Abstract
Suppression of thermal expansion is of great importance for industry. Negative thermal expansion (NTE) materials which shrink on heating and expand on cooling are therefore attracting keen attention. Here we provide a brief overview of NTE induced by intermetallic charge transfer in A-site ordered double perovskites SaCu3Fe4O12 and LaCu3Fe4−xMnxO12, as well as in Bi or Ni substituted BiNiO3. The last compound shows a colossal dilatometric linear thermal expansion coefficient exceeding −70 × 10−6 K−1 near room temperature, in the temperature range which can be controlled by substitution.
1. Introduction
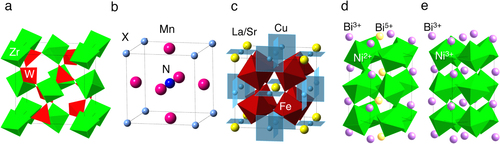
Thermal expansion originating from anharmonic vibration of atoms is a common feature of matter in solid, liquid and gas states. For example, the coefficient of linear thermal expansion (CTE) of iron is αL = (1/L)(ΔL/ΔT) = −11.6 × 10−6 K−1 leading to the 1.16 μm expansion of a 10 cm long rod on heating by 1 K. Nanoscale production of electronic devices and optical communications requires precise positioning, and thus, even such small amounts of thermal expansion can be a problem. Negative thermal expansion (NTE) materials which shrink on heating and expand on cooling are attracting much interest because these are expected to compensate for the thermal expansion of structure materials by making composites [Citation1–Citation4]. Useful NTE materials for zero or controlled expansion composites should show a smooth contraction while heating through a wide temperature range. The compounds with flexible frameworks in the crystal structures (mechanism 1) such as β-LiAlSiO4 [Citation1, Citation2] ZrW2O8 [Citation5], and Cd(CN)2 [Citation6] can be categorized into the first generation of NTE materials. Indeed, crystallized glass, where β-LiAlSiO4 crystallizes into a Li–Al–Si–O glass matrix, is widely used as a low thermal expansion material in cooktops and astronomical telescopes. The last decade has seen a remarkable development in materials with NTE resulting from phase transitions. In particular, a large NTE over αL = −30 × 10−6 K−1 coupled with a magnetic transition (mechanism 2) was discovered in an anti-perovskite manganese nitride [Citation7–Citation13]. PbTiO3 based perovskites were found to show NTE originating from a ferroelectric-paraelectric transition (mechanism 3) [Citation14–Citation19]. An intermetallic charge transfer transition was shown to cause volume shrinkage (mechanism 4) in A-site ordered double perovskites upon heating [Citation20–Citation23]. Colossal dilatometric linear thermal expansion coefficient over −70 × 10−6 K−1 is observed in the controlled temperature range near room temperature (RT) in Bi or Ni substituted perovskite compound BiNiO3 [Citation24–Citation26]. The properties of these typical NTE materials are summarized in table , and the crystal structures of selected compounds are shown in figure . In this review, we focus on the NTE induced by intermetallic charge transition in A-site ordered double perovskites and modified BiNiO3.
Table 1. Coefficients of linear thermal expansion and operating temperatures Toper of typical negative thermal expansion materials.
2. NTE induced by intermetallic charge transfer in A-site ordered double perovskites
LaCu3Fe4O12 has so-called A-site ordered double perovskite structure where A-site of perovskite ABO3 is periodically occupied by La3+ and Cu3+ as shown in figure (c). The formal valence state of this compound is La3+Cu3+3Fe3+4O12 at RT. Intermetallic charge transfer between Cu3+ and Fe3+ takes place on heating above 393 K resulting in La3+Cu2+3Fe3.75+4O12 high-temperature (HT) phase. Because of the shrinkage of Fe–O bonds, the unit cell volume shrinks by 1% [Citation20]. This transition is discontinuous first order one as shown in figure and the thermal expansion coefficient cannot be defined. However, replacement of La3+ by Sr2+ changes the nature of transition to second order and NTE with αL = 22.6 × 10−6 K−1 is observed between 200 and 230 K as shown in figure [Citation21]. The NTE was confirmed by dilatometric measurements with thermal mechanical analysis (TMA) and strain gauge (figure ) [Citation22]. Similar NTE is also realized in LaCu3Fe4−xMnxO12 as shown in figure [Citation23].
Figures 2. Temperature dependence of lattice constants of LaCu3Fe4O12 and SrCu3Fe4O12 determined by XRD measurements. Reproduced with permission from [Citation21], copyright 2011 John Wiley and Sons.
![Figures 2. Temperature dependence of lattice constants of LaCu3Fe4O12 and SrCu3Fe4O12 determined by XRD measurements. Reproduced with permission from [Citation21], copyright 2011 John Wiley and Sons.](/cms/asset/61d297ac-e5c1-40b7-8cce-8406a8cb9777/tsta_a_11661296_f0002_oc.jpg)
Figure 3. Thermal expansion of SrCu3Fe4O12 evaluated by XRD, SXRD, TMA and strain gauge. Reproduced with permission from [Citation22].
![Figure 3. Thermal expansion of SrCu3Fe4O12 evaluated by XRD, SXRD, TMA and strain gauge. Reproduced with permission from [Citation22].](/cms/asset/ad3ef2b8-8ce3-459a-881b-939ec0ab513a/tsta_a_11661296_f0003_oc.jpg)
Figure 4. Temperature dependence of unit cell volumes of LaCu3Fe4−xMnxO12 determined by XRD measurements. Reprinted with permission from [Citation23], copyright 2014, AIP Publishing LLC.
![Figure 4. Temperature dependence of unit cell volumes of LaCu3Fe4−xMnxO12 determined by XRD measurements. Reprinted with permission from [Citation23], copyright 2014, AIP Publishing LLC.](/cms/asset/4abf60b1-3bd7-4f38-9e83-da477286a255/tsta_a_11661296_f0004_oc.jpg)
3. NTE in A- or B- site substituted perovskite compound BiNiO3
3.1. Pressure induced intermetallic charge transfer in BiNiO3
BiNiO3 is a perovskite compound with a triclinically distorted crystal structure (space group P-1) stabilized by high-pressure (HP) synthesis at 6 GPa. Bi is a main group element, but it has Bi3+/Bi5+ charge degree of freedom depending on 6s2 and 6s0 electronic configurations. These occupy distinct crystallographic sites in BiNiO3 whose valence distribution is unusual Bi3+0.5Bi5+0.5Ni2+O3 as illustrated in figure (d) [Citation27]. Powder neutron diffraction (PND) [Citation28] and x-ray absorption spectroscopy (XAS) [Citation29] studies revealed a pressure-induced melting of the Bi-charge disproportionation at 3–4 GPa and a simultaneous Ni to Bi charge transfer accompanied by a structural change to the orthorhombic GdFeO3 type perovskite superstructure (figure (e)) with valence distribution Bi3+Ni3+O3 and an insulator to metal transition. The structural transition is accompanied by a discrete shrinkage of lattice parameters and a 2.5% decrease in the unit cell volume [Citation24]. This large change results from the dominant contraction of the Ni–O perovskite framework as Ni2+ is oxidized to the smaller Ni3+ at the transition, which outweighs the lattice expanding effects of reducing Bi5+ to Bi3+ and increases in the Ni–O–Ni angles.
The PND and XAS results have been used to construct the P–T phase diagram for BiNiO3 shown in figure . BiNiO3 decomposes above 500 K at ambient pressure (AP), but is stabilized up to at least 565 K at 1.8 GPa (and to ∼1300 K at 6 GPa under synthesis conditions). The boundary between the low pressure and temperature (LPT) and HPT phases has slope dTCT/dp = –140 KGPa−1. The 2.5–3.4% volume contraction occurs on both pressurizing and heating.
Figure 5. Pressure–temperature phase diagram of BiNiO3 determined by PND and XAS studies. Circles and squares show PND and XAS data, and blue and red symbols correspond to the low pressure and temperature (LPT) and high pressure and temperature (HPT) phases, respectively. Reproduced from [Citation24].
![Figure 5. Pressure–temperature phase diagram of BiNiO3 determined by PND and XAS studies. Circles and squares show PND and XAS data, and blue and red symbols correspond to the low pressure and temperature (LPT) and high pressure and temperature (HPT) phases, respectively. Reproduced from [Citation24].](/cms/asset/7036f143-db24-44f6-8d20-8b39bf192173/tsta_a_11661296_f0005_oc.jpg)
3.2. NTE in Bi0.95La0.05NiO3
The large ΔTCT of BiNiO3 shows that colossal NTE is feasible but the transition is only observed above pressures of 1.5 GPa in pure BiNiO3. Chemical substitutions for Bi is used to suppress the charge disproportionation in the Bi3+0.5Bi5+0.5Ni2+O3 phase and thereby shift the charge transfer transition to near ambient conditions. Partial substitution of La3+ without charge degree of freedom for Bi destabilizes the characteristic Bi3+/Bi5+ disproportionation and shift the charge transfer transition to around 350 K at AP in Bi0.95La0.05NiO3 [Citation24, Citation30].
Synchrotron x-ray diffraction (SXRD) patterns on Bi0.95La0.05NiO3 in figure (a) show merging of five main peaks characteristic for the triclinic phase with Bi3+/Bi5+ charge disproportionation into three indicating the transition to the orthorhombic phase with (Bi, La)3+Ni3+O3 valence distribution. The 2.9% volume shrinkage, which has a similar magnitude to that observed in undoped BiNiO3 under a pressure of 1.8 GPa, was observed as shown in figure (b). Coexistence of the low and high temperature phases is observed at three points in the transition region and a linear fit to the weighted average volumes is used to obtain the transition width of ΔTCT = 70 K. Such a coexistence of two phases changing the fractions as functions of temperature appears to against the Gibbs phase rule, but is commonly observed in ZrO2 and HfO2 [Citation31]. In these ceramics, low-temperature monoclinic and HT tetragonal phases coexist changing the phase fractions across the diffusionless (martensitic) phase transition. The deviation of the pressure from 1 atm at the domain boundary is thought to be the origin of such phenomena. The crystallographic volume thermal expansion coefficient of Bi0.95La0.05NiO3 between 300 and 370 K is αV = – 413 × 10−6 K−1 and the linear coefficient is –137 × 10−6 K−1, showing that colossal NTE magnitudes are observable in Bi1−xLaxNiO3. Crystallography predicts the upper limit of the magnitude of thermal expansion as the formation of pores and other microstructural defects can lessen the effect in bulk ceramics.
Figure 6. Selected SXRD data of Bi0.95La0.05NiO3 at various temperatures (a). Reproduced from [Citation30]. Temperature dependence of the unit cell volume (b). The dilatometric linear thermal expansion of Bi0.95La0.05NiO3 on heating and cooling (c) The inset shows the sample pasted on the strain gauge. Reproduced from [Citation24].
![Figure 6. Selected SXRD data of Bi0.95La0.05NiO3 at various temperatures (a). Reproduced from [Citation30]. Temperature dependence of the unit cell volume (b). The dilatometric linear thermal expansion of Bi0.95La0.05NiO3 on heating and cooling (c) The inset shows the sample pasted on the strain gauge. Reproduced from [Citation24].](/cms/asset/2b51444a-fd3a-4685-8e88-d05dce1085d5/tsta_a_11661296_f0006_oc.jpg)
Dilatometric measurements on a polycrystalline ceramic of Bi0.95La0.05NiO3 prepared at HP were made during heating and cooling cycles as shown in figure (c). The strain ΔL/L(400 K) increases with increasing temperature up to 270 K indicating the normal positive thermal expansion, but decreases above 270 K. The average observed αL between 270 and 400 K is −49 × 10−6 K−1 and the maximum negative slope between 320 and 380 K corresponds to a linear thermal expansion coefficient of −82 × 10−6 K−1 [Citation24]. It was confirmed that the oxidation of Ni ion from 2+ to 3+ was the origin of this volume shrinkage by XAS measurement [Citation32].
3.3. Tunable NTE in Bi1−xLnxNiO3 (Ln: Lanthanides)
The temperature range of NTE and the CTE can be controlled by tuning the composition, i.e., the element substituting Bi and the degree of substitution. Thermal expansion of Bi1−xLnxNiO3 (Ln: = La, Nd, Eu, Dy) were investigated [Citation25]. Figure (a) shows the temperature dependence of the weighted average volume calculated from the unit cell volumes and the phase fractions refined by Rietveld analysis of laboratory XRD data. NTE with temperature hysteresis is present in all samples. The dilatometric curves measured by TMA (figure (b)) are consistent with the volume change. Table summarizes the NTE parameters determined from the TMA data. For all systems, the temperature range of NTE upon heating shifts to the lower side and the volume shrinkage becomes more gradual as the Ln3+ content increases. Figure (a) summarizes the compositional dependence of the onset temperature of NTE (TNTE) and the temperature hysteresis width. The TNTE decreases almost linearly with the amount of substituted lanthanide, and this result shows that the temperature range can be controlled by chemical tuning. The transition temperature also depends on the ionic radius of Ln3+. Substitution with a small Ln3+ stabilizes the triclinic phase and maintains it at a HT. This lanthanide dependence is explained as follows. Under HPT synthesis conditions, Bi1−xLnxNiO3 is in the orthorhombic (Bi, Ln)3+Ni3+O3 state with an unique Bi/Ln site. The La3+ ions are homogeneously distributed, because the ionic radius of La3+ is close to that of Bi3+. Suppose such a sample is in the HT (Bi, Ln)3+Ni3+O3 state at AP. Charge disproportionation on cooling is suppressed by the presence of a La3+ ion at the Bi5+ site of the triclinic low-temperature phase. TNTE is therefore lowered by the La substitution, and the orthorhombic phase is dominant for Bi0.90La0.10NiO3. On the other hand, a large difference in ionic radius between Bi3+ and small Ln3+ ions should lead to a partial Bi3+/Ln3+ ordering, as schematically illustrated in figure (b). The charge transfer transition is less affected by a small Ln3+ because Bi5+ can periodically exist owing to the Ln/Bi ordering. Since the amount of substitution is small, the partial ordering is not observed as a super structure. La substitution decreases not only the transition temperature, but also the sharpness of the transition, which is also a consequence of randomness. The unit cell volume and ΔL/L curves for Bi1−xDyxNiO3 in figure show parallel shifts to a lower temperature with increasing x. Most importantly, the temperature hysteresis summarized in figure (a) is reduced by using smaller lanthanides.
Figure 7. Temperature dependence of the weighted average volume (a) and the dilatometric linear thermal expansion (b) of Bi1-xLnxNiO3 (Ln = La, Nd, Eu, Dy; x = 0.05, 0.075, 0.10) on heating and cooling. Reproduced from [Citation25].
![Figure 7. Temperature dependence of the weighted average volume (a) and the dilatometric linear thermal expansion (b) of Bi1-xLnxNiO3 (Ln = La, Nd, Eu, Dy; x = 0.05, 0.075, 0.10) on heating and cooling. Reproduced from [Citation25].](/cms/asset/a0d93293-257a-4ab0-b733-16051f227347/tsta_a_11661296_f0007_oc.jpg)
Table 2. The liner thermal expansion coefficient αL of Bi1−xLnxNiO3 (Ln = La, Eu, Nd, Dy) estimated by the dilatometric measurement on heating [Citation25].
Figure 8. (a) Composition dependence of TNTE on heating and temperature hysteresis width determined by XRD. (b) Illustration of the Bi/Ln layer of BiNiO3 (left) and Bi1−xLnxNiO3 (center and right) in the HT orthorhombic phase and low temperature triclinic phase (LT). Filled and open circles represent valence of 3+ and 5+, respectively. The large Ln3+ ions (red) are distributed homogeneously. On the other hand, the small Ln3+ ions (blue) have partial ordering. Reproduced from [Citation25].
![Figure 8. (a) Composition dependence of TNTE on heating and temperature hysteresis width determined by XRD. (b) Illustration of the Bi/Ln layer of BiNiO3 (left) and Bi1−xLnxNiO3 (center and right) in the HT orthorhombic phase and low temperature triclinic phase (LT). Filled and open circles represent valence of 3+ and 5+, respectively. The large Ln3+ ions (red) are distributed homogeneously. On the other hand, the small Ln3+ ions (blue) have partial ordering. Reproduced from [Citation25].](/cms/asset/8421cab8-9ab5-472e-8028-c83ea90907a3/tsta_a_11661296_f0008_oc.jpg)
3.4. NTE in LaNi1−xMxO3 (M: Al and Ga)
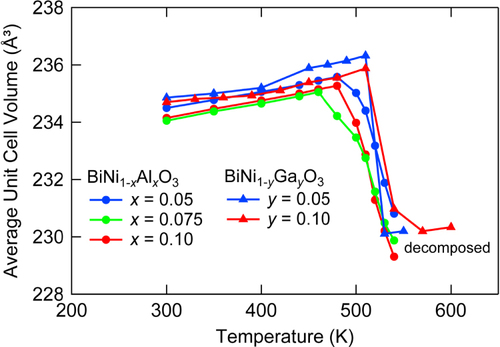
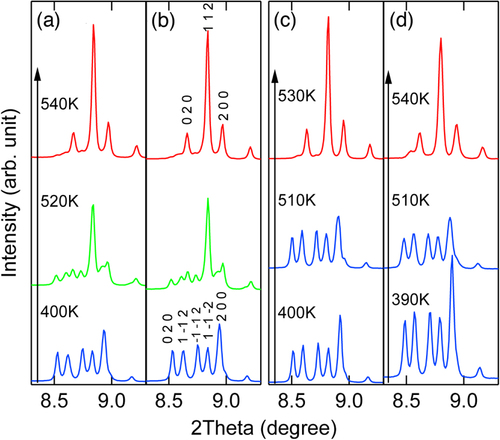
The above discussed NTE results from the temperature induced intermetallic charge transfer between Bi5+ and Ni2+. The presence of Ln3+ in Bi5+ site destabilizes the Bi3+/Bi5+ charge disproportionation in Bi3+0.5Bi5+0.5Ni2+O3 and HP phase of Bi3+Ni3+O3 appears on heating at AP. In this context, substitution of Ni2+ with a trivalent ion is also expected to stabilize Bi3+(Ni, M)3+O3 and thus leads to NTE. Figure shows the XRD patterns of BiNi1−xMxO3 (M = Ga, Al) on heating. They reveal transitions from triclinic to orthorhombic phases via coexistence of two phases, essentially the same as those for Bi1−xLnxNiO3.
Figure 9. SXRD patterns of BiNi0.925Al0.025O3 (a), BiNi0.9Al0.1O3 (b), BiNi0.95Ga0.05O3 (c) and BiNi0.9Ga0.01O3 (d) at various temperatures revealing the transition from triclinic to orthorhombic phases on heating.
The weighted average unit cell volumes obtained by the Rietveld analysis of the XRD data are plotted in figure . Note that only the data on heating are plotted since the samples partially decomposed after heating up to 550 K. These results indicate the presence of NTE, but TNTE is at around 500 K, well above RT and are almost independent of x. It is recently found that BiNi1-xFexO3 also shows large NTE with αL exceeding −150 × 10−6 K−1 in the controlled temperature range near RT [Citation26].
4. Conclusions
A brief overview of NTE induced by intermetallic charge transfer in A-site ordered double perovskites SrCu3Fe4O12 and LaCu3Fe4−xMnxO12 and A- or B-site substituted perovskite BiNiO3 is provided. The distinct volume contraction in LaCu3Fe4O12 is broadened by replacement of La3+ by Sr2+ or Mn substitution for Fe, leading to NTE. Substitution of Bi with Ln3+ or Ni with Al3+, Ga3+ stabilized the (Bi, Ln)3+(Ni, M)3+O3 phase which is present only in the HP condition for pure BiNiO3 and suppress the intermetallic charge transfer transition accompanied by volume contraction to ambient condition. Colossal NTE with CTE over −70 × 10−6 K−1 is observed by both diffraction and dilatometric measurements in the controlled temperature range for Bi1−xLnxNiO3 while TNTE is almost independent for BiNi1−xAlxO3 and BiNi1−xGaxO3. These compounds are promising for the suppression of the thermal expansion of structure materials, but the presence of emperature hysteresis owing to the first-order toransition is a problem for the practical applications. It is recently shown that the thermal hysteresis is suppressed in BiNi1−xFexO3. Moreover, 18 vol. % addition of BiNi0.85Fe0.15O3 with αL = −187 × 10−6 K−1 compensates for the thermal expansion of epoxy resin [Citation26].
Acknowledgments
This work was partially supported by Grants-in-Aid for Young Scientists (B) (26800180) and Creative Scientific Research (26106507) from the Japan Society for the Promotion of Science (JSPS). The synchrotron-radiation experiments were performed at SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (2012A1665).
References
- ChuC NSakaNSuhN P 1987 Mater. Sci. Eng. 95 303 10.1016/0025-5416(87)90523-4
- SleightA W 1998 Inorg. Chem. 37 2854 10.1021/ic980253h
- BarreraG DBrunoJ A OBarronT H KAllanN L 2005 J. Phys: Condens. Matter 17 R217 10.1088/0953-8984/17/4/r03
- TakenakaK 2012 Sci. Technol. Adv. Mater. 13 013001 10.1088/1468-6996/13/1/013001
- MaryT AEvansJ S OVogtTSleightA W 1996 Science 272 90 10.1126/science.272.5258.90
- PhillipsA EGoodwinA LHalderG JSouthonP DKepertC J 2008 Angew. Chem., Int. Edn Engl. 47 1396 10.1002/anie.200704421
- TakenakaKTakagiH 2005 Appl. Phys. Lett. 87 261902 10.1063/1.2147726
- TakenakaKAsanoKMisawaMTakagiH 2008 Appl. Phys. Lett. 92 011927 10.1063/1.2831715
- TakenakaKInagakiTTakagiH 2009 Appl. Phys. Lett. 95 132508 10.1063/1.3243340
- MatsunoJTakenakaKTakagiHMatsumuraDNishihataYMizukiJ 2009 Appl. Phys. Lett. 94 181904 10.1063/1.3129169
- HamadaTTakenakaK 2011 J. Appl. Phys. 109 07E309 10.1063/1.3540604
- TongPLoucaDKingGLlobetALinJ CSunY P 2013 Appl. Phys. Lett. 102 041908 10.1063/1.4790151
- TakenakaKHamadaTKasugaiDSugimotoN 2012 J. Appl. Phys. 112 083517 10.1063/1.4759121
- ChenJXingX RYuR BLiuG R 2005 J. Am. Ceram. Soc. 88 1356 10.1111/j.1551-2916.2005.00314.x
- ChenJXingXSunCHuPYuRWangXLiL 2008 J. Am. Chem. Soc. 130 1144 10.1021/ja7100278
- HuPKangHChenJDengJXingX 2011 J. Mater. Chem. 21 16205 10.1039/c1jm12410b
- ChenJNittalaKForresterJ SJonesJ LDengJYuRXingX 2011 J. Am. Chem. Soc. 133 11114 10.1021/ja2046292
- ChenJFanLRenYPanZDengJYuRXingX 2013 Phys. Rev. Lett. 110 115901 10.1103/PhysRevLett.110.115901
- ChenJWangF FHuangQHuLSongX PDengJ XYuR BXingX R 2013 Sci. Rep. 3 2458 10.1038/srep02458
- LongY WHayashiNSaitoTAzumaMMuranakaSShimakawaY 2009 Nature 458 60 10.1038/nature07816
- YamadaI 2011 Angew. Chem., Int. Edn Engl. 50 6579 10.1002/anie.201102228
- YamadaISiroKOkaKAzumaMIrifuneT 2013 J. Ceram. Soc. Japan 121 912 10.2109/jcersj2.121.912
- YamadaIMarukawaSMurakamiMMoriS 2014 Appl. Phys. Lett. 105 231906 10.1063/1.4903890
- AzumaM 2011 Nat. Commun. 2 347 10.1038/ncomms1361
- OkaKNabetaniKSakaguchiCSekiHCzapskiMShimakawaYAzumaM 2013 Appl. Phys. Lett. 103 061909 10.1063/1.4817976
- NabetaniK 2015 Appl. Phys. Lett. 106 061912 10.1063/1.4908258
- IshiwataSAzumaMTakanoMNishiboriETakataMSakataMKatoK 2002 J. Mater. Chem. 12 3733 10.1039/b206022a
- AzumaMCarlssonSRodgersJTuckerM GTsujimotoMIshiwataSIsodaSShimakawaYTakanoMAttfieldJ P 2007 J. Am. Chem. Soc. 129 14433 10.1021/ja074880u
- MizumakiMIshimatsuNKawamuraNAzumaMShimakawaYTakanoMUozumiT 2009 Phys. Rev. B 80 233104 10.1103/PhysRevB.80.233104
- IshiwataS 2005 Phys. Rev. B 72 045104 10.1103/PhysRevB.72.045104
- BansalG KHeuerA H 1972 Acta Metall. 20 1281 10.1016/0001-6160(72)90059-4
- OkaKSakaguchiCSinclairARitterCMizumakiMAttfieldJ PAzumaM 2013 Phys. Rev. B 88 014112 10.1103/PhysRevB.88.014112