Abstract
Two experiments were conducted to evaluate the inhibitory effects of 2-chloro-6 (trichloromethyl) pyridine (nitrapyrin) and dicyandiamide on nitrous oxide (N2O), a greenhouse gas, emission from soils amended with ammonium sulfate. In the two experiments, samples of an Andosol and a Gray Lowland soil were kept in glass vessels sealed with a butyl rubber cap and incubated at 25°C. In the first experiment, nitrapyrin (1 µg g−1 dry soil) and dicyandiamide (10 µg g−1 dry soil) were applied to samples of a water-saturated Andosol and a Gray Lowland soil to which ammonium sulfate had been applied at a rate of 0.1 mg N g−1 dry soil. Nitrapyrin decreased N2O emissions from the Andosol and the Gray Lowland soil by 71% and 24%, respectively. Dicyandiamide decreased N2O emissions from the Andosol and Gray Lowland soil by 31% and 18%, respectively. In the second experiment, nitrapyrin (1 µg g−1 dry soil) was applied to samples of an Andosol at 51% water-filled pore space to which ammonium sulfate had been applied at rates of 0.01, 0.1 and 0.5 mg N g−1 dry soil. Nitrapyrin decreased N2O emissions by 62%, 83% and 74%, respectively. Changes in the NH+ 4 and NO− 2 + NO− 3 concentrations in soil showed that nitrapyrin and dicyandiamide slowed down the nitrification process, but did not completely stop the process at any time. The results reveal the potential of nitrification inhibitors to decrease N2O emission from fertilized soil in a wide range of moisture conditions and nitrogen levels.
INTRODUCTION
The atmospheric concentration of nitrous oxide (N2O) has increased by 46 p.p.b. (17%) since 1750 and continues to increase at a rate of 0.25% year−1 (1980–1998). Radiative forcing amounts to 0.15 W m−2, which accounts for 6% of the total from all of the long-lived and globally mixed greenhouse gases (CitationIntergovernmental Panel on Climate Change 2001). Agricultural soil, a major source, accounts for approximately 6.2 Tg N2O-N year−1, or 35% of the annual global emission (CitationKroeze et al. 1999). Thus, it is necessary to reduce N2O emission from agricultural soil to mitigate global warming.
Nitrification inhibitors (ammonia oxidation inhibitors) contribute to the decrease in N2O emission from fertilized agricultural soil (CitationMcTaggart et al. 1997; CitationMinami 1994; CitationWolt 2004). Nitrification inhibitors such as nitrapyrin suppress the accumulation of nitrite-N in soil and thus are likely to indirectly decrease N2O emission via chemodenitrification or microbial denitrification of nitrite-N (CitationSahrawat 1989). Nitrification inhibitors also decrease nitrate leaching (CitationCookson and Cornforth 2002). However, the use of nitrification inhibitors on agricultural land is still limited because the cost of nitrification inhibitors is higher than the economical benefit for farmers. As the adverse effects of global warming are becoming evident, the demand for a decrease in N2O emission will increase. Practical techniques to decrease N2O emission from agricultural soil should be developed, and the use of nitrification inhibitors may achieve this objective. For that purpsose, it is necessary to elucidate the mechanisms whereby nitrification inhibitors affect N2O emission under various conditions.
In the present study, the effect of nitrapyrin and dicyandiamide on N2O emission was examined. Although there are numerous nitrification inhibitors (CitationSlangen and Kerkhoff 1984), most commonly inhibit NH3 monooxygenase (CitationMcCarty 1999). Nitrapyrin and dicyandiamide, which are two of the most common nitrification inhibitors (CitationBolan et al. 2004; CitationPrasad and Power 1995), were selected in the present study as typical nitrification inhibitors. Studies have shown that N2O emission increases as soil moisture increases (CitationBateman and Baggs 2005; CitationInubushi et al. 1999; CitationMcTaggart and Tsuruta 2003). However, it has not been determined whether nitrification inhibitors decrease N2O emission from soil under water-saturated conditions. Soil moisture conditions have been shown to affect the effectiveness of nitrification inhibitors (CitationSlangen and Kerkhoff 1984). The present study was conducted to clarify the effect of nitrification inhibitors on the decrease in N2O emission from water-saturated soils. Nitrification inhibitors were applied to samples of an Andosol and a Gray Lowland soil under water-saturated conditions. CitationBlackmer et al. (1980) reported that part of nitrified N evolved as N2O, and increased with increasing NH+ 4 concentration up to approximately 1 g NH+ 4-N L−1. Thus, the effect of nitrification inhibitors on N2O emission may change depending on the NH+ 4 concentration in the soil. Attempts were also made to test the effectiveness of nitrapyrin in decreasing N2O emission in samples of an Andosol amended with three different application rates of ammonium sulfate under aerobic conditions.
MATERIALS AND METHODS
Soil sample
Two soil types, an Andosol and a Gray Lowland soil, were used for the experiments. Samples of the Andosol were taken from the surface layer (0–10 cm) of the Hachiman-tai experimental upland field of the Japan International Research Center for Agricultural Sciences (JIRCAS; Tsukuba, Ibaraki) in March. Soybean was cultivated in the summer season, but no crops had been grown in the winter season for more than 5 years. There was no vegetation at the time of soil sampling. Total C and N concentrations were 33.5 g kg−1 and 2.8 g kg−1, respectively. The soil pH (KCl) was 5.44. Samples of the Gray Lowland soil were collected from the Hachiman-tai experimental paddy field of JIRCAS from the surface layer (0–10 cm) at the end of April, before puddling for rice cultivation was carried out. Rice had been cultivated for more than 20 years, and no crops were cultivated in the winter season. Total C and N concentrations were 17.5 g kg−1 and 1.7 g kg−1, respectively. The soil pH (KCl) was 4.55. Fresh samples of the two soil types were partially dried at room temperature and sieved using a 2 mm mesh sieve.
N2O analysis
The concentration of N2O in the vessel headspace was determined using a gas chromatograph fitted with an electron capture detector at 340°C (GC-14B, Shimadzu, Kyoto, Japan). The separating columns were packed with Porapak Q and the carrier gas consisted of 5% methane in argon. Three glass vessels for each treatment were sealed using butyl rubber caps. After measurement, the caps were removed immediately and air in the headspace was ventilated. The vessels were then resealed for further incubation.
NO− 2 + NO− 3 and NH− 4 analysis
NO− 2 + NO− 3 and NH+ 4 in soils were extracted with a 2 mol L−1 KCl solution. The extract was filtered, kept in a freezer and the concentrations of NO− 2 + NO− 3 and NH+ 4 were determined using an auto-analyzer (Auto Analyzer II, Bran+Luebbe, Tokyo, Japan).
Experiment 1
Experiment 1 examined the use of nitrapyrin and dicyandiamide in samples of an Andosol and a Gray Lowland soil under water-saturated conditions. Fresh soil samples (20 g on a dry weight basis) were placed in glass vessels with an inner diameter of 36.5 mm and 130 mm in height. Three types of treatments for each soil type were prepared as follows: ammonium sulfate (no-Ni), ammonium sulfate and nitrapyrin (1 mg g−1 dry soil) (Np), and ammonium sulfate and dicyandiamide (10 mg g−1 dry soil) (Dd). Ammonium sulfate was applied to all treatments at a rate of 0.1 mg N g−1 dry soil. After the application of the reagents, the moisture content of all samples was adjusted to the saturated condition using distilled water. These samples were incubated at 25°C and N2O emissions were determined at 2–4-day intervals for 45 days.
For the analysis of NO− 2 + NO− 3 and NH+ 4, treatment vessels were prepared in the same ways as the samples used to examine N2O emission. Two samples were extracted every week after the onset of incubation.
The preparation and incubation of the samples amended with nitrapyrin started 11 days after the other treatments. During that period, the fresh soil samples were put into plastic bags and kept in a refrigerator.
Experiment 2
Experiment 2 examined the use of nitrapyrin in samples of an Andosol amended with three levels of ammonium sulfate under aerobic conditions. Fresh Andosol samples (20 g on a dry weight basis) were placed in glass vessels as in experiment 1. Seven treatments were set up, including three levels of ammonium sulfate at application rates of 0.01, 0.1 and 0.5 mg N g−1 dry soil with or without 1 mg g−1 of nitrapyrin and a control treatment (no N and no nitrapyrin). The moisture content of all the samples was adjusted to 51% water-filled pore space (WFPS). Three glass vessels for each treatment were incubated at 25°C and the N2O concentration in the vessel headspace was determined using gas chromatography every 3 or 4 days until the NH+ 4 concentration in the soil decreased to the same level as that in the control.
For the analysis of NO− 2 + NO− 3 and NH+ 4, treatment vessels were prepared in the same way as samples examining N2O emission. Two samples were extracted with a 2 mol L−1 KCl solution every week after the onset of incubation.
RESULTS
Experiment 1
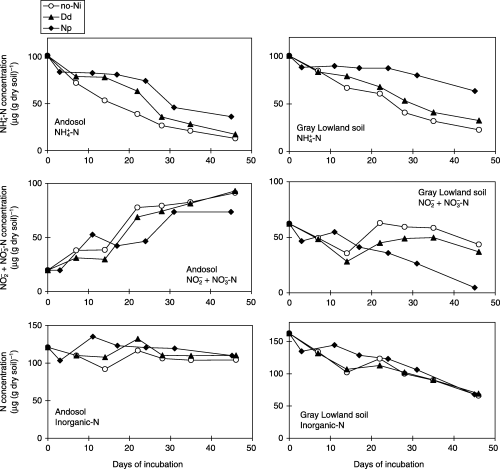
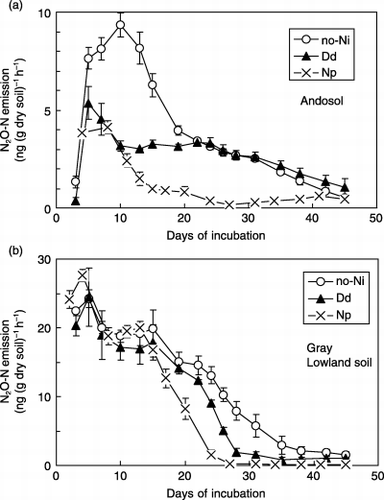
Nitrapyrin and dicyandiamide decreased the N2O emissions from the two soil types under water-saturated conditions (). In the Andosol, N2O emission from the Np treatment was always lower than that from the no-Ni treatment for 45 days, whereas the N2O emission from the Dd treatment was lower during the first 3 weeks
Figure 1 Nitrous oxide emission from an Andosol and a Gray Lowland soil amended with nitrification inhibitors under water-saturated conditions. (Bars represent the standard deviation.)
Changes in the NH+ 4 and NO− 2+NO− 3 concentrations showed that nitrapyrin and dicyandiamide retarded, but did not stop, nitrification in both soil types (). Although the concentration was lower, nitrapyrin inhibited nitrification more strongly than dicyandiamide. The decrease in the total inorganic nitrogen concentration (NH+ 4, NO− 2 and NO− 3) indicated that denitrification occurred in the Gray Lowland soil. However, the total inorganic nitrogen concentration in the Andosol did not decrease.
Experiment 2
Nitrapyrin decreased the emission of N2O at all three levels of ammonium sulfate treatment (). The ratio of emitted N2O to applied ammonium sulfate increased as the application rate increased ().
Changes in the NH+ 4 and NO− 2 + NO− 3 concentrations in soil showed that nitrapyrin did not stop, but slowed down, the nitrification process at any time (data not shown). Nitrification was completed within 7, 14 and 28 days in the Andosol amended with 0.01, 0.1 and
Table 1 Emitted N2O and N2O-N/applied nitrogen from soil amended with ammonium sulfate and nitrapyrin
DISCUSSION
Nitrification inhibitors decreased N2O emissions from the Andosol and Gray Lowland soils under water-saturated conditions. CitationPoth and Focht (1985) reported that N2O was produced by autotrophic NH3 oxidizers through a reductive process in which organisms use as an electron acceptor, particularly when O2 is a limiting factor. CitationWilliams et al. (1992) suggested that although energy is required to reduce NO− 2 to N2O, this mechanism enables organisms to conserve limited O2 for the oxidation of NH3, from which they gain energy for growth and regeneration while avoiding the possible accumulation of toxic levels of NO− 2. It appears that the nitrification inhibitors slowed down the conversion rate of NH+ 4 to in the soil, which contributed to a lower N2O emission ratio to nitrified nitrogen.
The N2O emitted from soil is mainly produced through nitrification and denitrification by soil microorganisms (CitationGranli and Bockma 1994; CitationWilliams et al. 1992). CitationInubushi et al. (1996) observed that most N2O was derived from autotrophic nitrification in two Andosols at 60% and 80% water-holding capacity with NH+ 4 amendment, whereas large amounts of N2O were emitted at 100% water-holding capacity mostly by denitrification. The decrease in the amount of total
Figure 3 Influence of nitrapyrin on nitrous oxide emission in an Andosol amended with different levels of ammonium sulfate. (Bars represent the standard deviation.)
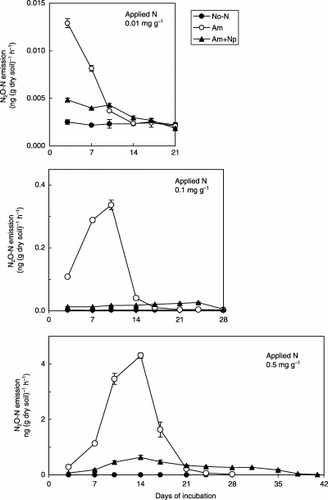
In experiment 2, nitrapyrin inhibited the oxidation of NH+ 4 to NO− 2 and decreased the ratio of N2O emission to accumulated NO− 3 in the Andosol amended with ammonium sulfate (0.01, 0.1 and 0.5 mg N g−1 dry soil). A higher ratio of N2O was emitted when more NH+ 4-N was applied to the soil (). CitationBlackmer et al. (1980) reported that part of the nitrified N evolved as N2O increased with increasing NH+ 4 concentration up to approximately 1 g NH+ 4-N L−1. The NH+ 4-N concentration in the soil water in experiment 2 was less than 1 g NH+ 4-N L−1 and this result supported previous results (CitationBlackmer et al. 1980). Nitrapyrin decreased N2O emission regardless of the NH+ 4-N concentration within the prepared range in the Andosol.
Nitrification inhibitors contribute to the alleviation of N2O emission from fertilized agricultural soil. CitationWolt (2004) indicated that in cornfields in Midwestern USA, nitrapyrin decreased greenhouse gas emissions by an average of 51%. However, the use of nitrification inhibitors on agricultural land is still limited. In addition to the cost-benefit problem, the limited use of nitrification inhibitors may be ascribed to variations in their effectiveness. It was observed that the temperature, pH value, organic matter content, clay content and moisture conditions of soil affect the effectiveness of nitrification inhibitors (CitationSlangen and Kerkhoff 1984). To enhance the effectiveness and reliability of nitrification inhibitors, the conditions under which the inhibitors decrease N2O emission and leaching loss of nitrogen need to be clarified. The present study showed that nitrapyrin effectively decreased N2O emission. However, nitrapyrin has not been used in Japan. Nitrapyrin has a relative high vapor pressure (CitationSlangen and Kerkhoff 1984) that excludes its addition to solid fertilizers. Alternative nitrification inhibitors that would be more effective than dicyandiamide and nitrapyrin need to be identified (CitationZerulla et al. 2001). It is also necessary to develop other methods of decreasing N2O emissions from fertilized soil, including improvement of land management, organic matter management and the use of controlled-release fertilizers (CitationAkiyama and Tsuruta 2003; CitationKoga et al. 2004; CitationSmith et al. 1997). CitationIshikawa et al. (1999) reported that a tropical grass growing in tropical pastures inhibits nitrification and decreases N2O emission from soil where the grass grows. We may utilize that biological resource to breed crops that inhibit nitrification and decrease N2O emission from arable land soil.
REFERENCES
- Akiyama , H and Tsuruta , H . 2003 . Nitrous oxide, nitric oxide, and nitrogen dioxide fluxes from soils after manure and urea application . JEnvironQual , 32 : 423 – 431 .
- Bateman , EJ and Baggs , EM . 2005 . Contribution of nitrification and denitrification to N2O emissions from soils at different water-filled pore space . BiolFertilSoils , 41 : 379 – 388 .
- Blackmer , AM , Bremner , JM and Schmidt , EL . 1980 . Production of nitrous oxide by ammonia-oxidizing chemoautotrophic microorganisms in soil . ApplEnvironMicrobiol , 40 : 1060 – 1066 .
- Bolan , NS , Saggar , S , Luo , J , Bhandral , R and Singh , J . 2004 . Gaseous emissions of nitrogen from grazed pastures . AdvAgron , 84 : 37 – 120 .
- Cookson , WR and Cornforth , IS . 2002 . Dicyandiamide slows nitrification in dairy cattle urine patches: effects on soil solution composition, soil pH and pasture yield . Soil BiolBiochem , 34 : 1461 – 1465 .
- Granli , T and Bockman , OC . 1994 . Nitrogen oxide from agriculture . Norwegian JAgricSci , 12 : 7 – 124 .
- Intergovernmental Panel on Climate Change . 2001 . “ Third Assessment/Summary for Policymakers ” . In Climate Change 2001 The Scientific Basis , Edited by: Houghton , JH , Ding , Y Griggs , DJ . 6 – 7 . Cambridge : Cambridge University Press .
- Inubushi , K , Naganuma , H and Kitahara , S . 1996 . Contribution of denitrification and autotrophic and heterotrophic nitrification to nitrous oxide production in andosols . BiolFertilSoils , 23 : 292 – 298 .
- Inubushi , K , Barahona , MA and Yamakawa , K . 1999 . Effects of salts and moisture content on N2O emission and nitrogen dynamics in Yellow soil and Andosol in model experiments . BiolFertilSoils , 29 : 401 – 407 .
- Ishikawa , T , Watanabe , T and Minami , K . 1999 . Possibility of nitrification suppression by a tropical grass . JpnJSoil SciPlant Nutr , 70 : 762 – 768 . (in Japanese with English summary)
- Koga , N , Tsuruta , H , Sawamoto , T , Nishimura , S and Yagi , K . 2004 . N2O emission and CH4uptake in arable fields managed under conventional and reduced tillage cropping systems in northern Japan . Global BiogeochemCycles , 18 : GB4025
- Kroeze , C , Mosier , A and Bouwman , L . 1999 . Closing the global N2O budget; A retrospective analysis 1500–1994 . Global BiogeochemCycles , 13 : 1 – 8 .
- McCarty , GW . 1999 . Modes of action of nitrification inhibitors . BiolFertilSoils , 29 : 1 – 9 .
- McTaggart , IP , Clayton , H , Parker , J , Swan , L and Smith , KA . 1997 . Nitrous oxide emissions from grassland and spring barley, following N fertilizer application with and without nitrification inhibitors . BiolFertilSoils , 25 : 261 – 268 .
- McTaggart , IP and Tsuruta , H . 2003 . The influence of controlled release fertilizers and the form of applied fertilizer nitrogen on nitrous oxide emissions from an andosol . NutrCyclAgroecosyst , 67 : 47 – 54 .
- Minami , K . 1994 . “ Effect of nitrification inhibitors and slow-release fertilizers on emissions of nitrous oxide from fertilized soils ” . In CH4and N2O , Edited by: Minami , K , Mosier , A and Sass , R . 187 – 196 . Tokyo : Yokendo Publishers .
- Prasad , R and Power , JF . 1995 . Nitrification inhibitors for agriculture, health, and the environment . AdvAgron , 54 : 233 – 281 .
- Poth , M and Focht , DD . 1985 . 15N kinetic analysis of N2O production by Nitrosomonus europaea: An examination of nitrifier denitrification . ApplEnvironMicrobiol , 49 : 1134 – 1141 .
- Sahrawat , KL . 1989 . Effects of nitrification inhibitors on nitrogen transformations, other than nitrification, in soils . AdvAgron , 42 : 279 – 309 .
- Slangen , JHG and Kerkhoff , P . 1984 . Nitrification inhibitors in agriculture and horticulture . FertilRes , 5 : 1 – 76 .
- Smith , KA , McTaggart , IP and Tsuruta , H . 1997 . Emissions of N2O and NO associated with nitrogen fertilization in intensive agriculture, and the potential for mitigation . Soil Use and Management , 13 : 296 – 304 .
- Williams , EJ , Hutchinson , GL and Fehsenfeld , FS . 1992 . NOx and N2O emissions from soil . Global BiogeochemCycl , 6 : 351 – 388 .
- Wolt , JD . 2004 . A meta-evaluation of nitrapyrin agronomic and environmental effectiveness with emphasis on corn production in the Midwestern USA . NutrCyclAgroecosyst , 69 : 23 – 41 .
- Zerulla , W , Barth , T Dressel , J . 2001 . 3,4-Dimethylpyrazole phosphate (DMPP) – a new nitrification inhibitor for agriculture and horticulture . BiolFertilSoils , 34 : 79 – 84 .