Abstract
A great deal of information on the efficiency of gypsum or phosphogypsum to ameliorate acidity in highly weathered soils is available, but only limited information is available on the efficiency in acid Andosols, which possess large amounts of active aluminum (Al). We examined the effectiveness of gypsum application to non-allophanic Andosols (one humus-rich A horizon and two B horizons poor in humus) using extractable soil Al analyses (batch and continuous extraction methods) and a cultivation test using burdock (Arctium lappa). With gypsum amendment, pH(H2O) values of the soil decreased from 4.5–4.7 to 4.2–4.4, whereas the treatment made almost no difference to the values of pH(KCl). Total active Al (acid oxalate-extractable Al) was hardly affected by gypsum for all samples. Potassium chloride-extractable Al definitely decreased with the addition of gypsum in all soils; however, the decrease was small (0.1–1.4 cmolc kg−1) and the values still exceeded “the threshold of 2 cmolc kg−1” for inducing Al toxicity in sensitive plants (4.4–8.6 cmolc Al kg−1). The change in Al solubility with gypsum application represented by Al release rates from soils using continuous extraction methods with a dilute acetate buffer solution (10−3 mol L−1, pH 3.5) differed greatly among the soil samples: The release rate of one of the B horizon samples decreased by 71%, certainly showing the insolubilization of Al compounds, whereas the release rates of the A horizon sample showed almost no change. These changes in Al solubility were well correlated with the plant root growth. Root growth was improved with gypsum in the B horizon sample, whereas improvement was not observed in the A horizon soil. The decrease in the rate of Al release of another B horizon soil with gypsum treatment was smaller (by 20–34%), possibly because of lower pH values after gypsum application (pH[H2O] of 4.2–4.3). In the B horizon soil, root growth improved only slightly. Thus, the effectiveness of gypsum application to acid Andosols appeared to be largely influenced by soil humus contents and slight differences in soil pH values, and corresponded to a decrease in Al release rates using the continuous extraction method.
INTRODUCTION
Andosols, the representative soils formed from volcanic ejecta, generally possess excellent physical properties such as low bulk density, high permeability and high water holding capacity (CitationNanzyo et al. 1993a). In contrast to these good physical properties, Andosols show high phosphate sorption capacity, which is the main chemical problem in their agricultural use. Another chemical problem of the soils is soil acidity. Acid injury to plant roots, mainly aluminum (Al) toxicity, usually occurs in Andosols poor in allophanic materials (non-allophanic Andosols) (CitationNanzyo et al. 1993b). Because exchange acidity (y1, 1 mol L−1 KCl exchangeable acidity) is a good indicator for Al toxicity, toxic Al is believed to be mainly the exchangeable Al on the permanent charge of 2:1 aluminosilicates (CitationSaigusa et al. 1980). However, in non-allophanic Andosols dominated by Al-humus complexes, the exchangeable Al is largely controlled by organically complexed Al (CitationTakahashi et al. 1995, Citation2003, Citation2006). This indicates that these soils contain a large number of precursors of toxic Al; nontoxic Al can easily be converted to toxic Al by decreasing the pH or increasing the ionic strength. Non-crystalline or poorly-crystalline minerals, such as allophane and imogolite, show a high buffer capacity to Al dissolution in their dominance (allophanic Andosols). In fact, allophanic Andosols seldom exhibit Al toxicity. In cultivated allophanic soils, however, lowering soil pH by the heavy application of fertilizers resulted in dissolution of Al from the allophanic materials (CitationMatsuyama et al. 2005).
Lime has generally been applied to acid soils to counter Al toxicity. However, liming of surface soils is not effective for the amelioration of subsoil acidity because of its low solubility. Gypsum or phosphogypsum, which have higher rates of solubility, are believed to ameliorate subsoil acidity with surface application. Considerable information on their efficiency to ameliorate acidity in highly weathered soils is available (CitationFarina and Channon 1988; CitationPavan et al. 1982; CitationShainberg et al. 1989; CitationSumner et al. 1986; CitationSumner and Carter 1988; CitationSumner 1993). In contrast, there is very little information on the efficiency of gypsum or phosphogypsum in Andosols or Andisols and the tendency of the efficiency among soils has not been clear. CitationMora et al. (1999) showed that gypsum treatment of acid Andisols rich in organic matter increased soil pH slightly, reduced exchangeable Al and increased the yield of ryegrass in a similar way to calcitic and dolomitic liming. The effect of gypsum is also related to the supply of sulfur (CitationMora et al. 1999). Saigusa and co-workers (CitationSaigusa et al. 1996; CitationSaigusa and Toma 1997; CitationToma and Saigusa, 1997) reported that phosphogypsum application to an Andosol with low humus content was effective for plant root elongation and reduction of exchange acidity, whereas the application to an Andosol with high humus content was barely effective. CitationInoue et al. (2001) also reported that the application of gypsum to an acid Andosol with high humus content scarcely reduced the exchangeable Al of the soil. Thus, consistency has not been shown in the effect of gypsum or phosphogypsum to Andosols or Andisols. Although CitationSaigusa et al. (1996), CitationSaigusa and Toma (1997) and CitationToma and Saigusa (1997) found an effect of phosphogypsum on one Andosol with low humus contents, as previously noted, the universality of the effectiveness on such Andosols has not yet been confirmed.
Variation in the efficiency of gypsum or phosphogypsum to acid Andosols may be related to the complexity of Al solubility of the soil. Acid Andosols contain large amounts of active Al in various forms, such as easily exchangeable Al, organically complexed Al and allophanic materials. The exchangeable Al in Andosols, which is used as an indicator of toxic Al, was largely influenced by organically complexed Al and by allophanic materials as well (CitationDahlgren et al. 2004). This indicates that exchangeable Al in Andosols is not the Al form directly reflecting the Al solubility or toxicity of soils, although many previous studies evaluated the efficiency of gypsum using exchangeable Al.
In the present study, we investigated the efficiency of gypsum application to non-allophanic Andosols (one A horizon sample and two B horizon samples) using soil analyses and plant culture tests in a closed experimental system in the laboratory. The effect on extractable Al from soil samples was evaluated not only by using batch extractions but also by using the Al release rate calculated from continuously extracted Al concentrations from soils using acidic buffer solution (pH 3.5, 10−3 mol L−1 sodium acetate) (CitationDahlgren and Walker 1993; CitationTakahashi et al. 1995). It was suggested that the method of Al release rates from soils would be a good indicator for the effectiveness of gypsum application to acid Andosols (CitationToma 1996).
MATERIALS AND METHODS
Soil samples and their characterization
One A horizon sample and two B horizon samples of non-allophanic Andosols were used in this study (). As a comparison, we used a Kakogawa Yellow soil sample.
Table 1 Sampling points, classification and clay mineralogy of the soil samples
Air-dried, fine-earth fractions (<2 mm) were used for the laboratory analyses that follow. The soil pH was potentiometrically measured in water and 1 mol L−1 KCl (soil:solution = 1:2.5) following a 30 min and 1 h equilibrium period, respectively. The amount of organic carbon was determined using dry combustion. Exchangeable bases were determined using extraction with 1 mol L−1 ammonium acetate (pH 7). Selective dissolution techniques were used to determine the various pools of Al, Fe and Si. They are: (1) 1 mol L−1 KCl extractable Al (exchangeable Al, AlK) (CitationBlakemore et al. 1981), (2) 0.5 mol L−1 CuCl2 extractable Al (AlCu) (CitationHargrove and Thomas 1981), (3) sodium pyrophosphate (pH 10) extractable Al and Fe (Alp, Fep) (CitationMcKeague 1967), (4) acid ammonium oxalate (pH 3) extractable Al, Fe and Si (Alo, Feo, Sio) (CitationMcKeague 1976), (5) dithionite–citrate–bicarbonate extractable Fe (Fed) (CitationMehra and Jackson 1960). The contents of Al and Fe in all the extracts were measured using atomic absorption spectrophotometry and the Si content was colorimetrically determined. All soil analyses were carried out in duplicate.
The rates of Al release from the <0.5 mm soil fraction were determined using a stirred flow cell as described by CitationDahlgren and Walker (1993) and CitationTakahashi et al. (1995). A reaction solution consisting of 10−3 mol L−1 sodium acetate adjusted to pH 3.5 was used for the Al release experiment. The reacted solution was continuously injected into an autoanalyzer using the pyrocatechol violet method for quantification of monomeric Al (CitationRogeberg and Henriksen 1985). The measurement was carried out in triplicate.
Gypsum treatment
Field moist, undried soil samples were used for the gypsum treatment. The following rates of gypsum (CaSO4·2H2O) were mixed well with the soil samples: 4.3 g kg−1 soil samples (Gypsum 1) and 8.6 g kg−1 soil samples (Gypsum 2). The rate of gypsum in the Gypsum 2 plot was based on the Ca amount of the lime requirement of Kawatabi A horizon soil. The mixture of soil samples and gypsum was incubated at field water capacity (60% of maximum water holding capacity) at room temperature for 30 days.
Subsamples of the mixture were air-dried and passed through a 2-mm or 0.5-mm sieve. The same soil characterization analyses used for the original samples were carried out for the gypsum-treated samples except for total carbon content and phosphate retention. The remaining subsamples were used for plant culture.
Plant culture
A plant culture was carried out in the gypsum-treated and original soils. The soil samples were added to 200 mL pots (50 g dry soil basis for Kawatabi soil and 100 g for the other soils). The water content was adjusted and kept at 70% of the maximum water holding capacity. Ten seedlings of burdock (Arctium lappa cv. Kantan) per one pot were sown and cultured at room temperature (23–25°C). After a 2-week culture, the plants were harvested and the length of the roots and shoots was measured. Withering individuals were excluded from the measurement.
Statistical analyses
We used JMP 4.0 (SAS Institute Inc.) for the statistical analyses. A two-way anova (gypsum treatments × soils) was carried out for Alo, AlCu–K and AlK values. Mean comparisons were carried out using Tukey's tests for the total released Al from the soil samples at 60 min calculated from the experiment of Al release rates, and for the results of the plant culture experiment.
RESULTS
Soil characterization
Kawatabi A, Fujisawa B and Rokuhara B soils showed characteristics for Andosols, such as high acid oxalate-soluble Al and Fe (Alo and Feo) concentrations and high phosphate retention (). Among these soils, the total carbon concentration of Kawatabi A soil was very high, whereas the concentration in Fujisawa B and Rokuhara B soils was low. All the soil samples contained small amounts of allophanic materials (Sio 0.7–2.8 g kg−1). The dominant crystalline minerals in the clay fractions of all the soil samples were chloritized 2:1 minerals ().
In contrast to the Andosols, Kakogawa Yellow soil contained small amounts of Alo and Feo ().
Table 2 Selected properties of the soil samples
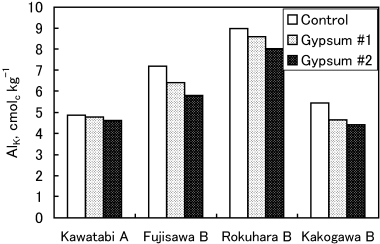
The soil pH(H2O) of the four soil samples ranged from 4.5 to 5.1, showing extreme acidities. The amounts of exchangeable Al (AlK) were very high (range: 4.8–9 cmolc kg−1) and exceeded the threshold of 2 cmolc kg−1 for inducing Al toxicity in sensitive plant roots (CitationSaigusa et al. 1980; CitationSoil Survey Staff 1999).
Effect of gypsum amendment on the chemical properties and Al solubility of soils
The pH(H2O) values decreased markedly in most soils with gypsum treatment, by as much as 0.7 units (). This is more attributable to the salt effect of gypsum leading to an increase in H+ and Al ions than to the opposite reaction of ligand exchange of OH− and SO2− 4 (CitationGarrido et al. 2003; CitationShainberg et al. 1989). The change in the pH(H2O) values of Kawatabi A soil was small, reflecting the stronger buffer reaction of this soil. The pH(KCl) values were not affected or were only slightly lower with gypsum treatment.
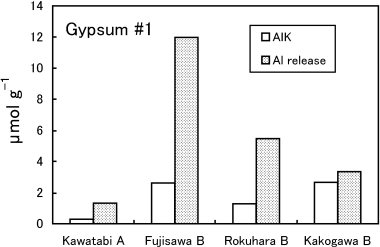
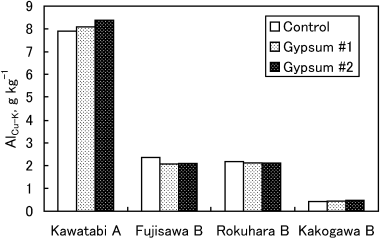
The concentration of Alo, which corresponds to total active Al, was scarcely changed by the application of gypsum in all soil samples (). The Alp concentration, oraganically complexed Al, was also barely affected (data not shown). The difference between copper chloride-extractable Al (AlCu) and exchangeable Al (AlK) (AlCu–K) is believed to be organically complexed Al for soils with high humus contents (CitationAitken 1992; CitationConyers 1990; CitationJuo and Kamprath 1979). The AlCu–K is also attributable to Al-polymeric species and metastable forms of Al hydroxides and hydrous oxcides in soils with low humus contents
Table 3 Soil pH values before and after gypsum treatment
Figure 1 Effect of gypsum treatments on acid oxalate-extractable Al (Alo). The results of a two-way anova showed no significant difference in the Alo values among gypsum treatments (P > 0.05), but there was a significant difference in Alo values among soils (P < 0.01).
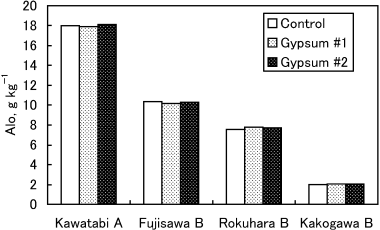
Figure 2 Effect of gypsum treatments on the value of the difference between CuCl2-extractable Al and KCl-extractable Al (AlCu–K). The results of a two-way anova showed no significant difference in the AlCu–K values among gypsum treatments (P > 0.05), but there was a significant difference in AlCu–K values among soils (P < 0.01).
Thus, all the batch methods of Al extraction were not significantly affected by the application of gypsum. On the contrary, Al release rates using continuous extraction of soils with a dilute acetate buffer solution (10−3 mol L−1,
Figure 3 Effect of gypsum treatments on KCl-extractable Al (AlK). The results of a two-way anova showed significant differences in the AlK values both among gypsum treatments (P < 0.01) and among soils (P < 0.01).
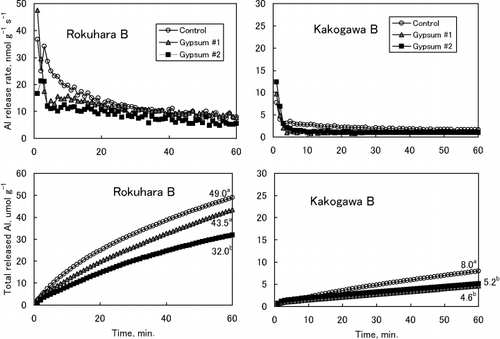
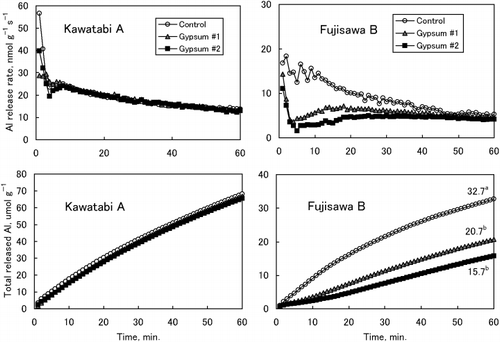
In contrast, gypsum treatment did not affect the Al release rates in Kawatabi A soil, which is abundant in organic matter (). The Al release rates from Kakogawa soil were very slow and the effect of gypsum was not clear. Nevertheless, the application of gypsum resulted in the acceleration of the Al release rates in the first few minutes and the subsequent decrease in the rates ().
The decrease in the Al release rates indicates insolubilization of Al and the increase indicates solubilization, although the change in the specific Al fractions cannot be identified.
Figure 4 Aluminium release rates (upper) and cumulative amounts of Al released (lower) from Kawatabi A and Fujisawa B soils. The numbers in the lower figure of Fujisawa B show the amount of total released Al at 60 min. Data with different letters are significantly different at P < 0.05 using a Tukey's test. There was no significant difference in the amount of total released Al in Kawatabi A soil among the treatments.
Effect of gypsum application on plant growth
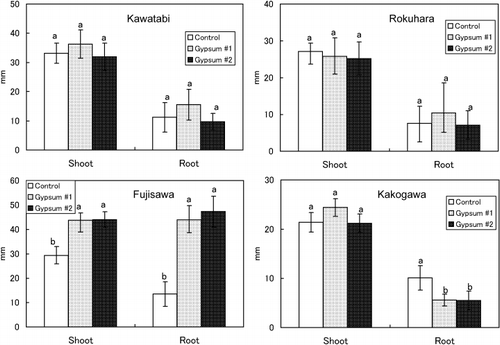
As shown in , the effects of gypsum application on the growth of plant roots and shoots differed greatly among the soils. In the Kawatabi soil, root growth was strongly restricted in the Control plot and the restriction of root growth was not improved with gypsum. In the Fujisawa soil, although Al injury was observed in roots in the Control, root growth recovered markedly in the Gypsum 1 and 2 plots and this influenced shoot growth. This improvement in root growth with the application of gypsum corresponds to the decrease in Al release rates from soils (). The decrease in Al release rates with gypsum in Rokuhara soil was remarkable compared with the rate in Fujisawa soil (). However, the effect of gypsum on plant root growth was not observed in Rokuhara soil (). Application of gypsum to Kakogawa soil reduced root growth (); this might correspond to the accelerated Al release from gypsum-amended soils during the first few minutes () or to the decrease in soil pH with gypsum ().
DISCUSSION
A decrease in the Al solubility of soil with the application of gypsum is attributable to the formation of hydroxy Al polymers and Al hydroxysulfate minerals (CitationGarrido et al. 2003; CitationSaigusa and Toma 1997; CitationSposito 1989; CitationSumner 1993). The formation of Al compounds is expected to reflect some soil chemical data. In particular, the formation of hydroxy Al polymers would reduce AlK and increase
Figure 7 Effect of the application of gypsum on shoot and root growth in burdock (n = 7–10). Error bars indicate standard deviation. Data with different letters are significantly different at P < 0.01 using a Tukey's test.
The change in the Al solubility of soils with gypsum that was demonstrated by the Al release rates definitely differed among the soils (,). The Al release rates in Kawatabi soil did not decrease with the application of gypsum. This can be explained by the fact that almost all the active Al is in Al-humus complexes and this form of Al is hardly reduced and even increased by the application of gypsum as revealed by the AlK and AlCu–K values. These results convince us that gypsum amendment to acid Andosol horizons rich in organic matter is scarcely effective (CitationInoue et al. 2001; CitationSaigusa et al. 1996). In contrast, Al release rates from soil with low humus contents (Fujisawa and Rokuhara soils) decreased greatly with gypsum treatment. This may be explained by the fact that easily exchangeable Al absorbed on permanent negative charges of 2:1 minerals was exchanged and precipitated as hydroxy Al polymers, and the polymers absorbed irreversibly on the soil surface (CitationSaigusa and Toma 1997). In the present study, however, the AlCu–K values did not increase with gypsum treatment and, thus, the formation of hydroxy Al polymers in these soil samples would be reduced. The decrease in the amounts of Al released by dilute acetate buffer largely exceeded the decrease in the amounts of AlK in Fujisawa and Rokuhara soils (). The formation of Al hydroxysulfate minerals is another possibility for lowering the Al release rates (CitationShainberg et al. 1989; CitationSumner 1993). Although X-ray diffraction analysis did not show the presence of crystalline Al hydroxysulfate minerals in Gypsum 1 and 2 plots of Fujisawa and Rokuhara soils (data not shown), the formation of amorphous Al-sulfate compounds might exist. It has been very difficult to directly confirm the existence of these minerals in soils. Recently, CitationDelfosse et al. (2005) found these minerals in the clay fractions of Andosols, simply by dispersing clays with Na-resins, with transmission electron microscopy coupled with energy-dispersive analysis.
Improvement in root growth with gypsum treatment was observed only in Fujisawa B soil, which contains small amounts of humus. An effect of gypsum was not observed in Rokuhara B soil with a low humus content and Kawatabi A with high humus content. In Kakogawa Yellow soil, gypsum application reduced root growth. The tendency of the gypsum effectiveness to root growth did not well harmonize with the decrease of exchangeable Al (AlK)d with the addition of gypsum. (,), although CitationSaigusa et al. (1996), CitationSaigusa and Toma (1997) and CitationToma and Saigusa (1997) concluded that the improvement of plant root growth with gypsum or phosphogypsum was explained by a decrease in exchange acidity, which is mostly composed of exchangeable Al. The AlK values in Fujisawa soil, in which root growth improved, decreased by up to 19% with gypsum (Gypsum 2 plot). However, the AlK values of Gypsum 1 and 2 were still greater than 2 cmolc kg−1, the threshold for inducing Al toxicity. Although other soils also decreased AlK values with gypsum, improvement of root growth was not observed in these soils.
A good correspondence between the effects of gypsum on root growth and Al release rate was observed in Kawatabi A and Fujisawa B soils (,). In Kawatabi soil, gypsum application did not affect either root growth or Al release rates. In contrast, Al release rates from Fujisawa soil decreased greatly with gypsum, and this reflected root growth. In Rokuhara soil, Al release rates also decreased in Gypsum 1 and 2 (), but root growth did not improve (). This may be because of the high level of Al release rates in Gypsum 1 and 2 plots, possibly because of the low pH values (pH[H2O] of 4.2–4.3) after gypsum application. Although Al release was very slow from Kakogawa soil, gypsum application lead to an increase in Al release during the first few minutes (). This increase in Al release is related to further restriction of root growth (). As expected from the very low pH values of Kawatabi, Rokuhara and Kakogawa soils (), proton injury might have occurred in these soils (CitationFoy 1984).
Acid Andosols contain a much greater amount of active Al compared with other acid soils. It was confirmed that, in the case of soils with high humus contents, organically complexed Al is dominant and this form of Al is hardly affected by gypsum application. It is unlikely to form enough hydroxy Al polymer even in B horizon soils poor in humus to consume high amounts of exchangeable and active Al when the soil pH does not increase with gypsum addition. In these cases, even H+ injury to plant roots may occur at pH levels below approximately 4.2 (CitationFoy 1984). Therefore, materials that result in an increase in soil pH would be more effective for acid Andosols. Otherwise, materials, such as chelate compounds, that can make strong complexes with Al and remove them from soils into below subsoils would be expected.
Notes
Present addresses: Johnson and Johnson K. K., 5-2 Nishi-kanda 3-chome, Chiyada-ku, Tokyo 101-0065, Japan.
Shizuoka Agricultural Experiment Station, 678-1 Tomigaoka, Iwata, Shizuoka 438-0803, Japan.
REFERENCES
- Nanzyo , M , Shoji , S and Dahlgren , RA . 1993a . “ Physical characteristics of volcanic ash soils ” . In Volcanic Ash Soils – Genesis, Properties and Utilization , Edited by: Shoji , S , Nanzyo , M and Dahlgren , RA . 189 – 207 . Amsterdam : Elsevier .
- Nanzyo , M , Dahlgren , RA and Shoji , S . 1993b . “ Chemical characteristics of volcanic ash soils ” . In Volcanic Ash Soils – Genesis, Properties and Utilization , Edited by: Shoji , S , Nanzyo , M and Dahlgren , RA . 145 – 187 . Amsterdam : Elsevier .
- Saigusa , M , Shoji , S and Takahashi , T . 1980 . Plant root growth in acid Andosols from northeastern Japan: 2. Exchange acidity Y1as a realistic measure of aluminum toxicity potential . Soil Science , 130 : 242 – 250 .
- Takahashi , T , Fukuoka , T and Dahlgren , RA . 1995 . Aluminum solubility and release rates from soil horizons dominated by aluminum-humus complexes . Soil SciPlant Nutr , 41 : 119 – 131 .
- Takahashi , T , Sato , T , Sato , A and Nanzyo , M . 2003 . Relationship between 1 M KCl-extractable aluminum and pyrophosphate-extractable aluminum in A horizons of non-allophanic Andosols . Soil SciPlant Nutr , 49 : 729 – 733 .
- Takahashi , T , Ikeda , Y , Fujita , K and Nanzyo , M . 2006 . Effect of liming on organically complexed aluminum of nonallophanic Andosols from northeastern Japan . Geoderma , 130 : 26 – 34 .
- Matsuyama , N , Saigusa , M , Sakaiya , E , Tamakawa , K , Oyamada , Z and Kudo , K . 2005 . Acidification and soil productivity of allophanic Andosols affected by heavy application of fertilizers . Soil SciPlant Nutr , 51 : 117 – 123 .
- Farina , MPW and Channon , P . 1988 . Acid-subsoil amelioration: II. Gypsum effects on growth and subsoil chemical properties . Soil SciSocAmJ , 52 : 175 – 180 .
- Pavan , MA , Bingham , FT and Pratt , PF . 1982 . Toxicity of aluminum to coffee in Ultisols and Oxisols amended with CaCO3 MgCO3 and CaSO4·2H2O . Soil SciSocAmJ , 46 : 1201 – 1207 .
- Shainberg , I , Sumner , ME , Miller , WP , Farina , MPW , Pavan , MA and Fey , MV . 1989 . Use of gypsum on soils: a review . AdvSoil Sci , 9 : 1 – 111 .
- Sumner , ME , Shahandeh , H , Bouton , J and Hammel , J . 1986 . Amelioration of an acid soil profile through deep liming and surface application of gypsum . Soil SciSocAmJ , 50 : 1254 – 1258 .
- Summer , ME and Carter , E . 1988 . Amelioration of subsoil acidity . CommunSoil SciPlant Anal , 19 : 1309 – 1318 .
- Sumner , ME . 1993 . Gypsum and acid soils: the world scene . AdvAgron , 51 : 1 – 32 .
- Mora , ML , Schnettler , B and Demanet , R . 1999 . Effect of liming and gypsum on soil chemistry, yield, and mineral composition of ryegrass grown in an acidic Andisol . CommunSoil SciPlant Anal , 30 : 1251 – 1266 .
- Saigusa , M , Toma , M and Nanzyo , M . 1996 . Alleviation of subsoil acidity in nonallophanic Andosols by phosphogypsum application in topsoil . Soil SciPlant Nutr , 42 : 221 – 227 .
- Saigusa , M and Toma , M . 1997 . Mechanism of reduction of exchangeable aluminum by gypsum application in acid Andosols . Soil SciPlant Nutr , 43 : 343 – 349 .
- Toma , M and Saigusa , M . 1997 . Effects of phosphaogypsum on ameliorating strongly acid nonallophanic Andosols . Plant and Soil , 192 : 49 – 55 .
- Inoue , K , Kondo , S , Tamano , Y and Yokota , H . 2001 . Amelioration of subsoil acidity in a nonallophanic Andosol by surface application of organic calcium salts . Soil SciPlant Nutr , 47 : 113 – 122 .
- Dahlgren , RA , Saigusa , M and Ugolini , FC . 2004 . The nature, properties and management of volcanic soils . AdvAgron , 82 : 113 – 182 .
- Dahlgren , RA and Walker , WJ . 1993 . Aluminum release rates from selected Spodosol Bs horizons: Effect of pH and solid-phase aluminum pools . Geochimica et Cosmochimica Acta , 57 : 57 – 66 .
- Toma , M . 1996 . “ Research on agricultural use of gypsums, especially phosphogypsum ” . Tohoku University, Japan . (in Japanese)(Doctoral dissertation)
- Blakemore , LC , Searle , PL and Daly , BK . 1981 . “ Soil Bureau Laboratory Methods: A. Methods for Chemical Analysis of Soils ” . New Zealand Soil Bureau Scientific Report . 10A (revised)
- Hargrove , WL and Thomas , GW . 1981 . Extraction of aluminum from aluminum-organic matter complexes . Soil SciSocAmJ , 45 : 151 – 153 .
- McKeague , JA . 1967 . An evaluation of 0.1 M pyrophosphate and pyrophosphate-dithionite in comparison with oxalate as extractants of the accumulation products in podzols and some other soils . CanJSoil Sci , 47 : 95 – 99 .
- McKeague , JA . 1976 . Manual on Soil Sampling and Methods of Analysis , Ottawa : Soil Resource Institute, Agriculture Canada .
- Mehra , OP and Jackson , ML . 1960 . Iron oxide removal from soils and clays by a dithionite–citrate system buffered with sodium bicarbonate . Clays and Clay Minerals , 5 : 317 – 327 .
- Rogeberg , EJS and Henrikson , A . 1985 . An automatic method for fractionation and determination of aluminum species in fresh waters . Vatten , 41 : 48 – 53 .
- Soil Survey Staff1999 Soil Taxonomy2nd edn USDA-NRCS, Agriculture Handbook No.436
- Garrido , F , Illera , V , Vizcayno , C and García-González , MT . 2003 . Evaluation of industrial by-products as soil acidity amendments: chemical and mineralogical implications . EurJSoil Sci , 54 : 411 – 422 .
- Aitken , RL . 1992 . Relationship between extractable Al, selected soil properties, pH buffer capacity and lime requirement in some acidic Queensland soils . AustJSoil Res , 30 : 119 – 130 .
- Conyers , M . 1990 . The control of aluminium solubility in some acidic Australian soils . JSoil Sci , 41 : 147 – 156 .
- Juo , ASR and Kamprath , EJ . 1979 . Copper chloride as an extractant for estimating the potentially reactive aluminum pool in acid soils . Soil SciSocAmJ , 43 : 35 – 38 .
- Vizcayno , C , Garcia-Gonzalez , MT , Fernandez-Marcote , Y and Santano , J . 2001 . Extractable forms of aluminum as affected by gypsum and lime amendments to an acid soil . CommunSoil SciPlant Anal , 32 : 2279 – 2292 .
- Sposito , G . 1989 . The Chemistry of Soils , New York : Oxford University Press .
- Delfosse , T , Elsass , F and Delvaux , B . 2005 . Direct evidence of basic aluminium sulphate minerals in an S-impacted Andosol . EurJSoil Sci , 56 : 281 – 286 .
- Foy , CD . 1984 . “ Physiological effects of hydrogen, aluminum, and manganese toxicities in acid soil ” . In Soil Acidity and Liming , Edited by: Adams , F . 57 – 97 . Madison : American Society of Agronomy, Inc., Crop Science Society of America, Inc., and Soil Science Society of America, Inc. .
- Ito , T and Saigusa , M . 1996 . Characteristics of nonallophanic Andisols at Tohoku University Farm . Bulletin of the Experimental Farm, Tohoku University , 12 : 91 – 103 .
- Nanzyo , M , Kobayashi , K and Shoji , S . 1995 . Reaction of phosphate and exchangeable Al in soils and Al-smectite . JClay SciSocJapan , 35 : 15 – 23 . (in Japanese)
- Shoji , S , Ito , T , Saigusa , M and Yamada , I . 1985 . Properties of nonallophanic Andosols from Japan . Soil Sci , 140 : 264 – 277 .
- Present addresses: Johnson and Johnson K. K., 5-2 Nishi-kanda 3-chome, Chiyada-ku, Tokyo 101-0065, Japan.
- Shizuoka Agricultural Experiment Station, 678-1 Tomigaoka, Iwata, Shizuoka 438-0803, Japan.