Abstract
The effects of salinity stress on vegetative growth in tobacco (Nicotiana tabacum) plants were examined in terms of the source–sink relationship. The effect of salinity stress on plant growth was continuously measured using a micromorphometric technique under greenhouse conditions. This technique is less cumbersome and more precise than conventional techniques used to measure plant growth under salinity conditions. The effect of salinity stress on photosynthetic rate, 13C partitioning and sugar and Na+ concentrations was also measured. Salinity stress was imposed by irrigating the plants with NaCl solution at 50 and 100 mmol L−1. Stem diameters of the control plants started to decrease around 07:00 am and reached a minimum at 03:00 pm. Salinity stress did not change the diurnal pattern of stem diameter dynamics, but increased the amplitude of daytime shrinking, and consequently stem growth estimated by the difference in stem diameter at night was inhibited. The effect of salinity stress on stems, a major sink, appeared within a few hours of treatment, whereas the effects on photosynthetic rate were observed after a lag period of 3–4 days of salt application. Salinity stress also reduced 13C export rate, but increased leaf sugar concentration and Na concentration in all plant parts. These results suggest that salinity impairs sink activity earlier than source activity. Based on the results of this study, the utilization of micromorphometric techniques for early diagnosis of growth in salt-prone environments is recommended.
INTRODUCTION
Salinity is an important environmental factor resulting in suboptimal growth. Despite the far-reaching impact in agronomic terms, however, only limited information about the effect of salinity on plant metabolism and source–sink relationships is available (CitationRoitsch 1999). It has been shown that salt stress not only alters photosynthesis and carbohydrate levels, at least transiently, but also alters the types of carbohydrates that are synthesized and exported by the source tissue (CitationGilbert et al. 1997; CitationGucci et al. 1998). The proposal that alterations in the type of carbohydrate transported in response to salt stress may act as a sugar signaling system in plants still lacks any experimental evidence (CitationRoitsch 1999). Salinity causes a deficiency of water in plant tissue, and low water potential reduces growth by inhibiting cell division and cell expansion (CitationHasegawa et al. 2000). In cell-specific responses, the water potential gradient between the cell and its stressful environment determines the water balance deficit of the cell. In tissue-specific responses, the water balance deficit of a plant is also influenced by its water storage capacity (CitationHuguet 1985).
Our earlier studies have shown that changes in the fruit and stem diameter of a specific plant can be continuously monitored with high sensitivity using a shrinkage type micro-displacement detector (CitationFujita et al. 2003; CitationIto et al. 2002). This method, therefore, can be used to estimate changes in sink activity of these plant parts under salt stress conditions. The method enables us to attain our objectives in the present study.
Recently, we reported that in a comparison between ectoine-transformed and wild tobacco plants, salt stress impairs plant growth mainly because of the depression of photosynthetic rate, which might be related to the suppression of nitrate-N transport to leaves through transpiration. However, we have not examined whether the behavior of carbon compounds, such as carbon partitioning and leaf sugar concentration, is involved in the depression of leaf photosynthetic rate under high salt stress. It is well known that photosynthetic activity is frequently associated with sink activity to receive photosynthate (CitationGifford and Evans 1981; CitationHo 1988).
Based on this information, the present study was undertaken to examine the effect of salinity on tobacco plant biomass production in terms of the source–sink relationship. Related parameters such as photosynthetic rate, stem diameter changes and 13C partitioning were also observed.
MATERIAL AND METHODS
Plant material and culture
Tobacco (Nicotiana tabacum SR1) seeds were surface sterilized and germinated on half-strength MS agar CitationMurashige and Skoog medium (MS; Murashige and Skoog 1962) at 25°C. Ten days after germination, the seedlings were transplanted to 500 mL pots filled with a soil mixture containing granite regosol, perlite and peat moss at the ratio 2:2:1 (v/v). The pots were kept in the greenhouse of the Graduate School of Biosphere Science, Hiroshima University, Hiroshima, Japan. The plants were grown under natural light irradiance. Temperature and light were automatically recorded. The plants were irrigated with nutrient solution containing 7.1 mmol L−1 N (KNO3/Ca(NO3)2), 0.48 mmol L−1 P (KH2PO4), 2.6 mmol L−1 K (KH2PO4/KNO3), 0.5 mmol L−1 Ca (Ca(NO3)2), 1 mmol L−1 Mg (MgSO4), 54 µmol L−1 Fe (Fe3+EDTA), 18 µmol L−1 Mn (MnSO4), 0.15 µmol L−1 Zn (ZnSO4), 0.16 µmol L−1 Cu (CuSO4), 4.6 µmol L−1 B (H3BO3) and 0.1 µmol L−1 Mo ((NH4)6Mo7O24·4H2O).
Salt treatment
The salinity treatment was conducted 60 days after germination by the administration of an irrigated nutrient solution including 0, 50 and 100 mmol L−1 NaCl. The solution was applied from the bottom of the pots through root uptake and transpiration.
Sampling and plant biomass
Sampling was conducted at 2, 5, 8 and 11 days after treatment. At each time, plants were separated into leaf blades, stem plus petioles and root. The samples were lyophilized for 72 h before dry-weight estimation. The lyophilized plant materials were ground to powder using a vibrating sampling mill (Model T1-100 Heiko Company, Fukushima, Japan) for chemical analysis (element and sugar concentration).
Measurement of Na, Ca and K
Aliquots of the powder were wet ashed with an acid mixture (HNO3:HClO4, 4:1, v/v) and the concentrations of Na, K and Ca were determined using a flame photometer (Tokyo Koden, ANA-135, Tokyo, Japan).
Determination of stem water potential
Measurements were conducted after 1, 4, 7 and 10 days of NaCl treatment. The water potential of the younger part of the plant stem was measured using a pressure chamber (Daiki Rika kogyo Company, Tokyo, Japan).
Measurement of photosynthesis
Photosynthetic rate (Po) of the second expanded leaf was measured using a portable infrared gas analyzer (Model LI-6400 model Li-Cor Company, Lincoln, NE, USA). The leaf chamber was an open type and measurements were done each day on both control and salt-treated plants over the treatment period. Photosynthetically active radiation (PAR) during measurement was adjusted to 1000 µmol m−2 s−1 and the CO2 concentration of the measurement chamber was adjusted to 360 p.p.m.
Measurement of stem diameter
Changes in stem diameter at 5 cm above the basal end of the stem were continuously monitored in both control and NaCl treatment plants from 1 day before treatment began until the end of the experiment using a shrinkage type micro-displacement detector (CitationFujita et al. 2003). The sensors were fastened to the growing stem and were connected to a computerized data acquisition system (NEC, Sanei Kogyo Company, Tokyo, Japan). A glass rod in place of a plant sample was the blank for the measurements and the sensitivity of the measurements was within a limit of 2 µm. All measurements were replicated using the three plants in which the photosynthetic rate was determined.
13C feeding
13CO2 feeding was given to the second expanded leaf 4 days after salinity treatment in both control and salt-treated plants. The leaf was enclosed in a transparent plastic bag and 350 µL of 13CO2 (99 13C atom %) was introduced from a cylinder. The leaf was allowed to assimilate 13CO2 for 1 h at PAR of more than 1000 µmol m−2 s−1. The plants were harvested 24 h after feeding and were separated into 13CO2-fed leaf, other leaves, stems plus petioles and roots. The plant parts were lyophilized, weighed and ground to powder for measurement of 13C abundance.
Determination of 13C abundance
The percentage of 13C atoms in the powdered plant sample was determined using a mass spectrometer (model Delta plus, Finigan Company, San Jose, CA, USA) (CitationNobuyasu et al. 2003). The excess percentage of 13C atom in the sample was calculated as the difference in 13C atom percentage between the 13CO2 fed and unfed samples. The amount of labeled C in the plant samples was calculated using the following equation:
The amount of total C in the sample was determined using the element analyzer facilitated in the mass spectrometer.
Measurement of sugar contents
Aliquots of the powdered leaves and roots were boiled with 80% (v/v) aqueous ethanol twice for the extraction of sugars. The sugar content in the ethanol-soluble extract was determined using the anthrone reagent according to the method of CitationYemm et al. (1954).
RESULTS
Dry mass accumulation
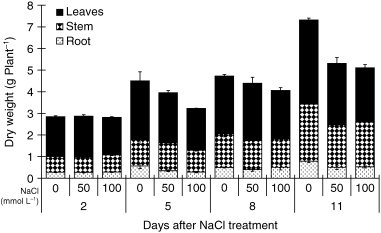
Whole plant biomass increased throughout the experimental period (). Treatments of 50 and 100 mmol L−1 NaCl depressed the whole plant biomass after 11 and 5 days of treatment, respectively. The decrease was mostly derived from a reduction in leaves and stem biomass. A similar trend was observed for root biomass except that there was no difference between 50 and 100 mmol L−1 at 5 days.
Water potential
Water potential of the younger part of the stem was not altered by NaCl treatment at any sampling time (data not shown).
Photosynthesis
The apparent photosynthetic rate remained similar in both control and salt-treated plants during the first 4 and 3 days for 50 and 100 mmol L−1 NaCl treatments, respectively (). However, activity in the treatment plants declined on the following days and remained lower than the control. When the photosynthetic rate decreased, an increase in the standard deviation (SD) was observed, suggesting that the decline occurred more rapidly in some plants than in others.
Figure 2 Effects of salinity (NaCl) treatment on the photosynthetic rate in tobacco plants. Values are means and standard deviations of 3 replicates.
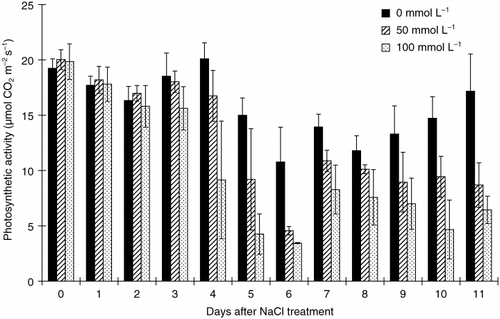
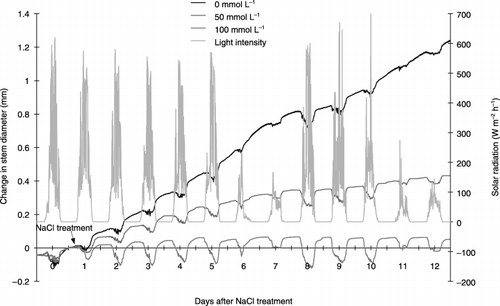
Figure 3 Effects of salinity (NaCl) treatment on the stem diameter of tobacco. Changes in stem diameter were continuously recorded using a shrinkage type micro-displacement detector in three control and three salt-treated plants. The changes in different plants treated with the same treatment were similar. The figure shows the change in a representative plant from each treatment.
Change in stem diameters
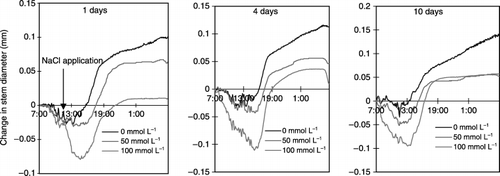
During the experimental period, the diameter of the stem in the control plant exhibited daytime shrinkage and night-time expansion (). There was a similar rhythmic shrinkage and expansion of the stem in the plants exposed to salt. However, the salt treatment led to increased stem shrinkage because of the enhanced daytime contraction of the stem diameter, and a reduction in increased expansion at night time. shows that among these components, the increase in stem diameter at night was more markedly depressed by the salt treatment than others. Therefore, the increase in stem diameter at night is a predominant factor depressing the daily increase in stem diameter and changing stem growth pattern during several days by the salt treatment. In the present study, we intended to analyze the factors involved in salt stress based on the daily increase in stem diameter measured at 06:00 am. The suppression of the stem diameter was obviously detected a few hours after salt treatment of 50 and 100 mmol L−1 (). In addition, the longer the duration of treatment and the higher the salt concentration the greater the reduction in stem diameter.
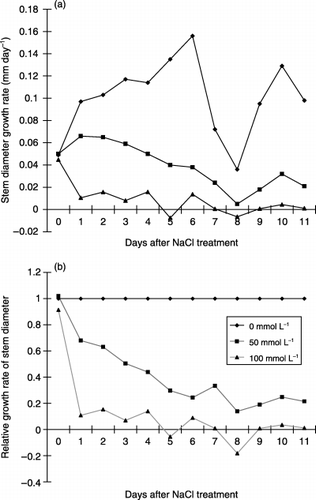
The salt treatment at 100 mmol L−1 decreased the stem growth rate rapidly up to the next day, whereas at 50 mmol L−1 growth rate decreased slowly for 6 days (). Subsequently, stem growth reached approximately zero in the former and was maintained at approximately 20% in the latter ().
13C partitioning
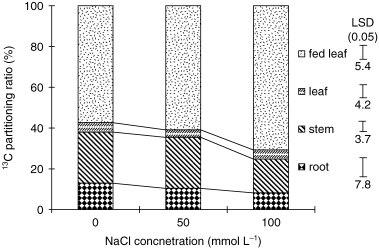
The export rate of 13C into other plant parts from the fed leaf in the control plant was approximately 42% at 4 days after the initiation of the treatment (). Salt treatment decreased the export rate only slightly at 50 mmol L−1, but the decrease was more pronounced at 100 mmol L−1.
Sugar concentration
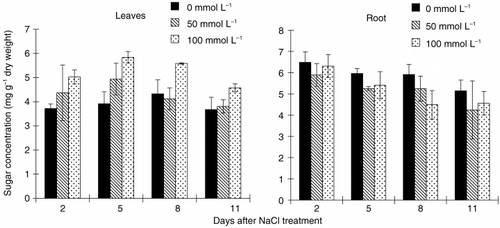
Salt treatment increased the leaf sugar concentration and the effect differed significantly between the two levels of salt treatment (). The sugar concentration remained significantly higher than the control from 2 days until the end of the experiment at 100 mmol L−1; however, at 50 mmol L−1 there was no significant difference between the treatment plants and the control. Root sugar concentration () decreased after 8 days of salt treatment at 100 mmol L−1.
Na, Ca, and K concentration
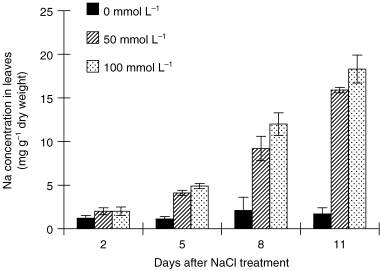
The Na concentration in leaves and the stem at 50 mmol L−1 and in leaves at 100 mmol L−1 NaCl did not change after 2 days of treatment. However, after 5 days the Na concentration significantly increased in the salt-treated plants: the higher the salt concentration in the culture media and the longer the treatment duration, the greater the Na concentration (, ).
The Ca concentration also increased with NaCl treatment (data not shown). However, K concentration was not affected by the accumulation of Na.
DISCUSSION
Plant growth, photosynthesis and salinity stress
The present data, which showed that salt treatment impaired plant growth (), support our earlier findings for tobacco plants (CitationMoghaieb et al. in press).
Figure 5 (a) Growth rate of the stem diameter in 0, 50 and 100 mmol L−1 NaCl treatments. Values were calculated as the stem diameter from 6:00 am to 6:00 am the following day. (b) Relative growth rate of the stem diameter at 50 and 100 mmol L−1 NaCl. The value of 0 mmol L−1 plant growth rate was indicated as 1. The relative growth rate was calculated as the proportion of the growth rate in the NaCl treatment relative to that of the control.
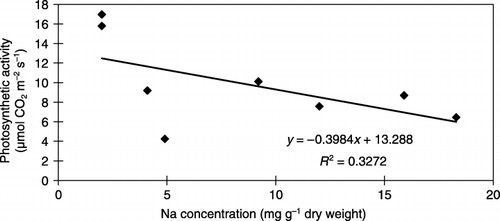
The results in the present study that showed that salt treatment decreased the photosynthetic rate, and subsequently the whole plant biomass suggest that the impairment of plant growth by salt stress mainly results from the suppression of photosynthetic activity. However, no significant correlation between photosynthetic rate and leaf Na concentration was observed ()
Current data showing that leaves and stem growth was depressed more than root growth by salt stress () suggests that sink activity in leaves and stems is
Figure 6 Effects of salinity (NaCl) treatment on 13C partitioning to different plant parts in tobacco. 13CO2 feeding was given to the second matured leaf 4 days after salinity treatment. Plant samples were collected 24 h after feeding. Values are means of 3 replicates. LSD, least significant difference.
These results suggest that salt stress depresses photosynthetic activity, which may suppress plant growth. However, it is not well understood why photosynthetic activity is impaired by salt stress, although it has been examined in relation to stomatal conductance and leaf internal CO2 concentration (CitationFujita et al. 2003; CitationHasegawa et al. 2000; CitationMoghaieb et al. 2000; CitationMoghaieb et al. in press).
Stem diameter and salinty stress
The present study showed that the stem diameter is composed of two components, a “reversible component” and an “irreversible component” (CitationSevanto et al. 2002) (,). The “reversible component” is a layer of extensible tissues surrounding the xylem that comprises matured cells with rigid cell walls (CitationBrough et al. 1986; CitationGenard et al. 2001; CitationKlepper 1971; CitationMolz et al. 1973). The results of the present study are consistent with the findings that the stem diameter shrinks during the day because of the loss of water in transpiration and swells at night owing to the uptake and storage of water (CitationHinekley et al. 1975).
Table 1 Effects of salinity on Na concentration in different parts of tobacco plants
In contrast, the “irreversible component” of the stem may be related to stem growth, as observed by the daily increase in stem diameter (). shows the change in the “irreversible component” of the stem with time, which is associated with the accumulation of photosynthates. The increase in the “irreversible component” was suppressed by salt stress.
Photosynthate behavior and salinity stress
The higher salt level (100 mmol L−1 NaCl) reduced the stem growth rate by 90% compared with the control plants during 24 h of salt treatment (). It also depressed the export rate of 13C from the fed-leaf (), but increased the leaf sugar concentration (). In contrast, the lower salt level (50 mmol L−1 NaCl) decreased the growth rate of the stem by less (32%), and the export
rate, and tended to increase the leaf sugar concentration. These results suggest that salt stress depressed the sink activity of the stem, which lead to the suppression of the export of photosynthate from a leaf, and consequently increased the leaf sugar concentration. This tendency is more pronounced at the higher salt level.Sugar accumulation and photosynthesis inhibition
Feedback inhibition of photosynthesis as a result of decreased sink demand is a well-known phenomenon (CitationRoitsch 1999). Different experimental approaches have shown that sugars play a key role in this regulatory mechanism by repressing the expression of the photosynthetic genes (CitationKoch 1996). The specific inhibitory effect of sugars on photosynthesis or on the expression of photosynthetic genes is further supported by current studies (CitationFelitti et al. 1998; CitationMorcuende et al. 1997; CitationWinder et al. 1998). In the present study, results showed that leaf sugar concentration () increased approximately 2 days earlier than the depression of photosynthetic rate () under a higher salt level, although such phenomena was not observed at a lower salt stress level. These results suggest that photosynthetic activity is possibly depressed by sugar accumulation in leaves.
CONCLUSION
Current results show that although salt stress impairs plant growth () because of the depression of photosynthetic rate, the earlier impairment of photosynthetic activity by salt stress may not result from Na accumulation in the leaves ().
Together all the results, a series of sequential responses to salt stress, can be summarized as follows: initially, sink activity of the stem is depressed by salt stress, then photosynthate transport is impaired, which results in sugar accumulation in leaves, and consequently photosynthetic activity decreases leading to suppression of plant growth.
Based on these results, we believe that the sink activity of vegetative plant parts like stems is directly inhibited by salt stress; leaf photosynthetic activity is indirectly suppressed by the limitation of the sink activity. In other words, the source–sink relationship is involved in the mechanism of impairment of plant growth by salt stress.
REFERENCES
- Roitsch , T . 1999 . Source–sink regulation by sugar and stress . CurrOpinPlant Biol , 2 : 198 – 206 .
- Girlbert , GA , Wilson , C and Madore , MA . 1997 . Root-Zone salinity alters raffinose oligosaccharide metabolism and transport in coleus . Plant Physiol , 115 : 1267 – 1276 .
- Gucci , R , Moing , A , Gravano , E and Gaudillere , JP . 1998 . Partitioning of photosynthetic carbohydrates in leaves of salt-stressed olive plants . AustJPlantPhysiol , 25 : 571 – 579 .
- Hasegawa , PM , Bressan , RA , Zhu , J-K and Bohnert , HJ . 2000 . Plant molecular responses to salinity . AnnRevPlant PhysiolPlant MolBiol , 51 : 463 – 499 .
- Huguet , JG . 1985 . Appreciation de l’etat hydrique d’une plante a partir des variations micrometriques de la dimension des fruits ou des tiges au cours de la fournee . Agonomie , 5 : 733 – 741 .
- Fujita , K , Ito , J Mohapatra , PK . 2003 . Circadian rhythm of stem and fruit diameter dynamics of Japanese persimmon (Diospyrus kakiThumb.) is affected by deficiency of water in saline environments . Funct. Plant Biol. , 30 : 747 – 754 .
- Ito , J , Hasegawa , K , Fujita , K , Ogasawara , S and Fujiwara , T . 2002 . Changes in water relations induced by CO2enrichment growth diurnal stem and fruit diameters of Japanese pear . Plant Science , 163 : 1169 – 1176 .
- Gifford , RM and Evans , LT . 1981 . Photosynthesis, carbon partitioning, and yield . AnnRevPlant Physiol , 32 : 485 – 509 .
- Ho , LC . 1988 . Metabolism and compartmentation of imported sugars in sink organs in relation to sink strength . AnnRevPlant PhysiolPlant MolBiol , 39 : 355 – 378 .
- Nobuyasu , H , Liu , S , Adu-Gyamfi , JJ , Mohapatra , PK and Fujita , K . 2003 . Variation in the export of 13C and 15N from soybean leaf: the effects of nitrogen application and sink removal . Plant Soil , 253 : 331 – 339 .
- Yemm , EW and Willis , AJ . 1954 . The estimation of carbohydrates in plant extracts by anthrone . BiochemJ , 57 : 508 – 514 .
- Moghaieb , REA , Tanaka , N Saneoka , H . Characterization of salt tolerance in ectoine-transformed tobacco plants (Nicotiana tabaccum): photosynthesis, osmotic adjustment, and nitrogen partitioning . Plant Cell Environ. , 29 173 – 182 .
- Moghaieb , REA , Tanaka , N Saneoka , H . 2000 . Expression of betaine aldehyde dehydrogenase gene in transgenic tomato hairy roots leads to the accumulation of glycine betaine and contributes to the maintenance of osmotic potential under salt stress . Soil SciPlant Nutr , 46 : 873 – 883 .
- Sevanto , S , Vesala , T , Peramaki , M and Nikinmaa , E . 2002 . Time lags for xylem and stem diameter variation in a scots pine tree . Plant Cell Environ. , 25 : 1071 – 1077 .
- Brough , DW , Jones , HG and Grace , J . 1986 . Diurnal changes in water content of stems of apple trees, as influenced by irrigation . Plant Cell Environ. , 9 : 1 – 7 .
- Genard , M , Fishman , S Vercambre , G . 2001 . A biophysical analysis of stem and root diameter variations in woody plants . Plant Physiol , 126 : 188 – 202 .
- Klepper , B , Browning , VD and Taylor , HM . 1971 . Stem diameter in relation to plant water status . Plant Physiol , 48 : 683 – 685 .
- Molz , FJ and Klepper , B . 1973 . On the mechanism of water stress-induced stem deformation . Agronomy , 65 : 304 – 306 .
- Hinckley , TM and Bruckerhoff , DF . 1975 . The effects of drought on water relations and stem shrinkage of Quercus alba . Can. J. Bot. , 53 : 62 – 72 .
- Koch , KE . 1996 . Carbohydrate-modulated gene expression in plants . AnnRevPlant PhysiolPlant MolBiol , 47 : 509 – 540 .
- Felitti , SA and Gonzalez , DH . 1998 . Carbohydrates modulate the expression of the sunflower cytochrome c gene at the mRNA level . Planta , 206 : 410 – 415 .
- Morcuende , R , Perez , P and Martinez-Carrasco , R . 1997 . Short-term feedback inhibition of photosynthesis in wheat leaves supplied with sucrose and glycerol at two temperatures . Photosynthetica , 33 : 179 – 188 .
- Winder , TL , Sun , J , Okita , TW and Edwerds , GE . 1998 . Evidence of the occurrence of feedback inhibition of photosynthesis in rice . Plant Cell Physiol , 39 : 813 – 820 .
- Murashige , T and Skoog , F . 1962 . A revised medium for rapid growth and bioassays with tobacco tissue culture . Physiol. Plant. , 15 : 473 – 479 .