings, for which there are several reports in the literature, the mechanics of inorganic compounds encapsulated in CNTs has still barely explored. In order to integrate these materials into nanoscale devices it is essential to know how they behave under external forces, such as compressive stress. Despite early theoretical reports, experimental evidence was provided only in 2009, in a pioneering study on the effect of the CNTs filling on the mechanical behavior of CNTs [Citation68]. The analyzed system was the above-described Zn0.92Ga0.08S@MWCNT. Using a combination of atomic force microscopy and transmission electron microscopy it was possible to estimate the elastic modulus of the filled CNTs before and after the release of the semiconducting core (figure ). It was found that, for this particular system, the stiffness of the composite is strongly dependent on the core.
Perspective, challenges and future outlook
Filled CNTs offer a new type of heterostructure with diverse properties and functionalities. Thus far, CNTs have been filled with a variety of materials such as magnetic and non-magnetic metals, alloys, ionic metal halides and inorganic functional materials such as oxides, sulfides, nitrides and phosphides. Their synthesis can be described as either a one-step or two-step process. In the former, the CNTs are usually larger in diameter, measuring about 100 nm, and are multi-walled. The two-step processes allow usage of thinner CNTs such as SWCNTs or DWCNTs. On the other hand, CNTs with diameters as large as several hundred nanometers have also been filled using CVD. The filling materials are usually polycrystalline in structure, although in a few cases, a single-crystalline filling has been obtained.
Uniform and size-controlled CNTs incorporating magnetic materials are of interest for many applications. For instance, they may be useful for drug delivery, because CNTs are non-immunogenic and the external surface of CNTs can be functionalized. Such systems can be manipulated using an external magnetic field, which should allow the controlled delivery of drugs loaded to the CNTs. In addition, magnetic metals encapsulated inside CNTs are of significant scientific interest because of their special physical properties, such as expanded lattice.
Amongst other applications, special mention can be made of nanothermometers with a high temperature range, such as Ga-filled CNTs, nanothermometers for biological applications with a possible sensitivity of 0.1 °C, and nanopipettes and spot-welding achieved with Cu-filled CNTs. In an interesting recent report, it was theoretically shown that nanoscale hydroelectric power generation is possible using water-filled CNTs [Citation69].
Overall, most CNTs encapsulated with compound materials are rather simple in structure and chemical composition. Owing to the inherent limitations of conventional filling methods, the number of highly complex structures and/or multi-elemental fillings has remained low and most successes in such syntheses were purely accidental. While the scope for further investigations and the impact of these materials on technology are undeniable, there are many challenges that must be overcome to realize the aforementioned technological goals. First, the synthesis procedure and growth mechanism of filled CNTs need to be understood in greater depth. This is essential to achieve better control of the size, diameter and wall thickness of the nanotube shells. The crystallinity of the filler is required to be controlled too, as most properties, such as mechanical, electrical and optical properties are determined by the crystal quality. Theoretical investigations dwell on SWCNTs. Therefore, to bridge the understanding of various phenomena in the experimental and theoretical works, it is important to develop strategies to fill SWCNTs at a large scale and with materials having various functionalities. A concomitant control of all the structural parameters and filling materials would lead to a new set of materials, F@CNT (where F = functionality), with immense potential for novel applications at the nanoscale.
Acknowledgment
This work was supported by the World Premier International (WPI) Initiative on Materials Nanoarchitectonics (MANA), MEXT, Japan.
References
- IijimaS 1991 Nature 354 56 http://dx.doi.org/10.1038/354056a0
- RaoC N RGovindarajA 2005 Nanotubes and Nanowires London Royal Society of Chemistry
- TasisDTagmatarchisNBiancoAPratoM 2006 Chem. Rev. 106 1105 http://dx.doi.org/10.1021/cr050569o
- DresselhausMDresselhausGAvourisP 2001 Carbon Nanotubes: Synthesis, Properties and Applications Berlin Springer
- KangiS JKocabasCOzelTShimMPimparakarNAlamM ARotkinS VRogersJ A 2007 Nat. Nanotechnol. 2 230 http://dx.doi.org/10.1038/nnano.2007.77
- AjayanP MIijimaS 1993 Nature 361 333 http://dx.doi.org/10.1038/361333a0
- UgarteDStöckliTBonardJ MChâtelainAde HeerW A 1998 Appl. Phys. A 67 101 http://dx.doi.org/10.1007/s003390050744
- MonthiouxMFlahautECleuziouJ P 2006 J. Mater. Res. 21 2774 http://dx.doi.org/10.1557/jmr.2006.0366
- AjayanP MEbbsenT WIchihashiTIijimaSTanigakiKHiuraH 1993 Nature 362 522 http://dx.doi.org/10.1038/362522a0
- DavisJ JGreenM L HHillH A OLeungY CSadlerP JSloanJXavierA VTsangS C 1998 Inorg. Chim. Acta 272 261 http://dx.doi.org/10.1016/S0020-1693(97)05926-4
- SeraphinSZhouDJiaoJWithersJ CLoutfyR 1993 Nature 362 503 http://dx.doi.org/10.1038/362503a0
- SloanJHammerJZwiefka-SibleyMGreenM L H 1998 Chem. Commun. 347
- SutterESutterP 2006 Adv. Mater. 18 2583 http://dx.doi.org/10.1002/adma.200600624
- EliSPeterSRaffaellaCTomaSRalphM 2007 Appl. Phys. Lett. 90 093118 http://dx.doi.org/10.1063/1.2710189
- LiuZ JCheRXuZPengL M 2002 Synth. Met. 128 191 http://dx.doi.org/10.1016/S0379-6779(02)00005-X
- SatishkumarB CGovindarajAVanithaP VRaychaudhuriA KRaoC N R 2002 Chem. Phys. Lett. 362 301 http://dx.doi.org/10.1016/S0009-2614(02)01082-5
- KimHSigmundW 2005 Carbon 43 1743 http://dx.doi.org/10.1016/j.carbon.2005.02.019
- LeonhardtARitschelMElefantDMatternNBiedermannKHampelSMullerCGemmingTBuchnerB 2005 J. Appl. Phys. 98 074315 http://dx.doi.org/10.1063/1.2058181
- DongLTaoXZhangLZhangXNelsonB J 2007 Nano Lett. 7 58 http://dx.doi.org/10.1021/nl061980+
- OhnoSObaYYabuuchiSSatoTKageshimaH 2008 J. Phys. Soc. Japan 77 104713 http://dx.doi.org/10.1143/JPSJ.77.104713
- ChenY KChuACookJGreenM L HHarrisP J FHeesomRHumphriesMSloanJTsangS CTurnerJ F 1997 J. Mater. Chem. 7 545 http://dx.doi.org/10.1039/a605652k
- ChanL HHongK HLaiS HLiuX WShihH C 2003 Thin Solid Films 423 27 http://dx.doi.org/10.1016/S0040-6090(02)00966-5
- GaoY HBandoY 2002 Nature 415 599 http://dx.doi.org/10.1038/415599a
- AjayanP MColliexCLambertJ MBernierPBarbedetteLTenceMStephanO 1994 Phys. Rev. Lett. 72 1722 http://dx.doi.org/10.1103/PhysRevLett.72.1722
- BaoJZhouQHongJXuZ 2002 Appl. Phys. Lett. 81 4592 http://dx.doi.org/10.1063/1.1526461
- SenRGovindarajARaoC N R 1997 Chem. Mater. 9 2078 http://dx.doi.org/10.1021/cm9700965
- MajectichS AArtmanJ OMcHenryM ENuhferN TStaleyS W 1993 Phys. Rev. B 48 16845 http://dx.doi.org/10.1103/PhysRevB.48.16845
- Guerret-PiecourtCLeBouarLoiseauAPascardH 1994 Nature 372 761 http://dx.doi.org/10.1038/372761a0
- LiuSZhuJMastalYFelnerIGedankenA 2000 Chem. Mater. 12 2205 http://dx.doi.org/10.1021/cm000062o
- HudziakSDarfeuilleAZhangRPeijsTMountjoyGBertoniGBaxendaleM 2010 Nanotechnology 21 125505 http://dx.doi.org/10.1088/0957-4484/21/12/125505
- GrobertNHsuW KZhuY QHareJ PKrotoH WWaltonD R MTerronesMTerronesHRedlichPRuhleM 1999 Appl. Phys. Lett. 75 3363 http://dx.doi.org/10.1063/1.125352
- RuskovT et al 2004 J. Appl. Phys. 96 7514 http://dx.doi.org/10.1063/1.1811781
- RuskovTSpirovIRitschelMMullerCLeonhardtARuskovR 2006 J. Appl. Phys. 100 084326 http://dx.doi.org/10.1063/1.2361090
- MarcoJ FGancedoJ RHernandoACrespoPPradosCGonzalezJ MGrobertNTerronesMWaltonD R MKrotoH W 2004 Hyperfine Interact. 139/140 535 http://dx.doi.org/10.1023/A:1021218727419
- WangX HOrikasaHInokumaNYangQ HHouP XOshimaHItohKKyotaniT 2007 J. Mater. Chem. 17 986 http://dx.doi.org/10.1039/b614300h
- LvRKangFWangWWeiJGuJWangKWuD 2007 Carbon 45 1433 http://dx.doi.org/10.1016/j.carbon.2007.03.032
- CostaP M F JSloanJHutchisonJ LGreenM L H 2003 Chem. Commun. 2276
- HutchisonJ L et al 2001 Carbon 39 761 http://dx.doi.org/10.1016/S0008-6223(00)00187-1
- BenzryadinC NLauATinkhamM Q 2000 Nature 404 971 http://dx.doi.org/10.1038/35010060
- NiliusNWallisT MHoW 2002 Science 297 1853 http://dx.doi.org/10.1126/science.1075242
- SloanJWrightD MWooH GBaileySBrownGYorkA P EColemanK SHutchisonJ LGreenM L H 1999 Chem. Commun. 699 http://dx.doi.org/10.1039/a901572h
- MuramatsuHHayashiTKimY AShimamotoDEndoMTerronesMDresselhausM H 2008 Nano Lett. 8 237 http://dx.doi.org/10.1021/nl0725188
- SloanJCookJChuASibleyM ZGreenM L HHutchisonJ L 1998 J. Solid State Chem. 140 83 http://dx.doi.org/10.1006/jssc.1998.7863
- SloanJTerronesMNuferSFriedrichsSBaileyS RWooH GRuhleMHutchinsonJ LGreenM L H 2002 J. Am. Chem. Soc. 124 2116 http://dx.doi.org/10.1021/ja0173270
- CostaP M F JFriedrichsSSloanJGreenM L H 2005 Chem. Mater. 17 3122 http://dx.doi.org/10.1021/cm050299q
- ChenGQiuaJQiuH 2008 Scr. Mater. 58 457 http://dx.doi.org/10.1016/j.scriptamat.2007.10.035
- KellerNPham-HuuCEstournèsCGrenècheJ MEhretGLedouxM J 2004 Carbon 42 1395 http://dx.doi.org/10.1016/j.carbon.2004.01.012
- TessonnierJ PWineGEstournesCLeuvreyCLedouxM JHuuC P 2005 Catal. Today 102 29 http://dx.doi.org/10.1016/j.cattod.2005.02.032
- YinL WBandoYZhuY CLiM S 2004 Appl. Phys. Lett. 84 5314 http://dx.doi.org/10.1063/1.1766079
- HuJBandoYZhanJZhiCGolbergD 2006 Nano Lett. 6 1136 http://dx.doi.org/10.1021/nl060245v
- GautamU KBandoYZhanJCostaPFangX SGolbergD 2008 Adv. Mater. 20 810 http://dx.doi.org/10.1002/adma.200701761
- GautamU KBandoYFangXZhanJGolbergD 2008 ACS Nano 2 1015 http://dx.doi.org/10.1021/nn800013b
- GautamU KBandoYBourgeoisLFangXCostaP M F JZhanJGolbergD 2009 J. Mater. Chem. 19 4414 http://dx.doi.org/10.1039/b903791h
- YangCZhaoJLuJ P 2003 Phys. Rev. Lett. 90 257203 http://dx.doi.org/10.1103/PhysRevLett.90.257203
- JoCLeeJ 2008 J. Magn. Magn. Mater. 320 3256 http://dx.doi.org/10.1016/j.jmmm.2008.06.022
- PradhanB KTobaTKyotaniTTomitaA 1998 Chem. Mater. 10 2510 http://dx.doi.org/10.1021/cm980266t
- KasaiKMorenoJ L VDavidiM YSarhanA A AShimojiNKasaiH 2008 Japan J. Appl. Phys. 47 2317 http://dx.doi.org/10.1143/JJAP.47.2317
- KishiTDavidMDinoW ANakanishiHKasaiH 2007 Japan J. Appl. Phys. 46 1788 http://dx.doi.org/10.1143/JJAP.46.1788
- DavidMKishiTKisakuMNakanishiHHasaiH 2007 Surf. Sci. 601 4366 http://dx.doi.org/10.1016/j.susc.2007.06.021
- DorozhkinP STovstonogS VGolbergDZhanJIshikawaYShiozawaMNakanishiHNakataKBandoY 2005 Small 1 1088 http://dx.doi.org/10.1002/smll.200500154
- SvenssonKOlinHOlssonE 2004 Phys. Rev. Lett. 94 145901 http://dx.doi.org/10.1103/PhysRevLett.93.145901
- HangB THayashiHYoonS HOkadaSYamakiJ 2008 J. Power Sources 178 393 http://dx.doi.org/10.1016/j.jpowsour.2007.12.012
- WinklerAMühlTMenzelSKosevaR KHampelSLeonhardtABüchnerB 2006 J. Appl. Phys. 99 104905 http://dx.doi.org/10.1063/1.2195879
- ZhanJBandoYHuJGolbergDNakanishiH 2005 J. Phys. Chem. B 109 11580 http://dx.doi.org/10.1021/jp050826t
- LiuZBandoYHuJRatinacKRingerS P 2006 Nanotechnology 17 3681 http://dx.doi.org/10.1088/0957-4484/17/15/010
- VyalikhA et al 2008 Nanomedicine 3 321 http://dx.doi.org/10.2217/17435889.3.3.321
- GolbergDCostaP M F JMitomeMHampelSHaaseDMuellerCLeonhardtABandoY 2007 Adv. Mater. 19 1937 http://dx.doi.org/10.1002/adma.200700126
- CostaP M F JGautamU KWangMBandoYGolbergD 2009 Carbon 47 541 http://dx.doi.org/10.1016/j.carbon.2008.11.031
- YuanQZhaoY P 2009 J. Am. Chem. Soc. 131 6374 http://dx.doi.org/10.1021/ja8093372