Abstract
This review describes recent efforts on the synthesis, dispersion and surface functionalization of the three dominating oxide nanoparticles used for photocatalytic, UV-blocking and sunscreen applications: titania, zinc oxide, and ceria. The gas phase and liquid phase synthesis is described briefly and examples are given of how weakly aggregated photocatalytic or UV-absorbing oxide nanoparticles with different composition, morphology and size can be generated. The principles of deagglomeration are reviewed and the specific challenges for nanoparticles highlighted. The stabilization of oxide nanoparticles in both aqueous and non-aqueous media requires a good understanding of the magnitude of the interparticle forces and the surface chemistry of the materials. Quantitative estimates of the Hamaker constants in various media and measurements of the isoelectric points for the different oxide nanoparticles are presented together with an overview of different additives used to prepare stable dispersions. The structural and chemical requirements and the various routes to produce transparent photocatalytic and nanoparticle-based UV-protecting coatings, and UV-blocking sunscreens are described and discussed.
1. Introduction
Successful implementation and optimized utilization of functional nanoparticles into commercial products ranging from catalysts, polishing media and magnetic fluids to cosmetics and sunscreens requires robust and cost-effective dispersion and surface functionalization routes [Citation1]. Indeed, the ability to prepare and handle concentrated and colloidally stable dispersions of nanoparticles in aqueous or non-aqueous media is important both in intermediate process steps and for the performance of the final product [Citation2, Citation3]. This review describes recent efforts in the dispersion and surface functionalization of the three dominating oxide nanoparticles used for photocatalytic [Citation4–Citation6], UV-blocking [Citation7–Citation9], and sunscreen applications: titania, zinc oxide, and ceria [Citation10].
Titanium dioxide and zinc oxide are wide bandgap semiconductors, which are transparent in the visible and absorb in the UV range. Upon UV irradiation, the photogenerated charge carriers can be used to generate a current, induce chemical reactions or emit light [Citation11, Citation12], One consequence of this process is photocatalysis, which can be defined as the light-induced degradation of organic molecules, driven by the formation of reactive radical species on the surface of the photocatalytic material (e.g. and
on illuminated TiO2) [Citation13, Citation14]. The radical formation also results in an increase in the surface density of hydroxyl groups that may change the wetting characteristics of TiO2 [Citation15, Citation16]. The photocatalytic activity increases with decreasing particle size [Citation17–Citation20] due to an increase in the specific surface area, down to a critical particle size, which is about 10 nm for titania, below which the wide bandgap and the high probability for electron/hole surface recombination results in a loss of photoactivity under visible light [Citation21].
Transparent, self-cleaning titania coatings on glass are today widely used and sold under several trade names [Citation22]. These coatings combine photocatalytic breakdown of absorbed organic dirt [Citation23] with light-induced superhydrophilic surface properties [Citation24] that promote water to evenly wet and rinse away the decomposition products. The titania films are normally applied by dip coating of an organic precursor followed by heat treatment to remove the organic residue and form the desired anatase phase. With the commercial success of self-cleaning glass in window frames of high-rise buildings, there is a growing interest in applying photocatalytic and self-cleaning coatings to the façades of buildings and to other construction materials in urban areas. This will not only keep the surfaces clean but also reduce the concentrations of harmful pollutants in the city air, such as NOx and other airborne contaminants (e.g. volatile organic compounds, VOCs) [Citation25]. The anti-bacterial properties of photocatalytic coatings are also attractive as a means to mitigate the problem of persistent bacteria, mainly in hospitals [Citation26]. In many cases, it is not possible to subject the façade or building material to the thermal treatment needed to transform a traditional sol–gel coating to a transparent anatase film. Hence, alternative low-temperature routes where transparent films can be formed directly, e.g. from dispersions containing photocatalytic nanoparticles, are needed.
Transparent UV-absorbing or UV-blocking coatings [Citation8, Citation9] have two main applications at present: as a UV-protecting lacquer for wooden surfaces, and as a UV-barrier coating deposited onto the surface of polymer-based products or devices to reduce the ageing of the polymer or internal components encased inside the polymer-based device. Transparent UV-blocking coatings are at an earlier stage of development compared to the established photocatalytic and self-cleaning coatings, but industrial interest is rapidly increasing with growing demands for durability without compromising the esthetic appearance of the underlying material. The limited thermal resistance of wood and most polymers means that UV-blocking coatings are normally produced directly from dispersions of UV-absorbing nanocrystals without a subsequent heating step.
Nanoparticle-based sunscreen formulations are already available commercially. Efficient utilization of the broad absorption in the UVB and UVA regions of titanium dioxide and zinc oxide in transparent sunscreens requires not only that the particle size be reduced far below the wavelength of visible light but also that agglomeration be minimized. The development of formulations of nanosized nanoparticles of titanium dioxide and zinc oxide with minimized light scattering in the visible region [Citation27, Citation28], together with reports on allergic reaction to some organic UV attenuators [Citation29], have resulted in a rapid increase of titania and zinc oxide in sunscreens. Currently, most of the commercial sunscreens incorporate titanium dioxide, zinc oxide, or both.
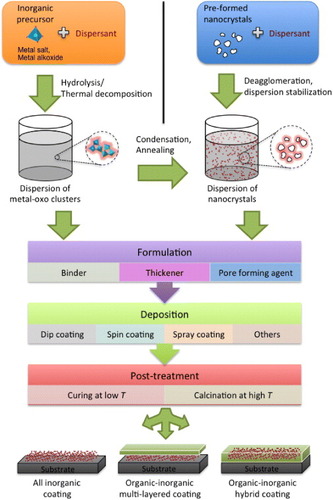
This review attempts to provide an overview of the fundamental principles of deagglomeration and stabilization of nanoparticles as well as describe the main processing steps for the production of photocatalytic and UV-blocking coatings and sunscreens, illustrated in figure . The main synthesis methods producing titania, zinc oxide and ceria nanoparticles are reviewed with a focus on recent liquid state studies that generate non-agglomerated powders. Agglomeration is often the major cause of poor performance and limited transparency. The deagglomeration process is described in detail and the challenges related to handling nanopowders are highlighted together with examples on primary titania nanoparticles. The main particle interactions—van der Waals, electrical double layer, and polymer induced forces—in dispersions of nanoparticles are described and important parameters for the three oxides, like the Hamaker constant and isoelectric point, are tabulated. Organic additives that are both efficient dispersants and can also serve to improve the compatibility of the inorganic particles with an organic matrix are identified.
Figure 1. Schematic illustration of the main processing steps involved in the formation of transparent coatings containing oxide nanoparticles.
The requirements for transparency of thin films are described and guidelines are presented for how the particle size or aggregates affect the transparency. The various methods used to deposit nanoparticle-containing films onto a substrate are briefly described with a discussion on the importance of the colloidal stability and rheological properties. The influence of the coating formulation and the post-processing treatment on the performance of photocatalytic coatings is illustrated by numerous examples from the literature. The structural and chemical requirements and the various routes to produce transparent nanoparticle-based UV-protecting coatings and UV-blocking sunscreens are described and discussed. An outlook discussing not only the technical performance but also concerns related to distributing nanoparticles in the environment is presented. Finally, the future prospects are described and promising materials, such as multifunctional coatings and hybrid films, are identified.
2. Synthesis of TiO2, ZnO and CeO2 nanoparticles
Oxide nanoparticles can be produced by several methods, which can be broadly classified by the physical state of the carrier or continuous media (gas or liquid). Gas-phase methods have in general a higher output capacity and lower production costs compared to liquid-phase routes. Hence, most of the commercially available oxide nanopowders intended for large-scale applications are produced by gas-phase techniques, which form nanocrystals with a high yield and high purity [Citation30, Citation31]. A noteworthy example is the Aeroxide P25 titania (anatase/rutile) powder used primarily for photocatalytic applications [Citation32]. However, oxide nanopowders produced in the gas phase are often heavily aggregated, which can severely limit the utilization of the inherent properties, pose substantial processing challenges and complicate the dispersion and functionalization of the nanoparticles [Citation33–Citation36]. Indeed, films and coatings produced from aggregated gas-phase powders are often hazy, with poor adhesion to the substrate [Citation37, Citation38].
In contrast to gas-phase reactions, where agglomeration is inherent and impossible to avoid, it is possible to design liquid-phase synthesis routes that also keep the particles colloidally stable as they grow. The production of non-agglomerated particles with a narrow size distribution [Citation39, Citation40] is typically achieved by adding organic additives that adsorb (weakly) onto the surface of the growing particles and simultaneously provide a means to control the growth rate and the extent of agglomeration [Citation41, Citation42]. Liquid-phase synthesis methods are also able to yield dispersed nanoparticles that can be functionalized in situ. However, the low concentration of reactants and the associated need to process large volumes of solvents, and time-consuming post-treatment routes are restricting and hampering the commercial introduction of liquid-phase-synthesized nanopowders for large-scale applications.
The liquid-phase synthesis of TiO2, ZnO and CeO2 nanoparticles has been described extensively in a number of reviews [Citation43–Citation46]. Some recent work has also reported the preparation of mesoporous TiO2, ZnO and CeO2 particles [Citation47–Citation49]. Here, we will present a concise account of synthesis routes for non-aggregated crystalline TiO2, ZnO and CeO2 nanoparticles. The methods were selected based on the demonstrated formation of non-aggregated particles smaller than 50 nm and a potential for up-scaling through the utilization of relatively concentrated precursor solutions (above 0.1 mol l−1).
Three polymorphs of TiO2 have been identified at atmospheric pressure: rutile, anatase and brookite. Anatase is metastable and tends to transform into rutile upon heating, typically above 600 °C for bulk anatase [Citation50, Citation51]. Brookite is metastable too and is rarely the main phase in synthetic powders; it has thus been less studied than the two other phases. Whereas rutile is the stable polymorph (in bulk form), anatase and brookite become thermodynamically stable when the crystal size decreases and are quite common in fine-grained powders [Citation52]. Anatase has often been identified as the most photoactive phase of TiO2 [Citation53, Citation54] with some notable exceptions [Citation55, Citation56], suggesting that comparison of the photocatalytic efficiency of anatase and rutile is not straightforward.
The synthesis of titania nanoparticles has been the subject of a very large research activity [Citation12, Citation43]. The large free energy of formation of titania is reflected in the high reactivity of most titania precursors, making it difficult to design synthesis routes with a high degree of size and shape control. Moreover, nanoparticles synthesized at near-ambient temperature are often amorphous or poorly crystallized in the anatase form [Citation57, Citation58]. Thus, significant efforts have been made to reduce the reactivity of the precursor and to improve the crystallinity [Citation59, Citation60].
Figure shows transmission electron microscopy (TEM) and scanning electron microscopy (SEM) images of TiO2 nanoparticles produced by different chemical routes. The hydrolysis and condensation of tetrabutyl orthotitanate Ti(O-tBu)4 at low temperature (60 °C) can be controlled in the presence of acetylacetone and paratoluene sulfonic acid, yielding anatase nanocrystals of 7 nm [Citation61]. Carboxylic acids are also commonly used to reduce the reactivity of the precursors and stabilize the particles. Barbé et al [Citation62] obtained anatase nanoparticles from the hydrolysis of titanium tetraisopropoxide (TTIP) with acetic acid, which results in a sol of acetate-capped particles that can be autoclaved without agglomeration. Oleic acid has been used as solvent to produce anatase nanorods from concentrated TTIP solutions with amines as crystallization promoters and shape-directing agents [Citation63, Citation64]. Benzyl alcohol is a versatile reaction medium that can act as both a solvent and capping agent. Niederberger showed that the reaction between titanium tetrachloride and benzyl alcohol [Citation65] yields anatase nanocrystals with a diameter of about 15 nm.
Figure 2. TEM and SEM images of titania nanoparticles synthesized by different chemical routes (a) Anatase nanocrystals obtained by hydrothermal treatment in acetic acid of amorphous electrospun TiO2 nanofibers. Reprinted with permission from Dai et al [Citation66], © (2009) American Chemical Society [Citation66]. (b) Anatase nanocrystals synthesized in benzyl alcohol, after annealing at 450 °C; reprinted with permission from Niederberger et al [Citation65], © (2002) American Chemical Society. (c) Acetate-capped anatase particles synthesized through hydrothermal treatment of titanium isopropoxide at 230 °C; reprinted from Barbé et al [Citation62], © (1997), with permission from John Wiley and Sons. (d) Tetramethylammonium-capped anatase particles synthesized by hydrothermal treatment of a titanium isopropoxide solution in water-propanol with tetramethylammonium hydroxide; reprinted from Chemseddine et al [Citation67], © (1999), with permission from John Wiley and Sons. (e) Tetramethylammonium-capped anatase nanoparticles produced by hydrothermal synthesis. Reprinted with permission from Yang et al [Citation68], © (2001), John Wiley and Sons. (f) Aeroxide P25 aggregated nanocrystals synthesized by flame pyrolysis. Reprinted with permission from Faure et al [Citation69], © (2010) Elsevier.
![Figure 2. TEM and SEM images of titania nanoparticles synthesized by different chemical routes (a) Anatase nanocrystals obtained by hydrothermal treatment in acetic acid of amorphous electrospun TiO2 nanofibers. Reprinted with permission from Dai et al [Citation66], © (2009) American Chemical Society [Citation66]. (b) Anatase nanocrystals synthesized in benzyl alcohol, after annealing at 450 °C; reprinted with permission from Niederberger et al [Citation65], © (2002) American Chemical Society. (c) Acetate-capped anatase particles synthesized through hydrothermal treatment of titanium isopropoxide at 230 °C; reprinted from Barbé et al [Citation62], © (1997), with permission from John Wiley and Sons. (d) Tetramethylammonium-capped anatase particles synthesized by hydrothermal treatment of a titanium isopropoxide solution in water-propanol with tetramethylammonium hydroxide; reprinted from Chemseddine et al [Citation67], © (1999), with permission from John Wiley and Sons. (e) Tetramethylammonium-capped anatase nanoparticles produced by hydrothermal synthesis. Reprinted with permission from Yang et al [Citation68], © (2001), John Wiley and Sons. (f) Aeroxide P25 aggregated nanocrystals synthesized by flame pyrolysis. Reprinted with permission from Faure et al [Citation69], © (2010) Elsevier.](/cms/asset/38b350fe-d513-4d59-a82e-49c89fd582ee/tsta_a_11661359_f0002_oc.jpg)
Zinc oxide precursors are less reactive than their titania counterparts and the formation of the oxide nanoparticles typically proceeds through a series of intermediate compounds [Citation44]. Hence, the as-synthesized particles often consist of a mixture of zinc hydroxide species that are sensitive to the water content and to ageing [Citation44, Citation70]. Figure shows some examples of ZnO nanoparticles prepared by different routes. Aqueous synthesis of spheroidal wurtzite ZnO nanoparticles can be achieved at near room temperature using zinc nitrate and tris(hydroxymethyl)aminomethane, which is a polydentate ligand that adsorbs strongly on the particles, inhibiting crystal growth [Citation71]. Concentrated zinc oxide nanocrystal suspensions can be obtained by adding a base (LiOH, NaOH or tetramethylammonium hydroxide) to zinc acetate dihydrate dissolved in alcohol [Citation72–Citation74]. The acetate-capped nanoparticles obtained by this sol–gel method exhibit photoluminescence both in the near UV (370 nm) and in the visible range (500–550 nm), the former being related to excitonic emission and the latter to the presence of oxygen vacancies on the surface. The visible emission might be problematic for some applications and can be quenched by a thermal post-treatment or copper doping [Citation44]. Alkylamines are often used as a base and shape-directing agent for the formation of rod-like ZnO particles or oriented films [Citation75, Citation76]. The solvothermal reaction between zinc acetate and ethanol at 120 °C results in wurtzite nanocrystals, formed upon the release of hydroxide ions during the formation of ethylacetate [Citation77]. Teardrop-shaped wurtzite nanocrystals can be obtained from the controlled dissolution-precipitation of ZnO in a mixture of oleic acid, octadecene and hexadecylamine [Citation78]. Bullet-shaped wurtzite nanoparticles were obtained from the thermal decomposition of zinc stearate in 1-octadecene in the presence of a small amount of octadecylamine [Citation79].
Figure 3. TEM micrograph of ZnO and CeO2 nanoparticle produced by various routes. (a) Wurtzite ZnO nanoparticles precipitated in aqueous solution at pH 8 and 37 °C. Reprinted from Bauermann et al [Citation71] © (2006) American Chemical Society. (b) ZnO wurtzite nanocrystals formed by solvothermal reaction in ethanol (scale bar 200 nm) [Citation77]; (c) bullet-shaped ZnO nanocrystals wurtzite nanocrystals prepared by thermal decomposition of zinc stearate in 1-octadecene in the presence of octadecylamine. Reprinted from Jana et al [Citation79] © (2004) American Chemical Society. (d) TEM and corresponding high-resolution-TEM images of CeO2 nanoparticles synthesized from cerium (IV) and ammonium carbonate aqueous solutions under reflux conditions. Reprinted from Sutradhar et al [Citation97] © (2011) American Chemical Society. (e) Hydrophilic ceria nanoparticles synthesized from ammonium cerium (IV) nitrate in water by supercritical flow method at 400 °C. Reprinted from Slostowski et al [Citation100] © (2012) American Chemical Society. (f) Hydrophilic ceria nanoparticles synthesized from the Ce(CO3)2·3H2O + NH4OH + H2O2 system using acetic acid in water by a hydrothermal method and corresponding electron diffraction pattern. Reprinted from Tok et al [Citation86], © (2007), with permission from Elsevier.
![Figure 3. TEM micrograph of ZnO and CeO2 nanoparticle produced by various routes. (a) Wurtzite ZnO nanoparticles precipitated in aqueous solution at pH 8 and 37 °C. Reprinted from Bauermann et al [Citation71] © (2006) American Chemical Society. (b) ZnO wurtzite nanocrystals formed by solvothermal reaction in ethanol (scale bar 200 nm) [Citation77]; (c) bullet-shaped ZnO nanocrystals wurtzite nanocrystals prepared by thermal decomposition of zinc stearate in 1-octadecene in the presence of octadecylamine. Reprinted from Jana et al [Citation79] © (2004) American Chemical Society. (d) TEM and corresponding high-resolution-TEM images of CeO2 nanoparticles synthesized from cerium (IV) and ammonium carbonate aqueous solutions under reflux conditions. Reprinted from Sutradhar et al [Citation97] © (2011) American Chemical Society. (e) Hydrophilic ceria nanoparticles synthesized from ammonium cerium (IV) nitrate in water by supercritical flow method at 400 °C. Reprinted from Slostowski et al [Citation100] © (2012) American Chemical Society. (f) Hydrophilic ceria nanoparticles synthesized from the Ce(CO3)2·3H2O + NH4OH + H2O2 system using acetic acid in water by a hydrothermal method and corresponding electron diffraction pattern. Reprinted from Tok et al [Citation86], © (2007), with permission from Elsevier.](/cms/asset/8094c733-a448-41bd-b211-64e7ab50ca53/tsta_a_11661359_f0003_oc.jpg)
Cerium dioxide (CeO2) in a loosely aggregated form is frequently produced by precipitation of cerium salts (e.g. cerium nitrate or cerium sulfate) in liquid media at elevated temperature. While the precipitation conditions (temperature, solvent, counter ions and final pH of the reaction), can have a profound effect on the size and shape of the cerium oxide nanoparticles, it is impossible to avoid agglomeration unless surfactants are introduced during the precipitation process [Citation80]. Sometimes, synthesis is performed under mechanical disintegration (ball-milling). Mechanical–chemical processes may not only deagglomerate the nanoparticles but also have an influence on the reaction kinetics and the crystallization behaviour [Citation81]. Interestingly, the use of both NH4OH and H2O2 results in the formation of intermediate species (e.g. Ce(OH)x(OOH)4−x), which transform into weakly-agglomerated nanosized ceria upon heating [Citation82].
Efforts to develop easy and scalable liquid-state synthesis routes of ceria have included a range of batch and continuous methods [Citation45, Citation83–Citation90]. Ceria nanoparticles can be produced in aqueous media by generating the precipitating agents in situ, e.g. by the slow decomposition of chemicals like urea [Citation91–Citation96], ammonium carbonate [Citation97], or hexamethylenetetramine (HMTA), but are often loosely aggregated [Citation20, Citation98, Citation99]. The decomposition of these salts can have a double effect on preventing agglomeration through (i) bridging of the metal ions and (ii) their decomposition into ammonia, carbamates, and aldehydes, which can result in electrosteric repulsion between the particles.
The simultaneous synthesis and surface modification of loosely aggregated dispersible CeO2 nanoparticles has been achieved in continuous flow reactors [Citation100–Citation105]. Figures (e) and (f) show examples of both water dispersible and solvent dispersible nanoparticles synthesized by supercritical flow synthesis in water. The process is also suitable for the production of complex and composite metal oxide nanoparticles [Citation103].
3. Deagglomeration of nanoparticles
Full utilization of the inherent properties of nanoparticle-based materials usually requires that the powders be deagglomerated down to a size that either represents the individual nanocrystallites or the smallest, primary, agglomerates. Minimization of the flaw size is, e.g., essential to obtain a high reliability and high strength, and transparency and other optical properties are highly sensitive to the presence of inhomogeneities with sizes in the order of the wavelength of the electromagnetic radiation of interest.
Most large-scale nanoparticle synthesis routes yield powders that are either weakly or strongly agglomerated. Agglomeration is commonly observed in nanopowders that have been produced in the gas phase or dried from the liquid phase, as shown for titania in figure . Indeed, agglomeration is inevitable during gas-phase synthesis, due to the ubiquitous attractive van der Waals interactions between the particles in the gas phase [Citation106]. If the synthesis temperature is high, partial sintering may reinforce the agglomerates by forming so-called inter-particle necks [Citation32–Citation34]. Agglomeration is also driven by the capillary forces generated by drying a dispersion; this effect is more pronounced for aqueous dispersions of hydrophilic nanoparticles, e.g. oxides. Moreover, the contact points between the grains may act as nucleation sites for the condensation of dissolved material, which leads ultimately to the formation of necks between the grains resulting in so-called ‘hard’ agglomerates.
Figure 4. The effect of high-pressure deagglomeration on the morphology and size of the aggregates of flame-made TiO2 particles; (A) as synthesized large agglomerates and aggregates, (B) smaller aggregates after high-pressure deagglomeration through a nozzle (1400 bar). Reprinted from Powder Technology Teleki et al [Citation34], © (2008), with permission from Elsevier.
![Figure 4. The effect of high-pressure deagglomeration on the morphology and size of the aggregates of flame-made TiO2 particles; (A) as synthesized large agglomerates and aggregates, (B) smaller aggregates after high-pressure deagglomeration through a nozzle (1400 bar). Reprinted from Powder Technology Teleki et al [Citation34], © (2008), with permission from Elsevier.](/cms/asset/c2327e7f-b591-48e5-9b64-678a16cc7036/tsta_a_11661359_f0004_oc.jpg)
Deagglomeration proceeds through the break-up of bonds between single nanoparticle crystallites in the aggregates. The adhesive interparticle forces that both cause the crystallites to aggregate and keep them together can be reduced by creating a repulsive interaction or simply by keeping the surfaces of the nanoparticles separated by an adsorbed adlayer that acts as a spacer. Milling, high-shear mixing and ultrasonication are commonly used to break down agglomerates. Upon milling, the mechanical impact between the particles and the grinding media result in both the breakup of agglomerates and the reagglomeration of particles, which broadens the particle size distribution. It is well known from experiments that the size reduction rates decrease with decreasing particle size [Citation107], e.g., due to the decrease of inertial and hydrodynamic forces and the increase of aggregate strength with decreasing particle size [Citation30, Citation66, Citation108]. In practice, this means that the milling efficiency is significantly reduced at particle sizes below 500 nm and further size reduction requires long milling times at very high collision speeds, which may increase the risk of contamination and unwanted phase transitions [Citation109, Citation110].
Milling, even at very large energy inputs, is best suited for deagglomeration of ‘soft’ agglomerates that are held together by physical interparticle bonds, i.e. van der Waals, hydrogen, electrostatic bonds, but less well suited to deagglomerate so-called ‘hard’ nanoparticle aggregates that are bonded together by interparticle necks [Citation111, Citation112]. Figure (b) shows that even high-pressure deagglomeration is not able to fully deagglomerate TiO2 nanoparticles, which strongly suggests that this gas-phase-produced material contains hard agglomerates [Citation111, Citation112].
Chemical approaches can complement mechanical routes to deagglomerate hard agglomerates. Chemical deagglomeration focuses on controlled dissolution of the interparticle necks by subjecting the nanoparticle aggregates to weakly aggressive conditions. Laarz et al [Citation113] showed how controlled dissolution of the silica necks between silicon nitride nanoparticle solutions could be accomplished by subjecting the material to alkaline solutions. Chemical deagglomeration of oxide nanoparticles requires it to be possible to dissolve or chemically attack the necks without dissolving or deteriorating the nanocrystals. Hence, differences in solubility between the interparticle necks and the ‘bulk’ nanocrystals should be utilized to chemically deagglomerate the nanoparticles. The difference in curvature between the neck and the nanocrystal and possible differences in degree of crystallinity could be sufficient to select conditions where the necks dissolve while the nanocrystal remains unaffected. The three oxides of interest in this review display significant differences in their degree of inertness in aqueous media, related, e.g., to the ionic potential of the metal cations [Citation71]. Thus, the solubility of ZnO in water depends strongly on the pH, and is high below pH 7 or above pH 13, while CeO2 and, in particular, TiO2 are relatively inert [Citation114–Citation116].
4. Stabilization and surface functionalization of nanoparticle dispersions
The utilization of photoactive and UV-blocking nanoparticles in transparent coatings and films relies on the ability to avoid agglomeration and to adapt and optimize the film forming and deposition processes to ensure that the nanosized functional particles are distributed either homogeneously or in desired patterns [Citation117]. Providing robust methods to prepare colloidally stable dispersions in polar or non-polar media is of pivotal importance and a process that requires a fundamental understanding of the nature and magnitude of the interparticle forces [Citation3]. Controlling the compatibility between the nanoparticles and the matrix, either polymeric or inorganic, is also very important in order to tune the microstructure of the final material.
4.1. Nanoparticle interactions
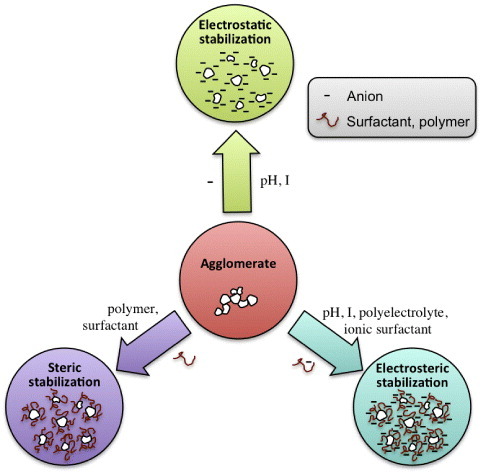
Interparticle forces play a pivotal role in determining the rheology and light-scattering behavior of nanoparticle dispersions. For example, concentrated nanoparticle dispersions can be transformed from an easily pourable liquid to a stiff paste by changing the interparticle forces from repulsive to attractive. Flocculation or aggregation will induce the formation of large clusters or aggregates that will scatter light. The ability to control and manipulate the sign of particle interactions represents a first step towards optimized nanoparticle processing. The dominating interparticle forces in most nanoparticle systems are the van der Waals, double layer (electrostatic), and steric (polymeric) forces, as shown in figure . We will give a brief account of these interactions with relevant examples for the three oxide systems of interest in this review.
Figure 5. Schematic illustration of the main mechanisms for stabilization of nanoparticle dispersions, assuming positively charged surfaces.
The van der Waals or dispersion force is an electrodynamic interaction that arises as a consequence of the interactions between fluctuating or permanent dipoles between molecular and macroscopic bodies (i.e. nanoparticles in this context) in close proximity [Citation3, Citation118, Citation119]. The magnitude of the van der Waals (vdW) pair interaction energy, VvdW , scales with the value of the Hamaker constant A [Citation120], and decreases with the separation distance, s. The exact form of the distance scaling depends on the geometry of the interacting particles, the pair interaction for two spheres of radius r, is shown in equation (Equation11 ).
1
The Hamaker constant is material dependent and increases with the contrast in dielectric properties between the particles and the solvent. It is possible to directly measure the van der Waals forces between inorganic particles using atomic force microscopy and also to estimate the magnitude of A from optical data using Lifshitz theory [Citation121, Citation122]. Table provides estimates of the Hamaker constants for oxide nanoparticles interacting in three commonly used solvents: water, isopropanol and hexane. The Hamaker constant for TiO2 is significantly larger than the Hamaker constant for CeO2, and ZnO.
Table 1. Material-dependent parameters involved in the colloidal interactions between CeO2, TiO2 and ZnO nanoparticles.
Colloidally stable nanoparticle dispersions require that some type of interparticle repulsion is introduced to overcome the ever-present van der Waals attraction. In a stable system, the maximum attractive interparticle energy should be sufficiently small, in the order of 1 - 2 kB T , to allow thermal motion or agitation to readily break all particle–particle bonds. Below, we will describe the two most common methods of stabilizing a nanoparticle dispersion; either by creating an electrical double layer at the solid–liquid interface or by adsorbing polymers or surfactants on the nanoparticle surfaces.
Electrical double layer interactions originate from the accumulation of counter ions near a charged surface. A net charge may build up on the surfaces of nanoparticles in liquids through various mechanisms such as the dissociation of surface groups, the specific adsorption or dissolution of ions, and the presence of crystalline defects [Citation132]. The dissociation of surface hydroxyl groups is the main charging-up mechanism for metal oxide surfaces in water and depends on the pH:
The pH at which the net surface charge is neutral is called the isoelectric point (IEP), and is typically close to pH 6.5 for CeO2 and TiO2, while the surface of ZnO is more alkaline with an IEP at pH 9, as shown in table [Citation123]. The adsorption of charged molecules with a high affinity for the surface can also be used to increase the surface charge. The range of these interactions is defined by the Debye length κ
- 1 and strongly decreases with increasing ionic strength I :
2 where ε
r is the dielectric constant of the solvent, ε0 the permittivity of vacuum, kB the Boltzmann constant, T the temperature, NA Avogadro's number and e the elementary charge. A high concentration of free ions will screen the repulsive double layer interactions and decrease their range. Therefore, electrical double layer stabilization, or electrostatic stabilization as it is frequently but somewhat erroneously called, is not recommended for the deposition of particles onto a substrate from evaporating aqueous suspensions. The ionic strength will increase upon drying, which may lead to agglomeration when the critical coagulation concentration is reached. Electrical double layer interactions can also be used to stabilize particles suspended in low-polar solvent (ε
r < 11 ) [Citation133]. Widegren and Bergström showed how the effective pH can be controlled and measured in ethanol and demonstrated that colloidally stable dispersions can be produced [Citation134].
In many nanoparticle systems, it is not possible to create a stable dispersion simply by controlling the pH. Hence, addition of suitable surfactants or polymeric dispersants is commonly used to provide a so-called polymer-induced or steric stabilization. Polymer-induced interactions arise when the adsorbed surfactants or polymers have segments or chains that protrude into the solvent and thus provide a protective adlayer on the nanoparticle surfaces. Several conditions should be fulfilled for efficient steric stabilization: the adsorbed layer should be thick enough to screen the attractive van der Waals interaction, the adsorbed molecules should be strongly adsorbed and cover the entire nanoparticle surface, the segments protruding into the solvent should be in so-called good solvent conditions [Citation135]. The polymer layers induce an increase in the pair-interaction energy when the adsorbed segments overlap at short separation distances [Citation136]. For metal oxide nanoparticles, the high surface density of hydroxyl groups is commonly used as the specific surface groups that are targeted for the adsorption groups of the surfactants or the polymer dispersants. High surface coverage is typically achieved through hydrogen-bonding moieties (e.g. ethers, alcohols and acrylamides), acid–base reactions (e.g. carboxylic acids) or electrostatic interactions (e.g. phosphates and carboxylates) [Citation137, Citation138]. Even if the affinity of each individual segment for the particle surface is low, the multiplicity of anchoring groups in the whole polymer chain provides a strong adsorption [Citation139].
4.2. Stabilization and surface functionalization of oxide nanoparticles
Colloidal dispersions of metal oxide particles can be stabilized by electrical double layer interactions, e.g., by adjusting the pH away from the pH value of the IEP (table ). Sometimes, simple ions can also promote the colloidal stability. Nitrate and acetate ions were found to specifically adsorb onto the surface of CeO2, and enhance the colloidal stability below pH 3 [Citation140, Citation141]. Due to the presence of hydroxyl groups on the surfaces of most oxides, similar additives can be used to stabilize aqueous dispersions of CeO2, TiO2 and ZnO, differing mainly in the pH conditions at which they are efficient. A list of organic additives displaying an affinity for the surfaces of the three oxides is provided in table . By combining the adsorbing moieties listed in the table with various steric moieties, stable dispersions can be prepared in a wide range of solvents [Citation135].
Table 2. List of additives for the dispersion of CeO2, TiO2 and ZnO.
Polyelectrolytes are frequently used as dispersants of metal oxide nanoparticles. The term electro-steric stabilization is often used to describe how polyelectrolytes act as dispersants (figure ). Electrosteric stabilization is a combination of a pure electrostatic repulsion and a polymer-induced repulsion. If the polyelectrolyte adsorbs in a flat conformation, the polymeric repulsion is short range, and the stabilization mechanism is mainly electrostatic. With thicker adsorbed layers, having chains protruding into the solution, the polymeric contribution will become more important. In addition to the steric contribution, there is always an electrostatic contribution since the adsorption of a highly charged polyelectrolyte on a weakly charged, amphoteric oxide surface usually results in an increase of the net surface charge density. Dispersants containing carboxylic groups (e.g., polyacrylic acid (PAA) and polyacrylamide (PAM)) interact with the surface through electrostatic interactions and H-bonding. Such dispersants have been used successfully to stabilize TiO2 and ZnO below their IEP [Citation114, Citation142, Citation143].
Figure 6. TiO2 particles prepared without (A) and with HPC (B). Reprinted from Park et al [Citation157], © (1997), with permission from John Wiley and Sons.
![Figure 6. TiO2 particles prepared without (A) and with HPC (B). Reprinted from Park et al [Citation157], © (1997), with permission from John Wiley and Sons.](/cms/asset/d5ff1f03-c402-4170-ab53-66bb8e39537e/tsta_a_11661359_f0006_oc.jpg)
It has been found that anionic, predominantly carboxylic acid-based polyelectrolytes have a significantly stronger interaction with metal oxide surfaces compared to other functional groups. The efficient stabilization of CeO2 by the addition of PAA at pH values between pH 6 and pH 9, has been related to the favorable interaction between the negatively charged carboxylate groups on the PAA and the weakly positively charged hydroxyl groups on the ceria surface [Citation80, Citation144]. Amines were found to have an overall weak affinity for TiO2 and ZnO in water [Citation145, Citation146]. Polyethers, which are commonly used as binders, have a low affinity for the surfaces of TiO2 and ZnO and presumably interact primarily by hydrogen bonding [Citation147, Citation148]. Combining polyether chains with a strongly adsorbing head group has resulted in efficient dispersants in water, e.g. phosphonated polyethlene glycol (PEG) for dispersions of CeO2 [Citation149]. The strongly adsorbed PEG-based dispersant provides a steric layer that is able to extend the stability range of the original ceria dispersion (that was stable only below pH 3) up to pH 9.
High-molecular weight polymers are commonly used as binders to enable handling and drying without cracking. However, sometimes the binder can also act as a dispersant, e.g., in recent studies on ZnO, where polyvinyl pyrrolidone (PVP), was found to be a better stabilizing agent than PVA and improved the optical properties significantly [Citation150, Citation151].
The adsorption of additives on hydroxylated metal oxide surfaces in non-aqueous systems relies on the same anchoring groups as the aqueous systems. In water–alcohol mixtures, acetate groups or ethers containing hydroxyl groups such as hydroxypropylcellulose (HPC) can be used as dispersant [Citation73, Citation157, Citation159]. As can be seen in figure , HPC prevents the aggregation of TiO2 nanoparticles.
Propylamine is beneficial for the dispersibility and film-forming ability of ZnO in chlorobenzene and chloroform, without preventing electron-transfer processes between the nanoparticles [Citation160, Citation170]. An amine-based dispersant has also been reported for TiO2 in styrene and hexane [Citation166].
5. Transparent photocatalytic and UV-absorbing coatings
This section will describe the requirements for transparency and provide guidelines for how the refractive index difference and the size of particles or aggregates control the degree of transparency of thin coatings and films. The various methods used to deposit nanoparticle-containing films onto a substrate are briefly described with a discussion on the importance of the colloidal stability and rheological properties for the preparation of transparent photocatalytic and UV-absorbing coatings.
5.1. Requirements for transparency
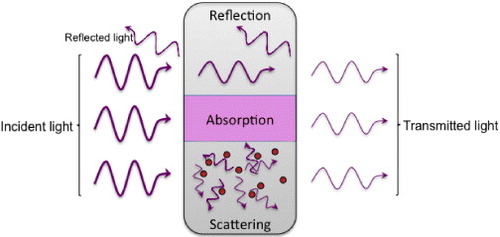
Transparent materials are characterized and defined by a small or insignificant loss in intensity of transmitted light. The transmittance of the sample can thus be defined as T = I/I0 , where I0 is the intensity of the incident light and I is the intensity of the transmitted light. This transmittance will be limited by a number of events, which will reduce T. These events, illustrated in figure , consist of (i) surface reflection, (ii) scattering and (iii) absorption.
The coefficient of reflection, often defined as the reflectivity, R , of a given material can be calculated from equation (Equation33 ) [Citation171]
3 where
is the complex refractive index, i.e.
, with n the refractive index and κ the extinction coefficient. In the absence of absorption, i.e.
, the reflectivity becomes
. In the absence of scattering, the transmittance is lowered only by the reflection at the sample's front and back surfaces, and can be expressed as
.
The scattering and absorption of light propagating in a solid has been observed to follow an exponential law as shown in equation (Equation44 ) [Citation172]
4 where I(x) is the intensity of the beam at a depth x and λ is the wavelength of the incident light. Considering that the refractive index
is a complex quantity, equation (Equation4
4 ) can thus be expanded as
5
The previous equation shows that the light attenuation can be separated into scattering and absorption, which are represented by the first and second factor of equation (Equation55 ), respectively. Using an analogy to the Lambert–Beer law, equation (Equation5
5 ) can be modified into
6 where α
abs and α
scat are the absorbance and scattering of the material, respectively. These can be expressed as a function of the number of absorbing/scattering centers per unit volume, N , and the absorbance and scattering cross-sections, Cabs and Cscat , respectively:
7
8 where r is the radius of the scattering center, and χ the efficiency factor for either absorbance or scattering.
The efficiency factors can be described under Mie's formalism as: 9
10 where
and the coefficients Ai depend on n and κ [Citation173].
In the special case when κ = 0 , A1 = A2 = A3 = 0 , the transmittance of light across a given material can be reduced to 11 where, in the interval
, with
12 Rayleigh's expression of scattering is obtained [Citation173].
All the materials of interest in this review, i.e., titanium dioxide (rutile, brookite, and anatase), zinc oxide, and cerium oxide, are transparent in the visible region. The optical transmittance can thus be estimated using equation (Equation1111 ) and using the reported refractive indices
,
,
,
, nZnO = 2.02 at λ = 589.3 nm [Citation174, Citation175]. Figure shows how the reflectivity and scattering (calculated using equation (Equation11
11 )) affect the transmittance of films composed of CeO2, TiO2 and ZnO nanoparticles with different particle sizes and refractive indices.
Figure 8. The dependence of transmittance of films containing nanoparticles of different particle size and refractive index. (Top) Decrease of transmittance of a film due to changes in reflectivity with the refractive index of the film calculated according to the first term in equation (Equation1111 ). (Middle) Decrease of transmittance of a film containing nanoparticles of different diameters: 20 nm (solid line), 50 nm (dashed line), and 100 nm (dotted line). The curves are calculated using a wavelength
, a volume fraction of 1, and a film thickness
according to the second term in equation (Equation11
11 ). (Bottom) Decrease of transmittance of a film due to both reflection and scattering using both terms according to the second term in equation (Equation11
11 ).
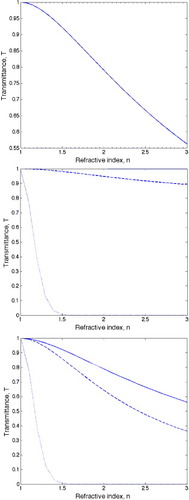
The scattering component has the most dramatic effect on the transmittance, where small deviations of the particle size (α
scat ∝r6 ) or of the wavelength of the incident light (α
scat ∝ λ
4 ) result in very large changes. Scattering is significantly reduced if nanoparticles with diameters below ≈10% of the wavelength are used, relating to β < 0.2 - 0.3 , and preferably β < 0.1 . This effect is reduced if the contrast in refractive index between the particle and the matrix is small. Hence, the lower refractive index of ZnO (nZnO = 2.02 ) compared to CeO2 ( and TiO2
is beneficial but the particle size has to be minimized and the number density of particles above about 100 nm has to be very small to achieve a high transparency of the films. The reflectance of the films is independent of the particle size or the film thickness and is only determined by the refractive index, i.e. the composition of the film. This parameter is difficult to tune but one possible way to avoid a strong film reflectance is to introduce nanosized porosity, which would result in a lower effective refractive index while avoiding excessive scattering.
5.2. Deposition of photocatalytic coatings
Deposition of nanoparticle suspensions onto a substrate of choice is a widely used method to produce nanostructured coatings [Citation176]. The deposition methods producing thin films can be classified into gas phase (e.g. chemical vapor deposition (CVD), sputtering) or liquid phase (e.g. dip coating, spin coating, spray coating) methods [Citation177]. Gas phase methods continue to be important for deposition on glass and metals [Citation22, Citation178, Citation179], while liquid phase deposition methods are widely used for deposition onto polymeric and heat sensitive substrates [Citation180, Citation181]. The liquid phase deposition of precursor solutions or nanoparticle dispersions [Citation117, Citation182–Citation186] offers several advantages, such as simple equipment, mild deposition conditions, and flexibility in tuning the properties of the films, that may be difficult to achieve using gas-phase deposition methods.
Dip coating and spin coating are suitable methods for the application of uniform and thin films onto flat substrates. In the dip-coating method (figure (a)), the substrate is immersed in the dispersion and then withdrawn at a constant speed under controlled temperature and atmospheric conditions [Citation187–Citation189]. The thickness of the film can be controlled by the withdrawal speed [Citation190, Citation191]. If the dispersion has a Newtonian behavior in the relevant shear rate range, the thickness can be calculated by the Landau–Levich equation 13 where
h is the coating thickness, η the dynamic viscosity, U the substrate withdrawal speed, γ
lv the liquid–vapor surface tension, ρ the density and g the gravity [Citation192]. The dip-coating method is generally characterized by a high optical quality and a homogeneous thickness of the coatings [Citation193].
Figure 9. Examples of liquid phase deposition methods; (a) dip coating; (b) spin coating and (c) spray coating.
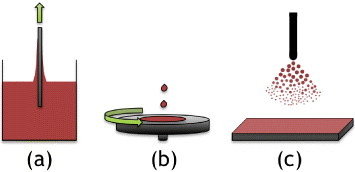
The spin coating process (figure (b)) relies on centrifugal forces to spread the liquid phase evenly across a spinning substrate. The solvent is usually volatile and therefore evaporates during the spinning process [Citation188]. The thickness of the film obtained by the spin-coating method depends on the rotation speed and the viscosity of the liquid phase. Newtonian liquids result in a uniform thickness, while the shear-thinning behavior commonly observed in particulate sols and gel-forming systems, results in spin-coated films that are thicker near the centre than at the edges. The thickness of an initially uniform film during spin-off is described by 14 where h0 is the initial thickness, t is the time, ω the angular velocity, ρ the density and η the dynamic viscosity.
High-boiling solvents with a high viscosity are commonly used for liquid phase deposition to suppress crack formation during drying and thermal post-processing, and to minimize the number of coating cycles [Citation194]. Alternatively, the addition of hydrated compounds (e.g. trehalose) that swell when the solvent is removed can reduce the induced tensile stresses in the coatings during evaporation [Citation195, Citation196]. Finally, polymers are commonly added to act both as plasticizers and to improve the wetting of the substrate. Uniform coatings have been prepared using, e.g., PEG [Citation62], methylcellulose (MC) [Citation197], hydroxypropoyl cellulose (HPC) [Citation184, Citation198, Citation199], and block co-polymers [Citation200, Citation201]. The spray coating process (figure (c)) offers some advantages over dip and spin coating, e.g. high deposition speed and a significant flexibility in the shape of the substrate [Citation193, Citation202]. However, the spray-coating method offers limited control of the uniformity of thickness.
The choice of substrate material, such as glass, ceramics, metals and polymers, has a profound influence on the deposition parameters and the properties of the coating. The most widely used material has been borosilicate glass due to its transparency, chemical and thermal stability, low cost and well-known optical properties. However, the non-crystalline nature of glass and the associated diffusion of Na+ and Ca2+ from the borosilicate glass substrate into a deposited titanium oxide layer have been shown to result in a drop of the photoactivity [Citation203, Citation204]. This can be prevented by coating the substrate with a SiO2 or Al2O3 barrier layer prior to the deposition of the TiO2 film [Citation26, Citation117, Citation205, Citation206], or by treating the substrate and the coating with sulfuric acid [Citation207, Citation208].
Coating of stainless steel used for construction and architectural design with transparent photocatalytic coatings offers a way to reduce corrosion and discoloration of façades, bridges and other structures. Such photocatalytic coatings applied to large constructions in cities could also contribute to a reduction in harmful airborne substances. Steel is a relatively inert material that is not altered during the deposition process except under extremely harsh chemical and thermal conditions [Citation209]. Steel, like other metallic substrates, can also be used for electrophoretic deposition of nanoparticles [Citation203]. Coating of ceramic substrates has also been studied for construction and indoor applications [Citation14, Citation210]. Similar to glass, coatings on ceramics can be heat treated at high temperature, which gives the coatings good adhesion properties [Citation211, Citation212].
Alternative deposition methods using, e.g., nanoparticle dispersions have been developed for heat-sensitive substrates such as polymers or organic fibers [Citation213, Citation214]. Many of the low-temperature deposition techniques suffer from poor adhesion of the coating to the substrate. The introduction of anionic hydrophilic groups by layer-by-layer impregnation, plasma etching, ion or UV irradiation, chemical etching or silanization can improve the mechanical adhesion of the films [Citation215–Citation222]. The uniformity and adhesion of deposited particle-based coatings can be significantly improved by using a layer-by-layer approach [Citation223–Citation226].
Attempts have been made to improve the properties of the coatings by embedding the particles in a matrix [Citation117, Citation227–Citation231]. The use of an inorganic matrix such as silica improves the optical transmittance, enhances the photoactivity and may also introduce superhydrophilic properties to the coatings [Citation176, Citation224, Citation232–Citation235]. In addition, the use of a silica matrix was found to prevent phase transitions and grain growth of the nanoparticles upon thermal post-treatment, and to improve the mechanical properties of the films [Citation224, Citation236, Citation237]. Organic additives have been used as pore-forming agents (PFA). Pore formation increases the photoactivity of the coatings by increasing the surface area, but reduces the transparency; hence, the porosity and the pore size needs to be tuned [Citation238, Citation239]. Long-chain non-ionic surfactants, such as PEG [Citation239–Citation242], diethyleneglycol (DEG) [Citation183], Tween 20 and Triton X-100 [Citation208, Citation243, Citation244] have been used as pore-forming agents and subsequently leached out by hot water treatment [Citation245]. Mesoporous photocatalytic coatings have been obtained using cationic surfactants [Citation246] and diblock [Citation247, Citation248], or triblock copolymers [Citation200, Citation201, Citation249, Citation250]. The rich structural polymorphism of block copolymers results in organic–inorganic cooperative self-assembly and yields ordered micro-mesoporous coatings upon removal of the organic component [Citation251]. Finally, silica-based sol–gel coatings made by incorporation of pre-formed N-doped nanoparticles [Citation252] have also been reported recently (for use as thin coatings on glass substrates), and have been shown to be highly active photocatalytic coatings under irradiation at 390 nm (figure ).
Figure 10. SEM images of TiO2 films deposited on glass (a) without PEG, (b) with PEG (200 g mol−1), showing reduced crack formation and increased porosity [Citation257].
![Figure 10. SEM images of TiO2 films deposited on glass (a) without PEG, (b) with PEG (200 g mol−1), showing reduced crack formation and increased porosity [Citation257].](/cms/asset/f48cd1a1-da19-44b0-bb0b-bbc4bc050f5f/tsta_a_11661359_f0010_oc.jpg)
An alternative approach has been to embed pre-formed titanium dioxide nanoparticles in a titanium dioxide matrix prepared by in situ hydrolysis of titanium alkoxide precursors. In recent years, this alternative sol–gel approach has been used both in the case of commercial P25 nanoparticles [Citation253] and in the case of other pre-synthesized nanoparticles of 4–5 nm in size [Citation254] for coating glass or silicon substrates (by either spin or dip coating), which were then heat treated up to 300–400 °C. In the latter case, the mesoporous coatings were found to display higher than expected activities, which were attributed to a synergic interaction between the crystalline and amorphous components.
Other more interesting sol–gel matrices for photocatalytic films embedding pre-formed or commercial (e.g. Hombikat, 5 nm) titanium dioxide nanoparticles are those based on silica–zirconia [Citation255] resulting from the hydrolysis of the zirconium and organosilane precursors. One added advantage in this case is the need for lower thermal treatment temperatures (about 100 °C), which make the process attractive for use on metallic substrates such as aluminum. Furthermore, these ZrOx:SiOx coatings, applied on glass and aluminum surfaces by dip coating, have been shown to possess high photocatalytic and biocidal activity.
Finally, a recent report [Citation256] has found that commercial titanium dioxide nanoparticles (about 25 nm in size) combined with PTFE can be sputtered (by radio frequency magnetron sputtering) onto substrates (such as quartz or structured Ti) and then heated at relatively mild temperatures (about 100 °C), to yield novel superhydrophobic surfaces with photocatalytic properties. These coatings display superhydrophobic rather than the usual superhydrophilic properties, commonly found in inorganic titania coatings, which widens the range of possible products that can be developed by incorporation of photocatalytic nanoparticles in different types of coating matrices.
5.3. UV-absorbing coatings
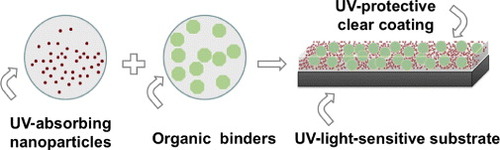
Transparent UV-absorbing or UV-protective clear coatings are primarily used as UV-protecting lacquer for wooden surfaces, and as a UV-barrier coating on polymer-based products or devices. The limited thermal resistance of wood and most polymers requires that UV-blocking coatings are produced directly from a dispersion that contains the UV-absorbing nanoparticles and the organic binder/matrix, schematically depicted in figure . Important considerations for the preparation of transparent UV-protective clear coatings are similar to the transparent photocatalytic coatings. However, because the UV-protective clear coatings are often significantly thicker than the photocatalytic coatings, issues related to scattering and absorption become very important. The UV-protective clear coatings are normally required to be colorless, which means that the light absorption in the visible range of the inorganic semiconducting nanoparticles and the organic additives should be negligible [Citation176, Citation258–Citation262].
Semiconducting nanoparticles such as TiO2, ZnO, SrTiO3, CeO2, WO3, Fe2O3, GaN, Bi2S3, CdS and ZnS are photoactive and UV absorbing due to their electronic structures, characterized by a filled valence band and an empty conduction band [Citation263]. However, a high photoactivity can also result in photocatalytic behavior, which brings about the production of unwanted reaction products and the possible degradation of the continuous phase the nanoparticles are dispersed in. Zinc oxide nanoparticles show a lower photocatalytic performance than TiO2 but are luminescent, radiating light in the visible region upon UV irradiation [Citation264, Citation265]. Recent work has suggested that CeO2 could be an interesting UV-absorbing additive in transparent UV-protective clear coatings due to a lower photocatalytic activity and a bandgap closer to the visible region compared to TiO2. However, CeO2 is frequently doped with divalent or trivalent cations to reduce the photocatalytic activity [Citation107]. Yabe and Sato [Citation107] suggested that the resulting low photocatalytic activity of doped ceria may be related to the oxygen vacancies.
The processes of charge separation and recombination in both TiO2 and CeO2 are schematically illustrated in figure . The photocatalytic activity of TiO2 can be suppressed, e.g., by surface modification with organic or inorganic compounds which prevents the recombination of the charge carriers with adsorbed species [Citation266, Citation267]. CeO2 can be used without surface modification and is considered as a better candidate for UV absorption in the cosmetic and coating formulations [Citation268]. Due to strict VOC regulations in the European Union, aqueous coating formulations using latex binders are preferred to solvent-based coatings.
Figure 12. Charge carrier formation (electron e− and hole h+) and recombination in TiO2 and CeO2 by UV light irradiation (adapted from [Citation269]).
![Figure 12. Charge carrier formation (electron e− and hole h+) and recombination in TiO2 and CeO2 by UV light irradiation (adapted from [Citation269]).](/cms/asset/ecdf4c68-2180-4f51-b77e-18f4a0d2d00a/tsta_a_11661359_f0012_oc.jpg)
Dispersions of CeO2 nanoparticles with diameters of 10–20 nm have been used to prepare UV-absorbing and abrasion-resistant nanocomposite coatings on polycarbonate substrates [Citation165]. The nanocomposite coatings were prepared by dispersing the nanoparticles into a matrix based on 3-glycidoxypropyltrimethoxysilane. The CeO2-based UV-protective clear coatings enhanced the scratch and abrasion resistance and the hardness of the polycarbonate substrate. Polyurethane nanocomposite films containing nanosized ceria functionalized with a phosphonated poly(oxyalkene) as UV absorber have also been studied [Citation163].
6. Nanoparticle-based sunscreens
Sunscreen (sometimes called sunblock) formulations have traditionally included a variety of organic and inorganic compounds with absorption bands in various regions of the UV–visible spectrum. The UV radiation from the sun is often divided into three different classes that relate to the response of the skin: UVC (270–290 nm), UVB (290–320 nm), and UVA, which is subdivided into UVA2 (320–340 nm) and UVA1 (340–400 nm). Many different UV-absorbing ingredients, primarily organic-based, have been developed and used in sunscreen formulations since the 1940s. Table presents a summary of the different FDA-approved ingredients in sunscreen formulations. In this review, we focus on sunscreens that utilize UV-absorbing inorganic nanoparticles, primarily titanium dioxide and zinc oxide, as the active ingredient.
Table 3. FDA-approvedFootnotea active sunscreen ingredients [Citation272].
The high refractive index and the broad absorption in the UVB and UVA regions of titanium dioxide and zinc oxide particles have been utilized in early and modern sunscreens and sunblock formulations [Citation270, Citation271]. The early use of a white zinc paste that consists of suspensions of micrometer sized zinc oxide particles in an oil-based medium on the skin was indeed an effective (but less attractive) sunscreen simply due to the pronounced light scattering in the visible region together with the UV absorption. However, the preparation of transparent sunscreens in the 1990s required that the particle size is reduced (see section 5.1) and that agglomeration is minimized. In the case of titanium dioxide, due to its very high refractive index, smaller particles are required whereas a somewhat larger particle size can be tolerated for zinc oxide.
Sunscreens can be formulated as sticks, ointments, gels, or aerosols with the dominating form being oil-in-water or water-in-oil emulsions, i.e. a mixture of two immiscible liquids. Emulsions offer a cost-effective and versatile formulation suitable for simple adjustment of the hydrophilic and hydrophobic UV-blocking organic components to tailor the rheology and the sun protection factor (SPF) [Citation272]. Emulsions are also cosmetically desired as they can make the skin smooth and avoid the greasy feeling of purely oil-based sunscreens [Citation29]. Many of the active ingredients are normally dissolved or dispersed in the hydrophobic oil phase. Titanium dioxide and zinc oxide in sunscreen formulations are usually coated with a dispersant, e.g. dimethicone (polydimethylsiloxane, PDMS) to be able to generate stable oil-based dispersions (see figure ). The major drawbacks with emulsions relate to their thermodynamic instability that eventually results in phase separation. Emulsions also provide a good medium for microbial attack, which needs to be suppressed by suitable preservatives. Sunscreen formulations contain additives to provide long-term stability. Most of these additives are either emulsifiers or thickeners. Emulsifiers reduce the interfacial tension between the oil and water phase and also adsorb and form an interfacial film on the emulsion drops that act as a barrier against coalescence. Emulsifiers are typically amphiphilic molecules like fatty acid soaps and nonionic ethoxylated surfactants. However, liquid crystal and particle-stabilized emulsions can also be used and provide very stable emulsions (see below). Thickeners increase the viscosity of the continuous phase, which reduces the coalescence rate and segregation rate. Commonly used thickeners include alginates, natural gums, cellulose derivatives, fatty alcohols, and inorganic materials like bentonite, laponite, and fumed silica. In the following subsections, we will give examples of nanoparticle-based formulations of both oil-in-water and water-in-oil type sunscreens.
Figure 13. TEM images of commercial sunscreen formulations containing (A) 10% titanium dioxide (T-Lite SF-S) and (B) 9% ZnO (Z-Cote) prepared as oil-in-water emulsions. Scale bar 200 nm. Reprinted from Gamer et al [Citation273], © (2006), with permission from Elsevier.
![Figure 13. TEM images of commercial sunscreen formulations containing (A) 10% titanium dioxide (T-Lite SF-S) and (B) 9% ZnO (Z-Cote) prepared as oil-in-water emulsions. Scale bar 200 nm. Reprinted from Gamer et al [Citation273], © (2006), with permission from Elsevier.](/cms/asset/90eaa21d-9c07-474d-b608-a903928c1872/tsta_a_11661359_f0013_oc.jpg)
6.1. Oil-in-water formulations
Oil-in-water formulations are emulsions with water as the continuous media with an oil-based dispersed phase. The active ingredients, including the nanoparticles, are normally dispersed or dissolved in the oil phase and the final composition can thus be adjusted by either varying the concentration in the oil phase and the relative concentration of the oil phase in the emulsion (i.e. the oil/water ratio). One of the practical advantages of this type of emulsion is that the oil content can be kept low. The nanoparticles also need to be functionalized with hydrophobic adlayers using, e.g., the techniques and additives discussed in section 4.2. This section contains a summary of some oil-in-water sunscreen formulations.
Monteiro-Riviere et al [Citation274] prepared a titanium dioxide-based sunscreen formulation consisting of 10% TiO2 nanoparticles (T-Lite SF, rutile nanocrystals with a primary size of 14–16 nm) coated with dimethicone/methicone copolymer. The treatment was only partially successful and resulted in the formation of agglomerates with a mean size of 200 nm with a relatively broad distribution. Hydrated silica and aluminum hydroxide were included as well, probably as thickeners. In comparison, a zinc oxide-based formulation was also prepared using 5% ZnO with a significantly larger particle size of 140 nm and a specific surface area of 12–24 m2 g-1 that was coated with triethoxycaprylsilane resulting in a stable dispersion in the non-polar medium. Gamer et al [Citation273] have also compared zinc oxide and titanium dioxide-based formulations. They prepared two titanium dioxide oil-in-water formulations of 10% T-Lite SF-S and T-Lite SF. The T-Lite SF-S titanium dioxide particles were needle-like with dimensions of 30–60 × 10 nm and coated with silica (2–5 wt%) and methicone (4.5–6.5%). The T-Lite SF titanium dioxide particles had the same dimensions as in T-Lite SF-S but were coated with methicone (3.5–5.5%) only. They prepared a zinc oxide-based formulation containing 10.3 wt% zinc oxide (Z-Cote) where the primary particles and loose agglomerates were present exclusively in the water phase with a substantial fraction of the primary particles being often adsorbed at the oil/water interface of the emulsion droplets. Bennat and Müller-Goymann [Citation275] prepared commercial TiO2 dispersions either in octyl palmitate or in water. The dispersions contained about 40% nanosized TiO2 and 1–2% of the polyelectrolyte, sodium polyacrylate. Formulations containing 5% TiO2 were manufactured using sodium carboxymethylcellulose (thickener), PEG 4000, lecithin (emulsifier) and two different silicon oils (dimethicone 100 and silicon oil AR 20 S, respectively).
Tyner et al [Citation276] showed that stable sunscreen formulations containing 5 wt% titania can be prepared using TiO2 particles with different surface properties (see figure ): (i) uncoated nanosized titanium dioxide (Degussa Aeroxide P25); (ii) titanium dioxide coated with aluminum hydroxide/dimethicone copolymer (BASF T-Lite SF); and (iii) titanium dioxide treated with aluminum hydroxide (Ishihara Tipaque CR-50). The versatile formulation consisted of three different phases in addition to the titanium dioxide particles: phase A (dibutyl adipate, cocoglycerides, sodium cetearyl sulfate (cationic emulsifier), lauryl glucoside (nonionic emulsifier), polyglyceryl-2 dipolyhydroxystearate, glycerin, cetearyl alcohol, C12–15 alkyl benzoate, octyl methoxycinnamate, and tocopheryl acetate; phase B glycerin, allantoin, xanthan gum (thickener), disodium EDTA, magnesium aluminum silicate and water; phase C phenoxyethanol, methylparaben, ethylparaben, butylparaben, propylparaben, and isobutylparaben. Sadrieh and coworkers [Citation277] followed up on this work and formulated sunscreens using an improved procedure. Phase A (dibutyl adipate, C12–15 alkyl benzoate, cocoglycerides, sodium cetearyl sulfate, lauryl glucoside, polyglyceryl-2 dipolyhydroxystearate, glycerine, cetearyl alcohol, octyl methoxycinnamate, tocopheryl acetate) was heated to 80 °C. The titanium dioxide was added to phase A and homogenized for 3 min. Phase B (glycerin, disodium ethylenediaminetetraacetic acid (EDTA), allantoin, xanthan gum, magnesium aluminum silicate, water) was heated to 80 °C and mixed with phase A (with the titanium dioxide) by vigorous stirring. The homogenized mixture was cooled to about 40 °C. Phase C (phenoxyethanol, methylparaben, ethylparaben, butylparaben, propylparaben, isobutylparaben) was added and then homogenized to yield the emulsion.
Figure 14. Stability of formulated sunscreens. (A) Representative P25 absorbance spectra before and after 1 h UV exposure. (B) Representative SF absorbance spectra before and after 1 h UV exposure. (C) Comparison of percentage change in area under the curve for formulated sunscreens from initial value after 1 h UV exposure. Only the sunscreen formulated with P25 shows significant reduction in the area under the curve, P < 0.05. Reprinted from Tyner et al [Citation276], © (2011), with permission from John Wiley and Sons.
![Figure 14. Stability of formulated sunscreens. (A) Representative P25 absorbance spectra before and after 1 h UV exposure. (B) Representative SF absorbance spectra before and after 1 h UV exposure. (C) Comparison of percentage change in area under the curve for formulated sunscreens from initial value after 1 h UV exposure. Only the sunscreen formulated with P25 shows significant reduction in the area under the curve, P < 0.05. Reprinted from Tyner et al [Citation276], © (2011), with permission from John Wiley and Sons.](/cms/asset/2c2a3896-5763-43b8-8df9-b648165c6b88/tsta_a_11661359_f0014_oc.jpg)
Zvyagin et al [Citation278] used a formula which includes 19 wt% 26–30-nm mean size ZnO particles with preservatives of phenoxyethanol (0.3 wt%) and hydroxybenzoates (0.3 wt%) suspended in caprylic capric triglycerides (liquid crystal formulation). Cross et al [Citation279] prepared (i) a ZnO dispersion with 60 wt% of siliconate-coated ZnO in caprylic capric triglyceride (ZinClearS\_60CCT), (ii) a typical oil-in-water emulsion sunscreen with 20 wt% ZnO (using ZinClear\_40CCT). Türkoglu and Yener [Citation280] made formulations with 5 or 10% TiO2 and/or ZnO using three different surfactants (2.7% cetyl trimethyl ammonium chloride, 1.5% sodium lauryl sulfate (anionic emulsifier) and 1.5% polysorbate 80, and 3.0% triethanolamine stearate). The rest of the formulation consisted of 64.2% water, 5% glycerol, 10% petrolatum, 2.5% isopropyl palmitate, 7% stearyl alcohol, 2.5% dimethicone, 0.5% sodium chloride, 0.3% methyl and propyl parabens. Villalobos-Hernández and Müller-Goymann [Citation281] investigated the use of carnauba wax (thickener) and cetyl oleate (emulsifier) for the dispersion of TiO2, BaSO4 or SrCO3. The suspensions were produced by dispersing the lipid phase into the aqueous phase using high-pressure homogenization. The lipid phase was composed of inorganic content 2–6 wt%, decyl oleate 5%, and carnauba wax 5–10% whereas the aqueous phase consisted of 1% Tween 80 (emulsifier) simethicone 0.01%, methylisothiazoline 0.0285% (added as preservative), and water. Those substances were mixed together by melting the wax at 90 ± 5 °C and applying magnetic stirring during 30 min at a rate of 300 rpm. The aqueous phase was prepared by dispersing the components at a speed rate of 300 rpm. Semenzato et al [Citation282] studied the UV-attenuating properties and the rheology of different emulsions. All emulsions were prepared according to the following formula: steareth-2 (3%); steareth-21 (2%); cetearyl alcohol (3%); mineral oil (17%); preservative (0.1%); inorganic sunscreen (5%); and water. The powder was pre-dispersed in the hot oil phase (70 °C) under vigorous stirring using a turbine mixer for 10 min; then the emulsion was prepared by adding the powder dispersed in oil to the aqueous phase under vigorous stirring for 5 min. The emulsions contained: (i) no inorganic component; (ii) 5% TiO2, inorganic-treated (iii) 5% ZnO; and (iv) TiO2, organic-treated (hydrophobized).
6.2. Water-in-oil formulations
Most metal oxide nanoparticles are hydrophilic and contain hydroxyl surface groups that develop a surface charge when immersed in water. This hydrophilicity is exploited in water-in-oil formulations where the inorganic nanoparticles are readily dispersed in an aqueous medium. Below is a summary of some water-in-oil sunscreen formulations.
Dussert et al [Citation283] investigated emulsions containing titanium dioxide, zinc oxide, and iron oxides. The emulsions contained decyl oleate (thickener), cyclomethicone and dimethicone copolyol (emulsifier), glycerin, cyclomethicone, C12–15 alkyl benzoate, mineral oil and caprylic/capric triglyceride (emulsifier), sodium chloride, octyldodecanol, phenoxyethanol, methylparaben, butylparaben, ethylparaben, propylparaben, petrolatum, quaternium-18 hectorite (thickener), polypropylene glycol (PPG) myristyl ether, tocopheryl acetate, glyceryl stearate, glyceryl laurate, PEG-30 dipolyhydroxystearate, silica, ozokerite, disodium EDTA, xanthan gum (thickener), o-cymen-5-ol, magnesium ascorbate, beta-carotene, orange oil and rosemary oil, lecithin and tocopherol and ascorbyl palmitate and hydrogenated tallow glyceride citrate.
Schulz et al [Citation284] compared formulations that utilized (i) the hydrophobization of T805 titanium dioxide (Degussa, particle size 20 nm) with trimethyloctylsilane, (ii) Eusolex T-2000 titanium dioxide (Merck, mean size of 10–15 nm) coated with Al2O3 (8–11%) and SiO2 (1–3%), and (iii), a hydrophilic dispersion of titanium dioxide (Tioveil AQ-10P, Solaveil, needle-shaped with a mean particle size of 100 nm) in water and propylene glycol, with alumina (4.25%) and silica (1.75%) as thickeners. The water-in-oil emulsions contained water, caprylic triglyceride, glycerin, butylene glycol, glyceryl stearate, octyldodecanol, dicaprylyl ether, cyclomethicone, stearic acid, trisodium EDTA, cetearyl alcohol, carbomer, lanolin alcohol and 4% ultra-fine titanium dioxide in either of the above-mentioned variants. Mavon et al [Citation285] designed a broad-spectrum UV water-in-oil emulsion containing water, glycerin, dimethicone, ethylhexyl methoxycinnamate, isododecane, cyclomethicone, C12-15 alkyl benzoate, PEG-30 dipolyhydroxystearate, decyl glucoside, dodecyl glycol copolymer, magnesium aluminum silicate, preservatives, zinc oxide, tocopheryl acetate, o-cymen-5-ol, xanthan gum and 3% ultrafine TiO2 (T805, Degussa, Germany) with a mean particle size of 20 nm (the TiO2 was hydrophobically coated with trimethyloctylsilane) and 8% methylene bis-benzotriazolyl tetramethylbutylphenol in a dispersion of decyl glucoside. Beasley et al [Citation286] prepared formulations of water-in-oil emulsions using 5% PEG-12 dimethicone cross-polymer as the emulsifier and containing either 3% avobenzone, 5% ZnO (Z-Cote HPl; BASF) or 5% TiO2 (TNP50M170; Kobo Products, Inc.,) as single sunscreen active ingredients. The oil phase additionally comprised 12% cyclopentasiloxane, 4% bis-hydroxyethoxypropyl dimethicone, and 7.0–31.5% di-isobutyl adipate. The water phase contained 5% propylene glycol and 0.5% sodium chloride, which was added to the oil phase under constant mixing to give final compositions.
6.3. Human risk
A great deal of work is also being dedicated to investigating the risk posed to humans by nanosized nanoparticles [Citation287]. Several recent investigations report that the inorganic TiO2 and ZnO nanoparticles do not penetrate into the dermis [Citation272–Citation279, Citation283–Citation285, Citation288]. However, the penetration distance varies with the state of the formulation; those encapsulated into liposomes that have a relative deeper penetration [Citation275]. In a recent review paper, authors from the major cosmetic companies indicate that the risk for humans from the use of nanosized titanium dioxide and zinc oxide is negligible [Citation289]. Nevertheless, although the authors did not find evidence of transdermal absorption, a recent report indicated that UV-damaged skin is more permeable to nanoparticles [Citation274]. Moreover, an increased amount of 68Zn has been found in blood and urine after exposure to 68Zn-enriched zinc oxide-containing sunscreens [Citation290].
7. Future prospects
Some of the early and commercially most important applications of nanoparticles like mechanochemical planarization, catalysis and sunscreens were realized through the development of efficient and cost-effective ways to formulate and process the nanoparticles. In this review, we have also shown how the applications of nanoparticles as part of photocatalytic and UV-absorbing coatings or as an ingredient in sunscreens rely on adequate strategies for stabilizing and functionalizing nanoparticles prior to their incorporation into an organic, inorganic or hybrid material, as well as for the final processing of the nanoparticle-containing product.
These successful cases give important guidance for the introduction and development of new, large-scale applications of nanoparticles. There is a need to develop versatile production routes that minimize or avoid the formation of hard, covalently bonded aggregates. Indeed, the inability to deagglomerate commercial nanoparticles to a sufficient extent to utilize the inherent size-specific properties is one of the major obstacles to introducing promising nanoparticles in prospective applications. In addition, many of the potential applications require that monodisperse nanoparticles with well-defined shapes can be produced in large quantities at moderate cost. While liquid-based colloidal routes have significant advantages over gas-phase routes with respect to size, shape and agglomeration control, the liquid-state routes are limited by poor yield and high cost. Development of cost-efficient colloidal nanoparticle production routes in aqueous media could open up more mundane application areas for the introduction of tailored nanoparticles.
The surface chemistry of nanoparticles needs to be much better understood. The surfaces of nanoparticles may differ significantly from the surface chemistry of larger, more well-characterized colloidal particles. Recent work that utilizes highly focused synchrotron light and high-resolution electron microscopy techniques is giving pivotal information on, e.g., the nucleation and growth of nanoparticles [Citation291, Citation292]. The rapid method development will certainly also provide important information on the highly dynamic surface chemistry and elucidate the mechanisms for efficient surface functionalization.
Finally, further development of new additives and innovative routes for the processing and preparation of stable dispersions of functionalized nanoparticles will be of pivotal importance for enabling and promoting new nanoparticle-based applications. Recent examples include, e.g., the functionalization of titanium dioxide nanoparticles for subsequent application in transparent nanocomposites [Citation287]. Other types of nanoparticles, such as lipid nanoparticles, have also been successfully functionalized for use in cellophane biocompatible hybrid films [Citation288].
References
- PitkethlyM J 2004 Mater. Today 7 20 10.1016/S1369-7021(04)00627-3
- GoesmannHFeldmannC 2010 Angew. Chem. Int. Edn. Engl. 49 1362 10.1002/anie.200903053
- IsraelachviliJ N 2011 Intermolecular and Surface Forces San Diego, CA Academic
- HoffmannM RMartinS TChoiWBahnemannD W 1995 Chem. Rev. 95 69 10.1021/cr00033a004
- GayaU IAbdullahA H 2008 J. Photochem. Photobiol. C 9 1 10.1016/j.jphotochemrev.2007.12.003
- FujishimaAZhangX T 2006 C. R. Chim. 9 750 10.1016/j.crci.2005.02.055
- NussbaumerR JCaseriW RSmithPTervoortT 2003 Macromol. Mater. Eng. 288 44 10.1002/mame.200290032
- ZhouSWuL 2009 Compos. Interfaces 16 281 10.1163/156855409X447101
- BlanchardVBlanchetP 2011 Bioresources 6 1219
- PiccinnoFGottschalkFSeegerSNowackB 2012 J. Nanopart. Res. 14 1109 10.1007/s11051-012-1109-9
- HagfeldtAGrätzelM 1995 Chem. Rev. 95 49 10.1021/cr00033a003
- CarpOHuismanC LRellerA 2004 Prog. Solid State Chem. 32 33 10.1016/j.progsolidstchem.2004.08.001
- LinsebiglerA LLuGYatesJ T 1995 Chem. Rev. 95 735 10.1021/cr00035a013
- FujishimaAZhangX TTrykD A 2008 Surf. Sci. Rep. 63 515 10.1016/j.surfrep.2008.10.001
- WangRHashimotoKFujishimaAChikuniMKojimaEKitamuraAShimohigoshiMWatanabeT 1997 Nature 388 431 10.1038/41233
- YuJ GZhaoX JZhaoQ N 2001 Mater. Chem. Phys. 69 25 10.1016/S0254-0584(00)00291-1
- BrusL 1986 J. Phys. Chem. 90 2555 10.1021/j100403a003
- KonstantatosGSargentE H 2010 Nature Nanotechnol. 5 391 10.1038/nnano.2010.78
- PesikaN SStebeK JSearsonP C 2003 Adv. Mater. 15 1289 10.1002/adma.200304904
- ZhangFJinQChanS-W 2004 J. Appl. Phys. 95 4319 10.1063/1.1667251
- ZhangZ BWangC CZakariaRYingJ Y 1998 J. Phys. Chem. B 102 10871 10.1021/jp982948+
- MillsALepreAElliottNBhopalSParkinI PO'NeillS A 2003 J. Photochem. Photobiol. A 160 213 10.1016/S1010-6030(03)00205-3
- PazYLuoZRabenbergLHellerA 1995 J. Mater. Res. 10 2842 10.1557/JMR.1995.2842
- ZhangXJinMLiuZNishimotoSSaitoHMurakamiTFujishimaA 2006 Langmuir 22 9477 10.1021/la0618869
- MaggosTBartzisJ GLevaPKotziasD 2007 Appl. Phys. A 89 81 10.1007/s00339-007-4033-6
- SunadaKKikuchiYHashimotoKFujishimaA 1998 Environ. Sci. Technol. 32 726 10.1021/es970860o
- PinnellS RFairhurstDGilliesRMitchnickM AKolliasN 2000 Dermatol. Surg. 26 309 10.1046/j.1524-4725.2000.99237.x
- MitchnickM AFairhurstDPinnellS R 1999 J. Am. Acad. Dermatol. 40 85 10.1016/S0190-9622(99)70532-3
- KaplanL A 1992 J. Wilderness Med. 3 173 10.1580/0953-9859-3.2.173
- StarkW JPratsinisS E 2002 Powder Technol. 126 103 10.1016/S0032-5910(02)00077-3
- TsuzukiT 2009 Int. J. Nanotechnol. 6 567 10.1504/IJNT.2009.024647
- RothP 2007 Proc. Combus. Inst. 31 1773 10.1016/j.proci.2006.08.118
- PratsinisS E 1998 Prog. Energy Combust. Sci. 24 197 10.1016/S0360-1285(97)00028-2
- TelekiAWengelerRWengelerLNirschlHPratsinisS E 2008 Powder Technol. 181 292 10.1016/j.powtec.2007.05.016
- TaniTMädlerLPratsinisS E 2002 J. Nanopart. Res. 4 337 10.1023/A:1021153419671
- MädlerLStarkW JPratsinisS E 2002 J. Mater. Res. 17 1356 10.1557/JMR.2002.0202
- ItoSChenPComtePNazeeruddinM KLiskaPPéchyPGrätzelM 2007 Prog. Photovolt. 15 603 10.1002/pip.768
- ChenYDionysiouD D 2006 Appl. Catal. B 62 255 10.1016/j.apcatb.2005.07.017
- MurrayC BKaganC RBawendiM G 2000 Annu. Rev. Mater. Sci. 30 545 10.1146/annurev.matsci.30.1.545
- YinYAlivisatosA P 2005 Nature 437 664 10.1038/nature04165
- GrubbsR B 2007 Polym. Rev. 47 197 10.1080/15583720701271245
- RozenbergB ATenneR 2008 Prog. Polym. Sci. 33 40 10.1016/j.progpolymsci.2007.07.004
- ChenXMaoS S 2007 Chem. Rev. 107 2891 10.1021/cr0500535
- SpanhelL 2006 J. Sol–Gel Sci. Technol. 39 7 10.1007/s10971-006-7302-5
- YuanQDuanH-HLiL-LSunL-DZhangY-WYanC-H 2009 J. Colloid Interface Sci. 335 151 10.1016/j.jcis.2009.04.007
- BumajdadAEastoeJMathewA 2009 Adv. Colloid Interface Sci. 147–148 56 10.1016/j.cis.2008.10.004
- JuanL V-EYa-DongCKevinC W WYusukeY 2012 Sci. Technol. Adv. Mater. 13 013003 10.1088/1468-6996/13/1/013003
- XuFZhangPNavrotskyAYuanZ-YRenT-ZHalasaMSuB-L 2007 Chem. Mater. 19 5680 10.1021/cm071190g
- NermeenNRenateSIngoLEmanuelKRobertFStefanKClemensK WKatharinaL 2011 Nanotechnology 22 135606 10.1088/0957-4484/22/13/135606
- NavrotskyAKleppaO J 1967 J. Am. Ceram. Soc. 50 626 10.1111/j.1151-2916.1967.tb15013.x
- BischoffB LAndersonM A 1995 Chem. Mater. 7 1772 10.1021/cM0058a004
- RanadeM RNavrotskyAZhangH ZBanfieldJ FElderS HZabanABorseP HKulkarniS KDoranG SWhitfieldH J 2002 Proc. Natl Acad. Sci. USA 99 6476 10.1073/pnas.251534898
- AugustynskiJ 1993 Electrochim. Acta 38 43 10.1016/0013-4686(93)80008-N
- TanakaKHisanagaTRivieraA P 1993 Photocatalytic Purification and Treatment of Water and Air OllisD FAl-EkabiH Amsterdam Elsevier pp 169
- AnderssonMOsterlundLLjungstromSPalmqvistA 2002 J. Phys. Chem. B 106 10674 10.1021/jp025715y
- GuillardCLachhebHHouasAKsibiMElalouiEHerrmannJ M 2003 J. Photochem. Photobiol. A 158 27 10.1016/S1010-6030(03)00016-9
- LivageJHenryMSanchezC 1988 Prog. Solid State Chem. 18 259 10.1016/0079-6786(88)90005-2
- WangC CYingJ Y 1999 Chem. Mater. 11 3113 10.1021/cm990180f
- DoeuffSHenryMSanchezCLivageJ 1987 J. Non-Cryst. Solids 89 206 10.1016/S0022-3093(87)80333-2
- GopalMChanW J MDeJongheL C 1997 J. Mater. Sci. 32 6001 10.1023/A:1018671212890
- ScolanESanchezC 1998 Chem. Mater. 10 3217 10.1021/cm980322q
- BarbéC JArendseFComtePJirousekMLenzmannFShkloverVGrätzelM 1997 J. Am. Ceram. Soc. 80 3157 10.1111/j.1151-2916.1997.tb03245.x
- CozzoliP DKornowskiAWellerH 2003 J. Am. Chem. Soc. 125 14539 10.1021/ja036505h
- JooJKwonS GYuTChoMLeeJYoonJHyeonT 2005 J. Phys. Chem. B 109 15297 10.1021/jp052458z
- NiederbergerMBartlM HStuckyG D 2002 Chem. Mater. 14 4364 10.1021/cm021203k
- DaiYCobleyC MZengJSunYXiaY 2009 Nano Lett. 9 2455 10.1021/nl901181n
- ChemseddineAMoritzT 1999 Eur. J. Inorg. Chem. 235 10.1002/(SICI)1099-0682(19990202)1999:2%3C235::AID-EJIC235%3E3.0.CO;2-N
- YangJMeiSFerreiraJ M F 2001 J. Am. Ceram. Soc. 84 1696 10.1111/j.1151-2916.2001.tb00901.x
- FaureBSæderup Lindel⊘vJWahlbergMAdkinsNJacksonPBergströmL 2010 Powder Technol. 203 384 10.1016/j.powtec.2010.05.033
- BahnemannD WKormannCHoffmannM R 1987 J. Phys. Chem. 91 3789 10.1021/j100298a015
- BauermannL PBillJAldingerF 2006 J. Phys. Chem. B 110 5182 10.1021/jp056830m
- SpanhelLAndersonM A 1991 J. Am. Chem. Soc. 113 2826 10.1021/ja00008a004
- MeulenkampE A 1998 J. Phys. Chem. B 102 5566 10.1021/jp980730h
- WoodAGiersigMHilgendorffMVilas-CamposALiz-MarzanL MMulvaneyP 2003 Aust. J. Chem. 56 1051 10.1071/CH03120
- OhyamaMKozukaHYokoT 1997 Thin Solid Films 306 78 10.1016/S0040-6090(97)00231-9
- KamalasananM NChandraS 1996 Thin Solid Films 288 112 10.1016/S0040-6090(96)08864-5
- DuHYuanFHuangSLiJZhuY 2004 Chem. Lett. 33 770 10.1246/cl.2004.770
- ZhongX HKnollW 2005 Chem. Commun. 1158 10.1039/b414948c
- JanaN RChenYPengX 2004 Chem. Mater. 16 3931 10.1021/cm049221k
- SehgalALalatonneYBerretJ FMorvanM 2005 Langmuir 21 9359 10.1021/la0513757
- TsuzukiTMcCormickP G 2001 J. Am. Ceram. Soc. 84 1453 10.1111/j.1151-2916.2001.tb00859.x
- LeeJ-SChoiS-C 2004 Mater. Lett. 58 390 10.1016/S0167-577X(03)00508-1
- DjuricicBPickeringS 1999 J. Eur. Ceram. Soc. 19 1925 10.1016/S0955-2219(99)00006-0
- MyeongW-JSongK-HParkS-WLeeJ-HBaikJ-SChungC-M 2007 Metal oxide solid solution, preparation and use thereof US Patent US20070042526 A1
- SunCLiHZhangHWangZChenL 2005 Nanotechnology 16 1454 10.1088/0957-4484/16/9/006
- TokA I YBoeyF Y CDongZSunX L 2007 J. Mater. Process. Technol. 190 217 10.1016/j.jmatprotec.2007.02.042
- CuiM YHeJ XLuN PZhengY YDongW JTangW HChenB YLiC R 2010 Mater. Chem. Phys. 121 314 10.1016/j.matchemphys.2010.01.041
- MasuiTHiraiHHamadaRImanakaNAdachiG-ySakataTMoriH 2003 J. Mater. Chem. 13 622 10.1039/b208109a
- AhniyazAWatanabeTYoshimuraM 2005 J. Phys. Chem. B 109 6136 10.1021/jp050047c
- AhniyazAFujiwaraTFujinoTYoshimuraM 2004 J. Nanosci. Nanotechnol. 4 233 10.1166/jnn.2004.028
- MatijevicEHsuW P 1987 J. Colloid Interface Sci. 118 506 10.1016/0021-9797(87)90486-3
- HsuW PRonnquistLMatijevicE 1988 Langmuir 4 31 10.1021/la00079a005
- AikenB A RHsuW PMatijevićE 1988 J. Am. Ceram. Soc. 71 845 10.1111/j.1151-2916.1988.tb07534.x
- TsaiM-S 2005 J. Cryst. Growth 274 632 10.1016/j.jcrysgro.2004.10.022
- TsaiM-SXiaoX-Z 2006 J. Cryst. Growth 289 351 10.1016/j.jcrysgro.2005.11.081
- TsaiM-S 2004 Mater. Sci. Eng. B 110 132 10.1016/j.mseb.2003.11.024
- SutradharNSinhamahapatraAPahariSJayachandranMSubramanianBBajajH CPandaA B 2011 J. Phys. Chem. C 115 7628 10.1021/jp200645q
- SpanierJ ERobinsonR DZhangFChanS-WHermanI P 2001 Phys. Rev. B 64 245407 10.1103/PhysRevB.64.245407
- ZhangFChanS-WSpanierJ EApakEJinQRobinsonR DHermanI P 2002 Appl. Phys. Lett. 80 127 10.1063/1.1430502
- SlostowskiCMarreSBabotOToupanceTAymonierC 2012 Langmuir 28 16656 10.1021/la303265t
- CabanasADarrJ ALesterEPoliakoffM 2000 Chem. Commun. 901 10.1039/b001424i
- CabanasADarrJ ALesterEPoliakoffM 2001 J. Mater. Chem. 11 561 10.1039/b008095k
- WengXZhangJWuZLiuYWangHDarrJ A 2011 Appl. Catal. B 103 453 10.1016/j.apcatb.2011.02.009
- VeriansyahBParkHKimJ-DMinB KShinY HLeeY-WKimJ 2009 J. Supercrit. Fluids 50 283 10.1016/j.supflu.2009.06.007
- TakamiSOharaSAdschiriTWakayamaYChikyowT 2008 Dalton Trans. 5442 10.1039/b801099d
- AkuratiK K 2008 Synthesis of TiO2 Based Nanoparticles for Photocatalytic Applications Göttingen Cuvillier Verlag
- YabeSSatoT 2003 J. Solid State Chem. 171 7 10.1016/S0022-4596(02)00139-1
- CampbellC TPedenC H 2005 Science 309 713 10.1126/science.1113955
- ChoHWatersM AHoggR 1996 Int. J. Miner. Process. 44–45 607 10.1016/0301-7516(95)00069-0
- Bégin-ColinSGirotTLe CaërGMocellinA 2000 J. Solid State Chem. 149 41 10.1006/jssc.1999.8491
- MandzyNGrulkeEDruffelT 2012 Powder Technol. 160 121 10.1016/j.powtec.2005.08.020
- ChungS JLeonardJ PNettleshipILeeJ KSoongYMartelloD VChyuM K 2009 Powder Technol. 194 75 10.1016/j.powtec.2009.03.025
- LaarzEZhmudB VBergströmL 2000 J. Am. Ceram. Soc. 83 2394 10.1111/j.1151-2916.2000.tb01567.x
- DangeCPhanT N TAndréVRiegerJPerselloJFoissyA 2007 J. Colloid Interface Sci. 315 107 10.1016/j.jcis.2007.03.068
- DegenAKosecM 2000 J. Eur. Ceram. Soc. 20 667 10.1016/S0955-2219(99)00203-4
- LiRYabeSYamashitaMMomoseSYoshidaSYinSSatoT 2002 Mater. Chem. Phys. 75 39 10.1016/S0254-0584(02)00027-5
- SatoruISong-ZhuCKenjiWDiLHajimeH 2003 Sci. Technol. Adv. Mater. 4 269 10.1016/S1468-6996(03)00059-7
- FrenchR H 2000 J. Am. Ceram. Soc. 83 2117 10.1111/j.1151-2916.2000.tb01527.x
- ParsegianV A 2006 Van der Waals Forces: A Handbook for Biologists, Chemists, Engineers, and Physicists Cambridge Cambridge University Press
- HamakerH 1937 Physica 4 1058 10.1016/S0031-8914(37)80203-7
- MeurkALuckhamP FBergstromL 1997 Langmuir 13 3896 10.1021/la9610967
- BergströmLMeurkNArwinHRowcliffeD J 1996 J. Am. Ceram. Soc. 79 339 10.1111/j.1151-2916.1996.tb08126.x
- KosmulskiM 2009 Adv. Colloid Interface Sci. 152 14 10.1016/j.cis.2009.08.003
- BergstromL 1997 Adv. Colloid Interface Sci. 70 125 10.1016/S0001-8686(97)00003-1
- FaureBSalazar-AlvarezGBergströmL 2011 Langmuir 27 8659 10.1021/la201387d
- GuoSArwinHJacobsenS NJärrendahlKHelmerssonU 1995 J. Appl. Phys. 77 5369 10.1063/1.359225
- AnsariA ASolankiP RMalhotraB D 2009 J. Biotechnol. 142 179 10.1016/j.jbiotec.2009.04.005
- Ponce OrtizRFacchettiAMarksT J 2010 Chem. Rev. 110 205 10.1021/cr9001275
- DagastineR RPrieveD, C.WhiteL R 2000 J. Colloid Interface Sci. 231 351 10.1006/jcis.2000.7164
- BellNDimosD 2000 MRS Proc. 625 129 10.1557/PROC-625-129
- HoughD BWhiteL R 1980 Adv. Colloid Interface Sci. 14 3 10.1016/0001-8686(80)80006-6
- PughR J 1994 Surface and Colloid Chemistry in Advanced Ceramics Processing PughR JBergströmL New York Marcel Decker p 127
- VanderhoevenP H CLyklemaJ 1992 Adv. Colloid Interface Sci. 42 205 10.1016/0001-8686(92)80024-R
- WidegrenJBergströmL 2000 J. Eur. Ceram. Soc. 20 659 10.1016/S0955-2219(99)00199-5
- NapperD H 1977 J. Colloid Interface Sci. 58 390 10.1016/0021-9797(77)90150-3
- AlexanderS 1977 J. Physique 38 983 10.1051/jphys:01977003808098300
- NeouzeM-ASchubertU 2008 Monatsh. Chem. 139 183 10.1007/s00706-007-0775-2
- FarrokhpayS 2009 Adv. Colloid Interface Sci. 151 24 10.1016/j.cis.2009.07.004
- PetterssonAMarinoGPursiheimoARosenholmJ B 2000 J. Colloid Interface Sci. 228 73 10.1006/jcis.2000.6939
- NabaviMSpallaOCabaneB 1993 J. Colloid Interface Sci. 160 459 10.1006/jcis.1993.1417
- SpallaOKékicheffP 1997 J. Colloid Interface Sci. 192 43 10.1006/jcis.1997.4964
- DeissJ LAnizanPEl HadiguiSWeckerC 1996 Colloids Surf. A 106 59 10.1016/0927-7757(95)03353-X
- LiufuSXiaoHLiY 2005 J. Colloid Interface Sci. 281 155 10.1016/j.jcis.2004.08.075
- KimS-KLeeSPaikUKatohTParkJ-G 2003 J. Mater. Res. 18 2163 10.1557/JMR.2003.0302
- BoddenbergBEltznerK 1991 Langmuir 7 1498 10.1021/la00055a036
- ShimMGuyot-SionnestP 2001 J. Am. Chem. Soc. 123 11651 10.1021/ja0163321
- MathurSMoudgilB M 1997 J. Colloid Interface Sci. 196 92 10.1006/jcis.1997.5192
- LiufuSXiaoHLiY 2004 Powder Technol. 145 20 10.1016/j.powtec.2004.05.007
- QiLSehgalACastaingJ-CChapelJ-PFresnaisJBerretJ-FCousinF 2008 ACS Nano 2 879 10.1021/nn700374d
- MahamuniSBendreB SLeppertV JSmithC ACookeDRisbudS HLeeH W H 1996 Nanostruct. Mater. 7 659 10.1016/0965-9773(96)00043-8
- GuoLYangS HYangC LYuPWangJ NGeW KWongG K L 2000 Appl. Phys. Lett. 76 2901 10.1063/1.126511
- MudunkotuwaI AGrassianV H 2010 J. Am. Chem. Soc. 132 14986 10.1021/ja106091q
- PeiróA MPeralJDomingoCDoménechXAyllónJ A 2001 Chem. Mater. 13 2567 10.1021/cm0012419
- ChibowskiSPaszkiewiczM 2001 J. Dispersion Sci. Technol. 22 281 10.1081/DIS-100105215
- ChuW-BYangJ-WLiuT-JTiuCGuoJ 2007 Colloids Surf. A 302 1 10.1016/j.colsurfa.2007.01.041
- TangE JChengG XMaX L 2006 Powder Technol. 161 209 10.1016/j.powtec.2005.10.007
- ParkH KKimD KKimC H 1997 J. Am. Ceram. Soc. 80 743 10.1111/j.1151-2916.1997.tb02891.x
- SunDMiyatakeNSueH J 2007 Nanotechnology 18 215606 10.1088/0957-4484/18/21/215606
- BobowskaIWojciechowskiPHalamusT 2008 Polym. Adv. Technol. 19 1860 10.1002/pat.1216
- RhodesRHorieMChenHWangZTurnerM LSaundersB R 2010 J. Colloid Interface Sci. 344 261 10.1016/j.jcis.2009.12.062
- ChenLXuJHolmesJ DMorrisM A 2010 J. Phys. Chem. C 114 2003 10.1021/jp9085766
- LeiteE RRibeiroC 2012 Crystallization and Growth of Colloidal Nanocrystals Berlin Springer pp 83
- Saadat-MonfaredAMohseniMTabatabaeiM H 2012 Colloids Surf. A 408 64 10.1016/j.colsurfa.2012.05.027
- KhrenovVKlapperMKochMMullenK 2005 Macromol. Chem. Phys. 206 95 10.1002/macp.200400213
- MosherB PWuCSunTZengT 2006 J. Non-Cryst. Solids 352 3295 10.1016/j.jnoncrysol.2006.05.026
- ErdemBSudolE DDimonieV LEl-AasserM S 2000 J. Polym. Sci. A Polym. Chem. 38 4419
- ComparelliRFanizzaECurriM LCozzoliP DMascoloGPassinoRAgostianoA 2005 Appl. Catal. B 55 81 10.1016/j.apcatb.2004.07.011
- DemirM MKoynovKAkbeyUBubeckCParkILieberwirthIWegnerG 2007 Macromolecules 40 1089 10.1021/ma062184t
- NussbaumerR JCaseriWTervoortTSmithP 2002 J. Nanopart. Res. 4 319 10.1023/A:1021141124257
- BeekW J EWienkM MKemerinkMYangXJanssenR A J 2005 J. Phys. Chem. B 109 9505 10.1021/jp050745x
- FoxM 2001 Optical Properties of Solids Oxford Oxford University Press
- HiemenzP CRajagopalanR 1997 Principles of Colloid and Surface Chemistry New York Marcel Dekker
- PenndorfR B 1962 J. Opt. Soc. Am. 52 896 10.1364/JOSA.52.000896
- ShannonR DShannonR CMedenbachOFischerR X 2002 J. Phys. Chem. Ref. Data 31 931 10.1063/1.1497384
- PatsalasPLogothetidisSMetaxaC 2002 Appl. Phys. Lett. 81 466 10.1063/1.1494458
- EbertDBhushanB 2012 Langmuir 28 11391 10.1021/la301479c
- GranqvistC G 2007 Sol. Energy Mater. Sol. Cells 91 1529 10.1016/j.solmat.2007.04.031
- SunH FWangC YPangS HLiX PTaoYTangH JLiuM 2008 J. Non-Cryst. Solids 354 1440 10.1016/j.jnoncrysol.2007.01.108
- JungJ MWangMKimE JParkCHahnS H 2008 Appl. Catal. B 84 389 10.1016/j.apcatb.2008.04.020
- SchubertUHüsingN 2005 Synthesis of Inorganic Materials Chichester Wiley
- AegerterM AAlmeidaRSoutarATadanagaKYangHWatanabeT 2008 J. Sol–Gel Sci. Technol. 47 203 10.1007/s10971-008-1761-9
- TakahashiYMatsuokaY 1988 J. Mater. Sci. 23 2259 10.1007/BF01115798
- FretwellRDouglasP 2001 J. Photochem. Photobiol. A 143 229 10.1016/S1010-6030(01)00526-3
- NegishiNMatsuzawaSTakeuchiKPichatP 2007 Chem. Mater. 19 3808 10.1021/cm070320i
- ChaeS YParkM KLeeS KKimT YKimS KLeeW I 2003 Chem. Mater. 15 3326 10.1021/cm030171d
- SonawaneR SHegdeS GDongareM K 2003 Mater. Chem. Phys. 77 744 10.1016/S0254-0584(02)00138-4
- BornsideD EMacoskoC WScrivenL E 1987 J. Imaging Technol. 13 122
- ScrivenL E 1988 Better Ceramics Through Chemistry III: Symp. 5–8 April (Reno, Nevada, USA, 1988) BrinkerC Jet al Pittsburgh, PA Materials Research Society p 717
- BrinkerC JFryeG CHurdA JAshleyC S 1991 Thin Solid Films 201 97 10.1016/0040-6090(91)90158-T
- StrawbridgeIJamesP F 1986 J. Non-Cryst. Solids 82 366 10.1016/0022-3093(86)90153-5
- DislichHHussmannE 1981 Thin Solid Films 77 129 10.1016/0040-6090(81)90369-2
- LandauL DLevichV G 1942 Acta Physicochim. URSS 17 42
- AegerterM APuetzJGasparroGAl-DahoudiN 2004 Opt. Mater. 26 155 10.1016/j.optmat.2003.11.023
- NegishiNTakeuchiK 2001 Thin Solid Films 392 249 10.1016/S0040-6090(01)01036-7
- BalliranoPSadunC 2009 Struct. Chem. 20 815 10.1007/s11224-009-9473-5
- MikiTNishizawaKSuzukiKKatoK 2004 Mater. Lett. 58 2751 10.1016/j.matlet.2004.04.015
- ChenWZhangJ YFangQLiSWuJ XLiF QJiangK 2004 Sensors Actuators B 100 195 10.1016/j.snb.2003.12.053
- MohammadiM RFrayD JCordero-CabreraM C 2007 Sensors Actuators B 124 74 10.1016/j.snb.2006.11.048
- NagpalV JDavisR MRiffleJ S 1994 Colloids Surf. A 87 25 10.1016/0927-7757(93)02735-W
- LiuK SZhangM LZhouWLiLWangJFuH G 2005 Nanotechnology 16 3006 10.1088/0957-4484/16/12/046
- BoscFAyralAAlbouyP AGuizardC 2003 Chem. Mater. 15 2463 10.1021/cm031025a
- Uzunova-BujnovaMKralchevskaRMilanovaMTodorovskaRHristovDTodorovskyD 2010 Catal. Today 151 14 10.1016/j.cattod.2010.02.058
- FernándezALassalettaGJiménezV MJustoAGonzález-ElipeA RHerrmannJ MTahiriHAit-IchouY 1995 Appl. Catal. B 7 49 10.1016/0926-3373(95)00026-7
- WatanabeTFukayamaSMiyauchiMFujishimaAHashimotoK 2000 J. Sol–Gel Sci. Technol. 19 71 10.1023/A:1008762121743
- YuJ GZhaoX J 2000 Mater. Res. Bull. 35 1293 10.1016/S0025-5408(00)00327-5
- ArconadaNDuránASuárezSPortelaRCoronadoJ MSánchezBCastroY 2009 Appl. Catal. B 86 1 10.1016/j.apcatb.2008.07.021
- PazYHellerA 1997 J. Mater. Res. 12 2759 10.1557/JMR.1997.0367
- YuJ CYuJ GZhaoJ C 2002 Appl. Catal. B 36 31 10.1016/S0926-3373(01)00277-6
- BalasubramanianGDionysiouD DSuidanM T 2004 Titanium dioxide coatings on stainless steel Encyclopedia of Nanoscience and Nanotechnology SchwarzJ AContescuC I Boca Raton, FL CRC Press ch 311
- MillsALeeS-K 2002 J. Photochem. Photobiol. A 152 233 10.1016/S1010-6030(02)00243-5
- WangRHashimotoKFujishimaAChikuniMKojimaEKitamuraAShimohigoshiMWatanabeT 1998 Adv. Mater. 10 135 10.1002/(SICI)1521-4095(199801)10:2%3C135::AID-ADMA135%3E3.0.CO;2-M
- BondioliFTaurinoRFerrariA M 2009 J. Colloid Interface Sci. 334 195 10.1016/j.jcis.2009.02.054
- LangletMKimAAudierMGuillardCHerrmannJ M 2003 J. Mater. Sci. 38 3945 10.1023/A:1026150213468
- QiK HDaoudW AXinJ HMakC LTangW ZCheungW P 2006 J. Mater. Chem. 16 4567 10.1039/b610861j
- PizemHSukenikC NSampathkumaranUMcIlwainA KDe GuireM R 2002 Chem. Mater. 14 2476 10.1021/cm010776e
- DutschkeADiegelmannCLobmannP 2003 J. Mater. Chem. 13 1058 10.1039/b212535h
- TsugeYKimJSoneYKuwakiOShiratoriS 2008 Thin Solid Films 516 2463 10.1016/j.tsf.2007.04.084
- ChanC MKoT MHiraokaH 1996 Surf. Sci. Rep. 24 1 10.1016/0167-5729(96)80003-3
- LatellaB ATrianiGZhangZShortK TBartlettJ RIgnatM 2007 Thin Solid Films 515 3138 10.1016/j.tsf.2006.08.022
- AslanKHolleyPGeddesC D 2006 J. Mater. Chem. 16 2846 10.1039/b604650a
- OhkoYUtsumiYNiwaCTatsumaTKobayakawaKSatohYKubotaYFujishimaA 2001 J. Biomed. Mater. Res. 58 97 10.1002/1097-4636(2001)58:1%3C97::AID-JBM140%3E3.0.CO;2-8
- BozziAYuranovaTKiwiJ 2005 J. Photochem. Photobiol. A 172 27 10.1016/j.jphotochem.2004.11.010
- SánchezBCoronadoJ MCaudalRPortelaRTejedorIAndersonM ATompkinsDLeeT 2006 Appl. Catal. B 66 295 10.1016/j.apcatb.2006.03.021
- ZhangX TSatoOTaguchiMEinagaYMurakamiTFujishimaA 2005 Chem. Mater. 17 696 10.1021/cm0484201
- LeeDRubnerM FCohenR E 2006 Nano Lett. 6 2305 10.1021/nl061776m
- SrivastavaSKotovN A 2008 Acc. Chem. Res. 41 1831 10.1021/ar8001377
- SanchezCSoler-IlliaGRibotFGrossoD 2003 C. R. Chim. 6 1131 10.1016/j.crci.2003.06.001
- BoucléJRavirajanPNelsonJ 2007 J. Mater. Chem. 17 3141 10.1039/b706547g
- IketaniKSunR DTokiMHirotaKYamaguchiO 2003 J. Phys. Chem. Solids 64 507 10.1016/S0022-3697(02)00357-8
- GardinSSignoriniRPistoreADella GiustinaGBrusatinGGuglielmiMBozioR 2010 J. Phys. Chem. C 114 7646 10.1021/jp911495h
- KatsumataK-iOhnoYTomitaKTaniguchiTMatsushitaNOkadaK 2012 ACS Appl. Mater. Interfaces 4 4846 10.1021/am301183t
- YuJ GYuJ CZhaoX J 2002 J. Sol–Gel Sci. Technol. 24 95 10.1023/A:1015258105966
- BennaniJDillertRGesingT MBahnemannD 2009 Sep. Purif. Technol. 67 173 10.1016/j.seppur.2009.03.019
- GaoXWachsI E 1999 Catal. Today 51 233 10.1016/S0920-5861(99)00048-6
- GuanK 2005 Surf. Coat. Technol. 191 155 10.1016/j.surfcoat.2004.02.022
- SmithaV SManjumolK ABaijuK VGhoshSPerumalPWarrierK G K 2010 J. Sol–Gel Sci. Technol. 54 203 10.1007/s10971-010-2178-9
- HataSKaiYYamanakaIOosakiHKazuoHYamazakiS 2000 JSAE Rev. 21 97 10.1016/S0389-4304(99)00075-2
- ChaM AShinCKannaiyanDJangY HKochuveeduS TRyuD YKimD H 2009 J. Mater. Chem. 19 7245 10.1039/b907922j
- YuJZhaoXZhaoQ 2000 Thin Solid Films 379 7 10.1016/S0040-6090(00)01542-X
- BuS JJinZ GLiuX XYangL RChengZ J 2005 J. Eur. Ceram. Soc. 25 673 10.1016/j.jeurceramsoc.2003.12.025
- ChenYDionysiouD D 2007 Appl. Catal. A 317 129 10.1016/j.apcata.2006.10.025
- MaYYaoJ N 1998 J. Photochem. Photobiol. A 116 167 10.1016/S1010-6030(98)00295-0
- ChoiHStathatosEDionysiouD D 2006 Thin Solid Films 510 107 10.1016/j.tsf.2005.12.217
- ChenY JDionysiouD D 2008 Appl. Catal. B 80 147 10.1016/j.apcatb.2007.11.010
- MatsudaAKotaniYKogureTTatsumisagoMMinamiT 2000 J. Am. Ceram. Soc. 83 229 10.1111/j.1151-2916.2000.tb01178.x
- YusufM MImaiHHirashimaH 2002 J. Sol–Gel Sci. Technol. 25 65 10.1023/A:1016045111857
- ChengY JGutmannJ S 2006 J. Am. Chem. Soc. 128 4658 10.1021/ja0562853
- HwangY KLeeK CKwonY U 2001 Chem. Commun. 1738 10.1039/b104699n
- TschirchJBahnemannDWarkMRathouskyJ 2008 J. Photochem. Photobiol. A 194 181 10.1016/j.jphotochem.2007.08.005
- AllainEBessonSDurandCMoreauMGacoinTBoilotJ P 2007 Adv. Funct. Mater. 17 549 10.1002/adfm.200600197
- NovotnáPZitaJKrýsaJKalousekVRathouskyJ 2008 Appl. Catal. B 79 179 10.1016/j.apcatb.2007.10.012
- GohinMMaurinIGacoinTBoilotJ-P 2010 J. Mater. Chem. 20 8070 10.1039/c0jm01499k
- ManiJSakeekHHaboutiSDietzeMEs-SouniM 2012 Catal. Sci. Technol. 2 379 10.1039/c1cy00302j
- SzeifertJ MFattakhova-RohlfingDGeorgiadouDKalousekVRathouskýJKuangDWengerSZakeeruddinS MGrätzelMBeinT 2009 Chem. Mater. 21 1260 10.1021/cm8029246
- SkorbE VShchukinD GMöhwaldHSviridovD V 2009 J. Mater. Chem. 19 4931 10.1039/b821827g
- KamegawaTShimizuYYamashitaH 2012 Adv. Mater. 24 3697 10.1002/adma.201201037
- GuoBLiuZHongLJiangH 2005 Surf. Coat. Technol. 198 24 10.1016/j.surfcoat.2004.10.055
- CaseriW 2000 Macromol. Rapid Commun. 21 705 10.1002/1521-3927(20000701)21:11%3C705::AID-MARC705%3E3.0.CO;2-3
- CaseriW 2003 Nanocomposites Chemistry of Nanostructured Materials YangP Singapore World Scientific p 359
- CaseriW 2004 Nanocomposites of polymers and inorganic particles Encyclopedia of Nanoscience and Nanotechnology vol 6 YangP London World Scientific p 235
- CaseriW 2009 Chem. Eng. Commun. 196 549
- SchützCSortJBacsikZOliynykVPellicerEFallAWågbergLBerglundLBergströmLSalazar-AlvarezG 2012 PLoS One 7 e45828 10.1371/journal.pone.0045828
- KubackaAFernández-GarcíaMColónG 2011 Chem. Rev. 112 1555 10.1021/cr100454n
- XiongH M 2010 J. Mater. Chem. 20 4251 10.1039/b918413a
- MatsuyamaKMishimaKKatoTIrieKMishimaK 2012 J. Colloid Interface Sci. 367 171 10.1016/j.jcis.2011.10.003
- KobayashiMKalriessW 1997 Cosmetics Toiletries Mag. 112 83
- UkajiEFurusawaTSatoMSuzukiN 2007 Appl. Surf. Sci. 254 563 10.1016/j.apsusc.2007.06.061
- GermanS F-RKenjiYBenD BIosifS GAnatolyI KStephenC VMyriamH AGoulnaraDJoseL E 2012 Sci. Technol. Adv. Mater. 13 043001 10.1088/1468-6996/13/4/043001
- ChenHNanayakkaraC EGrassianV H 2012 Chem. Rev. 112 5919 10.1021/cr3002092
- SerponeNDondiDAlbiniA 2007 Inorg. Chim. Acta 360 794 10.1016/j.ica.2005.12.057
- WeirAWesterhoffPFabriciusLHristovskiKvon GoetzN 2012 Environ. Sci. Technol. 46 2242 10.1021/es204168d
- SambandanD RRatnerD 2011 J. Am. Acad. Dermatol. 64 748 10.1016/j.jaad.2010.01.005
- GamerA OLeiboldEvan RavenzwaayB 2006 Toxicol. In Vitro 20 301 10.1016/j.tiv.2005.08.008
- Monteiro-RiviereN AWienchKLandsiedelRSchulteSInmanA ORiviereJ E 2011 Toxicol. Sci. 123 264 10.1093/toxsci/kfr148
- BennatCMüller-GoymannC C 2000 Int. J. Cosmet. Sci. 22 271 10.1046/j.1467-2494.2000.00009.x
- TynerK MWokovichA MGodarD EDoubW HSadriehN 2011 Int. J. Cosmet. Sci. 33 234 10.1111/j.1468-2494.2010.00622.x
- SadriehNet al 2010 Toxicol. Sci. 115 156 10.1093/toxsci/kfq041
- ZvyaginA VZhaoXGierdenASanchezWRossJ ARobertsM S 2008 J. Biomed. Opt. 13 064031 10.1117/1.3041492
- CrossS EInnesBRobertsM STsuzukiTRobertsonT AMcCormickP 2007 Skin Pharmacol. Physiol. 20 148 10.1159/000098701
- TürkogluMYenerS 1997 Int. J. Cosmet. Sci. 19 193 10.1111/j.1467-2494.1997.tb00182.x
- Villalobos-HernándezJ RMüller-GoymannC C 2005 Eur. J. Pharm. Biopharm. 60 113 10.1016/j.ejpb.2004.11.002
- SemenzatoADall'aglioCBoscariniG MOngaroABetteroASangalliM EBrunettaF 1994 Int. J. Cosmet. Sci. 16 247 10.1111/j.1467-2494.1994.tb00101.x
- DussertA SGoorisEHemmerleJ 1997 Int. J. Cosmet. Sci. 19 119 10.1111/j.1467-2494.1997.tb00175.x
- SchulzJHohenbergHPflückerFGärtnerEWillTPfeifferSWepfRWendelVGers-BarlagHWitternK P 2002 Adv. Drug Deliv. Rev. 54 S157 10.1016/S0169-409X(02)00120-5
- MavonAMiquelCLejeuneOPayreBMorettoP 2007 Skin Pharmacol. Physiol. 20 10 10.1159/000096167
- BeasleyD GMeyerT A 2010 Am. J. Clin. Dermatol. 11 413 10.2165/11537050-000000000-00000
- AuffanMRoseJBotteroJ-YLowryG VJolivetJ-PWiesnerM R 2009 Nat Nano 4 634 10.1038/nnano.2009.242
- NewmanM DStotlandMEllisJ I 2009 J. Am. Acad. Dermatol. 61 685 10.1016/j.jaad.2009.02.051
- SchillingKBradfordBCastelliDDufourENashJ FPapeWSchulteSTooleyIvan den BoschJSchellaufF 2010 Photochem. Photobiol. Sci. 9 495 10.1039/b9pp00180h
- GulsonBMcCallMKorschMGomezLCaseyPOytamYTaylorAMcCullochMTrotterJKinsleyLGreenoakG 2010 Toxicol. Sci. 118 140 10.1093/toxsci/kfq243
- PolteJ rErlerRThünemannA FSokolovSAhnerT TRademannKEmmerlingFKraehnertR 2010 ACS Nano 4 1076 10.1021/nn901499c
- YukJ MParkJErciusPKimKHellebuschD JCrommieM FLeeJ YZettlAAlivisatosA P 2012 Science 336 61 10.1126/science.1217654