Abstract
Plant species and cultivars elicit a diverse array of sophisticated metabolic and developmental strategies to enhance phosphorus (P) solublization and acquisition from P-deficient rooting media. Scavenging of phosphate (Pi) from extracellular sparingly soluble P sources may be aided by organic acids (OAs) exudation and root-mediated pH changes under a P-stress environment. Root exudates were collected to quantify short-term (4 h, 8 h) carboxylate exudation using hydroponically grown Brassica cultivars. Malic and citric acids were the dominant OAs and efficient cultivars exuded 2–3-fold more OAs than inefficient cultivars under P-deficient conditions. However, the exudation rate of both resistant and sensitive cultivars decreased with time. Experiments in nutrient solution were conducted to evaluate growth responses and the P solublization and acquisition ability of six genetically diverse Brassica cultivars. Pre-germinated seedlings were grown in nutrient solution containing ammonium di-hydrogen phosphate (AP), tri-calcium phosphate (TCP) and rock phosphate (RP) (TCP at 0.2 g L−1 and RP at 2 g L−1, in a bid to maintain deficiently buffered solution-P concentration) as different P sources. The cultivars exhibited substantial growth differences (P < 0.001) in terms of biomass accumulation, P acquisition and P utilization efficiency (PUE). Biomass production correlated significantly (P < 0.01) with their shoot P uptake and PUE at both P sources, indicating that cultivars efficient in P solublization accumulated more biomass. Higher P uptake by all cultivars was significantly related to the drop in root medium pH, which was presumably owing to H+ efflux from the roots supplied with TCP and RP. Higher shoot dry matter of the cultivars at TCP was related to better P acquisition ability, which in turn was related to higher Ca uptake. Thus, cultivars with efficient Ca accumulation ability can acquire higher amounts of P from P-deficient soils and can adapt well to such low-P soil conditions.
INTRODUCTION
Phosphorus (P) plays a central role as a reactant and effector molecule in plant cell metabolism at the nexus of photosynthesis, energy conservation and carbon metabolism. Phosphorus is taken up by plants in the orthophosphate (Pi) forms H2PO− 4 and HPO2− 4, which occur in soil solutions at very low concentrations (0.1–10 µmol L−1; CitationVance et al. 2003). Pi is extremely insoluble in most soils because it forms Ca salts or is complexed by constituents such as Fe or Al oxides or fixed into organic forms that render Pi largely inaccessible to plants. There is great disparity in the distribution of Pi between plant cells (mmol L−1) and soil solution (µmol L−1) because of its strong reactions with soil components. Pi is principally supplied to plant roots by diffusion rather than mass flow, and the diffusion of Pi in soils is slow (10−12 to 10−15 m2 S−1; CitationRausch and Bucher 2002); hence, P is one of the most dilute and inaccessible macronutrients in the soil. Pi deprivation frequently limits plant growth affecting more than 2 billion hectares in tropical and subtropical soils and 5.7 billions hectares worldwide.
The amelioration of Pi deficiency by the application of costly and environmentally hazardous P fertilizers is not an ideal solution and problematic for both the intensive and extensive agriculture of the developed and developing worlds, respectively (CitationNarang et al. 2000). In addition, run-off from P-loaded soils is a primary factor in eutrophication and hypoxia of lakes and marine estuaries of the developed world. Imbalanced and inadequate fertilizer application, low use efficiency, organic and inorganic fixation, fear of depletion of the world's reserves of inexpensive rock P coupled with day in/day out increasing prices of P fertilizers impel us to devise alternate environmentally friendly and economically feasible strategies to improve crop production in low P soils. This might be achieved by developing crops that either acquire P or use P more efficiently or by developing more precise methods to monitor crop P status, such that P fertilization can be managed efficiently. Therefore, the selection of cultivars efficient in P acquisition and utilization is an important strategy in a P-stress environment. It includes exploitation of genetic differences in plants with respect to absorption, translocation, assimilation and utilization of nutrients in a resource poor environment.
Plant species and cultivars have evolved an elegant array of morphological, physiological, biochemical and molecular adaptations triggered by P deficiency that enable them to acquire P from sparingly soluble P soil fractions. These adaptations include modifications in root architecture (CitationWu et al. 2005), increased organic acid exudation (CitationRengel 2002), rhizosphere acidification (CitationHinsinger et al. 2003), increased production of phosphatases and RNAses (CitationVance et al. 2003), enhanced phosphate uptake rate (CitationHinsinger et al. 2005) and an increase in the synthesis of Pi transporters (CitationHammond et al. 2004; CitationVance et al. 2003).
Under Pi deprivation stress, the roots enhance secretion of protons or organic acids to enhance the solublization of insoluble inorganic P complexes. In calcareous soils, rhizosphere acidification by H+ extrusion causes dissolution of poorly available Ca–P minerals. Various origins of pH changes are cation–anion exchange balance, organic anions (OAs) release, root exudation and respiration, and redox-coupled processes. Carboxylates are OAs of varying chain length with one or more carboxylic acid groups. Typical carboxylates found in root exudates include citrate, malate, malonate, acetate, fumarate, succinate, lactate and oxalate (CitationRengel 2002). Malate and citrate are the major root exudates in many plant species and are part of the primary plant metabolism. Exuded OAs are able to mobilize inorganic P into the soil solution by competing with phosphate groups for the same binding/adsorption sites in soil and forming stronger complexes with Al3+, Fe3+ and Ca2+ than phosphate does. Phosphorus can be liberated from Ca–P minerals as an OAs complex with Ca or block the sorption of P to other charged sites or through the ligand exchange process.
Plant species such as buckwheat, oilseed rape and legumes are quite efficient in utilizing rock P by releasing OAs because of changes in cellular metabolism as a Pi starvation response. Anion channels such as Cl− channels and multidrug and toxin extrusion (MATE) proteins have been suggested as potential candidates for OAs excretion (CitationVance et al. 2003). Plant adaptations allowing for improved growth in low P soils are related to the ability of a plant to take up more P from a deficient soil (higher P-acquisition efficiency) and/or its ability to produce more dry matter for a given quantity of P (higher P-use efficiency). All these changes improve the capacity of the plant root system to better explore and mine the soil for P. While differences in the ability of various species to solublize sparingly soluble P sources have been shown (CitationHinsinger 2001; CitationNeumann and Martinoia 2002; CitationRengel and Marschner 2005), differences between cultivars within a given species are poorly documented. Exploitation of intraspecific variation to identify efficient cultivars is an area of research priority for managing P efficiently and for providing an excellent resource for genetic model system to isolate genes that determine the P-efficiency traits.
Solution culture experiments were conducted to evaluate relative P solublization and P-use efficiency of Brassica cultivars using sparingly soluble P sources. Categorization of cultivars for P solublization, acquisition and use efficiency will open the possibility to bioengineer high P-uptake efficient cultivars as a way to bring more sparingly soluble P into cycling in crop production and obtain capitalization of soil P reserves.
MATERIALS AND METHODS
Plant material and culture conditions
The different cultivars of Brassica tested were: “Brown Raya”, “Con-1”, “Peela Raya”, “Rain Bow”, “Toria Selection” and “Sultan Raya” in experiments 1, 2 and 3 and “B. S. A.”“Toria”, “Brown Raya” and “Con-1” in experiment 4. Seeds were germinated in polyethylene-lined iron trays containing pre-washed riverbed sand and irrigated with distilled water for seed germination and seedling establishment. In experiment 1, 7-day-old uniform-sized seedlings were transplanted in foam-plugged holes in thermopal sheets floating on continuously aerated 200 L half-strength modified Hoffland's solution (CitationHoffland et al. 1989) in two polyethylene lined iron tubs (1 m × 1 m × 0.3 m). The composition of the solution was: (in mmol L−1): KNO3 (2), NH4NO3 (1), Ca(NO3)2·4H2O (2), MgSO4·7H2O (0.5) and K2SO4 (0.5), and (in µmol L−1): Fe(III)-EDTA (Ferric Dihydrogen Ethylene diamine Tetra-acetic Acid) (50), H3BO3 (25), MnSO4·H2O (2), ZnSO4·7H2O (2), CuSO4·5H2O (0.5), KCl (50) and H2MoO4 (0.5). The solutions in these tubs were modified by adding 200 µmol L−1 P NH4H2PO4 as a control treatment and Ca3(PO4)2 (TCP) (at 0.2 g L−1), respectively. The solutions were renewed at 3-day intervals to maintain nutrient concentrations being exhausted because of plant uptake. Six cultivars were grown in each P level by using a completely factorial randomized design (CRD) with eight repeats of each cultivar.
In experiments 2 and 3, 1-week-old pre-germinated, uniform-sized seedlings were transplanted to foam plugged holes made on plastic lids fitted on 3 L capacity plastic pots containing continuously aerated Hoffland's solution modified to contain no P. Experiments 1, 2 and 3 were conducted in a glasshouse in winter and the temperature during the growth period varied from a minimum of 5°C to a maximum of 25°C. For experiment 2, the solution was prepared in bulk and the pots were filled after adjusting the pH to 7. Phosphorus was applied to the individual pots as TCP at 0.2 g L−1. Each cultivar was transplanted in three pots with three plants per pot. The level of solution in each pot was marked and maintained every morning by using distilled water. The pH of the solutions was noted on days 6, 12, 16 and 20 after transplanting (DAT), while the P level in solutions was noted on 12 and 16 DAT. In experiment 3, powdered rock phosphate (RP) was added to the pots at 2 g L−1. The RP imported from Jordon was finely ground (0.15 mm) and contained 13.6% total P, 4.5% citrate-soluble (2% citric acid) P and no water soluble P. This is one of the medium reactive RPs known. The initial pH of the solution after the addition of RP was 6.5 ± 0.2 and was monitored regularly in all pots. The treatments were carried out in triplicate according to CRD. In experiments 2 and 3, nitrogen was supplied as NO3 and NH4 at a 3:1 ratio and TCP and RP in all pots were stirred mechanically twice per day.
Whole plants were harvested at 31 DAT in experiment 1 and 21 DAT in experiments 2 and 3. Plants were washed with distilled water (taking care that no grains of TCP or RP were attached to the roots), blotted dry with tissue paper and separated into shoots and roots. The samples were dried at 70°C for 48 h in a forced air-driven oven and dry mass (g plant−1) was recorded. The shoot and root samples were ground to pass through a 0.42 mm screen (40-mesh) for further analysis.
Measurement of various growth parameters
The plant samples (0.5 g each) were digested in a mixture of HNO3 and HClO4 (3:1). Phosphorus concentrations in shoots and roots were estimated using the vanadate–molybdate yellow color method (CitationChapman and Pratt 1961) using a spectrophotometer. Phosphorus uptake (mg plant−1) was calculated on a dry weight basis by multiplying P concentration in the respective tissue with its dry matter, and on a whole plant basis by adding the two.
The P-stress factor (PSF) for SDM was calculated by the formula given below:
where SDM is shoot dry matter (g plant−1) in the respective treatments.
Phosphorus-utilization efficiency (PUE) was determined by the formula given below:
In experiment 2, the Ca concentration (mg g−1) in root and shoot samples was estimated using an atomic absorption spectrophotometer. To determine the role of acidification in rock phosphate solublization in experiment 3, pH changes in the growth medium were measured regularly using a pH meter.
Exudation sampling technique
The cultivars used in this study were genetically diverse with respect to PUE for biomass synthesis and categorized into two groups in an earlier study on the basis of their PUE in a P-stress environment (CitationAkhtar et al. 2005). Group II cultivars (non-efficient): “B.S.A” and “Toria” and group I cultivars (efficient): “Brown Raya” and “Con-1”.
Seeds were sown on moist river sand and germinated in a dark chamber at 25°C. Six-day-old, uniformly sized seedlings were transferred to a complete nutrient solution for 7 days using KH2PO4 as a P source. The pH of the solution was daily monitored and maintained at 5.5 ± 0.5. The nutrient solution was renewed every 3 days to maintain the nutrients exhausted by plant uptake. The seedlings were grown in a cultivation chamber (CFH-405; Tomy Seiko Co., Tokyo, Japan) at a cycle of 14 h/20°C night and 10 h/25°C day at a light intensity of 40 µmol m−2 S−1 (approximately 3800 l×). The relative humidity of the chamber was adjusted to 60%. After 7 days in a complete nutrient solution, the seedlings were transferred to an aerated nutrient solution with (+P) or without (–P) 0.20 mmol L−1 P in 3.5-L pots. Each cultivar was transplanted in three pots maintaining three plants per pot using factorial CRD and each set of experiments was repeated at least twice. The plants were grown for an additional 12 days in a cultivation chamber. Plant roots were washed with deionized water and then submerged in 300 mL of aerated solution with 0.5 mmol L−1 CaCl2 (pH = 5.5) for 4 h and 8 h, respectively and root exudates were collected for organic anions.
Analysis of organic anions
The solution was evaporated to approximately 5 mL under reduced pressure at 45°C on a rotary evaporator (Vacuum controller NVC-1100, Eyela, Tokyo Rikakikai Co., Tokyo, Japan). The solution containing root exudates was allowed to pass first through a cation exchange column (16 mm × 14 cm) filled with 5 g Dowex 50 W × 8 (100–200, H+ form) resin (Muromachi Kagaku Kogyo Co., Tokyo, Japan) and then through an anion exchange column filled with 2 g Dowex 1 × 8 resin (0.15–0.06 mm, Cl− form) without adjusting the pH. This procedure was carried out under non-sterile conditions and the OAs retained in the anion exchange resin were eluted with 8 mol L−1 formic acid. The eluent was concentrated to dryness under reduced pressure using a rotary evaporator. The residue was re-dissolved in 1 mL ultra pure water adjusted to pH 2.1 with HClO4 and filtered with 0.45 µmol L−1 filter. The OAs were detected by High pressue liquid chromatograph (HPLC) (LC-6A, Shimadzu, Kyoto, Japan equipped with the ion-exclusion column Chemoo-pack Nucleosil 5C18, 4.6 × 250 (6 A), Japan).
Statistical background
Data were subjected to statistical analyses according to standard procedures (CitationSteel and Torrie 1980) using the “MSTAT-C” computer program and the methods described by CitationGomez and Gomez (1984). Factorial CRD was used for anova. Treatment means were separated using Duncan's multiple range test (DMRT). Correlation coefficient (r) values were determined among various parameters using the treatment means.
RESULTS
Experiment 1: Solublization and utilization of tri-calcium phosphate (Ca3 (PO4)2) by Brassica cultivars
Biomass production
Different P sources and Brassica cultivars had a significant (P < 0.01) main and interactive effect on shoot growth, root development and total biomass production (). Shoot dry matter was 2.5-fold lower in plants grown with TCP compared with the control (NH4H2PO4; AP). “Brown Raya”, “Con-1” and “Rain Bow” were the most efficient cultivars. Averaged over all cultivars, root dry matter (RDM) was lower, but statistically similar in both treatments. “Brown Raya” and “Con-1” responded quite differently compared with other cultivars and their RDM was slightly higher in TCP treatment. Root : shoot ratio (RSR) was significantly higher (1.8-fold) in plants grown at TCP compared with control plants ().
Cultivars differed significantly for relative reduction in biomass accumulation (P-stress factor; PSF) when grown with TCP (), indicating their relative tolerance
Table 1 Growth parameters of Brassica cultivars at 31 days after transplantation in solutions containing ammonium phosphate (AP) and sparingly soluble tri-calcium phosphate (TCP)
Phosphorus concentration, content and utilization efficiency
Cultivars and P sources had significant main and interactive effects on P concentration and it was approximately 2.75-fold lower in shoots of plants grown with TCP compared with AP. “Toria selection” and “Peela Raya” showed maximum P concentration at AP. Cultivars differed for P uptake and P utilization at both P sources (). Phosphorus uptake was lower, but PUE was higher in efficient cultivars grown in TCP compared with AP. “Brown Raya” and “Con-1” exhibited maximum P uptake, while “Peela Raya” and “Toria Selection” exhibited lower P uptake when grown with TCP. PUE can be used to classify the cultivars into efficient and inefficient utilizers of P and cultivars can be categorized into groups in terms of responsiveness and efficiency on the basis of the relationship between SDM and PUE (). Maximum PUE was exhibited by “Brown Raya” and “Con-1” at both P-levels (). PSF as a function of PUE of cultivars grown with TCP revealed that the cultivars “Brown Raya” and “Con-1”, showing lower PSF values, are considered more efficient than other cultivars ().
Experiments 2 and 3: P acquisition by Brassica cultivars under deficiently buffered P conditions (Ca3 (PO4)2 and rock phosphate)
Biomass accumulation
Statistically significant differences in root and shoot growth were obvious () among various cultivars grown in nutrient solutions buffered at deficient levels of P supply using TCP and RP as P sources. Maximum SDM was accumulated by “Brown Raya” followed by “Con-1” and “Rain Bow”, while the remaining three cultivars (“Peela Raya”, “Toria Selection” and “Sultan Raya”) produced statistically similar SDM and were inferior. Mean differences in root : shoot ratios of cultivars were also statistically significant and a positive correlation existed between mean values of RDM and SDM of the cultivars (r = 0.89** and r = 0.91** at TCP and RP, respectively: ** = significant at 1% level).
Tissue P concentrations, uptake and utilization efficiency
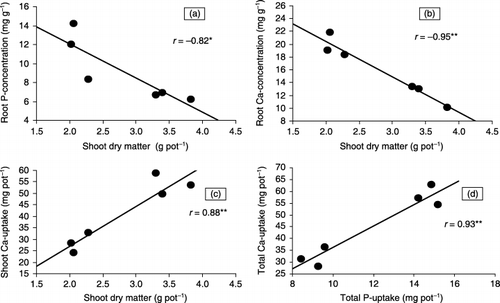
Phosphorus concentration in roots of the cultivars was higher than that in shoots () and the cultivar differences were of statistical importance only in the case of roots at both P sources. Cultivars with lower P concentration in their roots (implying a smooth translocation of absorbed P to above ground parts) yielded higher SDM (r = −0.82*: * = significant at 5% level) at
Figure 1 Ordination plot to classify Brassica cultivars for P utilization efficiency (PUE) as a function of (a) shoot dry matter (SDM) and (b) P stress factor (PSF) at different P sources (NH4H2PO4 and Ca3(PO4)2) at 31 days after transplanting in solution. ER, efficient and responsive; NER, non-efficient but responsive; ENR, efficient but non-responsive; NENR, non-efficient and non-responsive.
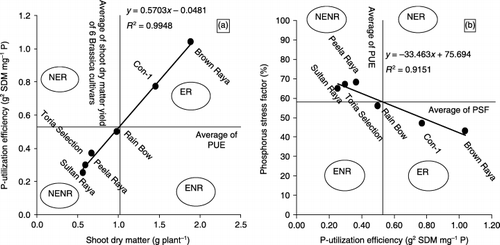
Table 2 Growth parameters of Brassica cultivars grown with sparingly soluble P sources
The cultivar “Brown Raya” was able to synthesize the highest amount of SDM per pot per unit of tissue P concentration (PUE) () followed by “Con-1” and “Rain Bow” at both P sources. The other three cultivars remained statistically similar in this respect and yielded lower SDM per unit of P. It can also be noted from that despite fairly uniform shoot P concentration of the cultivars, the amount of SDM synthesized per unit of P was quite variable. The cultivars differed significantly with respect to P uptake on a root, shoot and whole plant basis (). The cultivars depicting higher shoot P uptake yielded higher SDM (r = 0.92**at TCP and r = 0.88** at RP), whereas root P uptake had no correlation with SDM or RDM (r < 0.30) at both P sources.
Tissue Ca concentration and uptake
shows Ca concentrations in shoots and roots of tested Brassica cultivars after 21 DAT in solutions containing TCP. An approximately twofold difference existed between the minimum (in “Brown Raya”) and the maximum (in “Sultan Raya”) values of root Ca concentration, while shoot Ca concentration of the cultivars was fairly uniform except in “Rain Bow”, which showed a statistically higher Ca concentration than three out of the six cultivars. A negative correlation existed between root Ca concentration and SDM of the cultivars (r = −0.95**; ), whereas the impact of root Ca concentration was positive on root P concentration (r = 0.90**). Cultivar differences were statistically significant for Ca uptake on a shoot and whole plant basis (), while differences of root Ca uptake were not statistically different. The cultivars that had taken up higher Ca in their shoots also showed higher shoot P uptake and were able to produce more SDM ().
pH and P concentrations of nutrient solutions
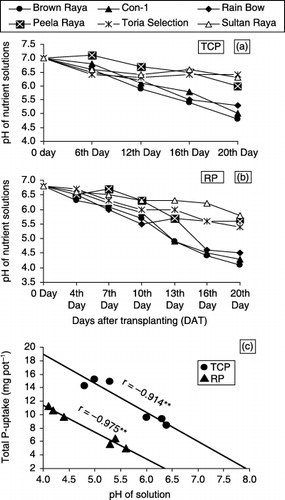
Phosphorus-deficiency induced pH changes were compared in cultivars with a differing ability to mobilize sparingly soluble P sources. A substantial decrease in solution media pH observed among tested Brassica cultivars during the growth period at both P sources and changes
in media pH with time by six cultivars grouped into two classes are depicted in . “Brown Raya”, “Con-1” and “Rain Bow” showed a decrease in pH to the tune of two points, while the other three cultivars showed only a slight decrease in pH (). Total P uptake of plants also had a significantly (P < 0.01) negative correlation (r = −0.975** and r = −0.914** at TCP and RP, respectively) with the pH of the nutrient solution measured at 20 DAT (). Cultivars accumulating higher amounts of total P in plants had a lower pH in the rooting media. Concentrations of P in the nutrient solutions containing TCP observed at 16 DAT, however, differed significantly among cultivars (). The differences of solution-P concentration were statistically non-significant at 12 DAT.Experiment 4: Organic acids (OAs) exudation
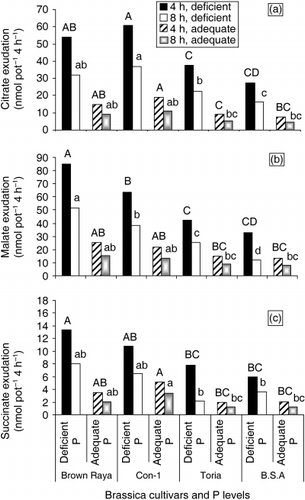
Organic acids (OAs) released from the roots of P-efficient plants increase the availability of P by mobilizing sparingly soluble forms of P such as Ca phosphate. To determine if a similar mechanism operates in Brassica, four genetically diverse cultivars contrasting in vegetative and grain yield under P-deficient conditions were examined for OAs root exudation triggered by P deficiency. Cultivars were grouped into two categories: “Brown Raya” and “Con-1” (efficient; group I) and “Toria” and “B.S.A” (inefficient; group II). Group I cultivars are relatively well adapted to P-limited conditions and accumulate nearly twice as much biomass as group II cultivars when both are under severe P conditions (CitationAkhtar et al. 2005). Citric, malic and succinic acids were measured in the root exudates of 25-day-old plants. The amount of OAs released from roots of P-starved plants differed from those of plants grown in P-sufficient conditions. When plants were supplied with adequate P, OAs were released in low amounts by all cultivars. In contrast, these acids were released in higher concentrations when plants were re-cultured for 12 days under P-deficient conditions. The amounts of OAs released from the roots differed among cultivars (). In all cultivars, malate and citrate were the predominant OAs released. In P-starved plants, the amount of exuded malic acid was the highest of the three. The low P-tolerant cultivars “Brown Raya” and “Con-1” showed the highest increase in malic and citric acid exudation, respectively, than all other cultivars with P deficiency (). The types and amounts of exuded OAs appeared to be cultivar and time specific. In the cultivars “Brown Raya”, “Con-1” and “Toria”, malate exudation is higher than the exudation of citrate after 4 h and 8 h exudation, whereas in the cultivar “Con-1”, citrate is released in higher amounts than malate after 4 h and 8 h exudation in both treatments (). In cultivar “B.S.A”, citrate exudation is 1.4-fold higher than malate under low P supply after 8 h exudation. Succinic acid exuded by cultivar “Con-1” is slightly higher than “Brown Raya” under adequate P supply (). In the
efficient cultivars “Brown Raya” and “Con-1”, the amounts of OAs in root exudates are higher than those of “Toria” and “B.S.A”. However, the exudation rate of both resistant and sensitive cultivars decreased with time. The highest exudation rate was found after the first 4 h of collection and then tended to decrease ().DISCUSSION
Two major mineral components, Fe oxides and CaCO3, largely control the dynamics of P in calcareous environments. Nevertheless, plant species and cultivars differ
Figure 4 (a) Citrate, (b) malate and (c) succinate exudation by the roots of four Brassica cultivars. The seedlings were raised on moist sand for 6 days and were cultured in complete nutrient solution for 7 days. The seedlings were then transferred to the solutions with or without 0.20 mmol L−1 P using KH2PO4 for 12 days. Root exudates were collected after 4 h and 8 h, respectively, to evaluate time-course effect and analyzed using HPLC. Means with different letter(s) on top of the bars differed significantly according to Duncan's multiple range test (P = 0.05).
In experiment 1, tested Brassica cultivars differed in SDM, RDM, TDM, RSR, P concentration, P uptake and PUE when grown with TCP, indicating the existence of useful genetic differences among Brassica cultivars for solublization of P from sparingly soluble P. The RSR of cultivars grown in TCP was much higher compared with the control. Higher RSR is often reported in P-deficient plants when compared with P-sufficient plants. This is attributed to higher export rates of photosynthates to the roots and utilization of photoassimilates in the roots. Preferential root growth helps the plants to acquire more P in P-stress conditions.
The PSF can be used as an index in assessing relative tolerance of Brassica cultivars. Cultivars such as “Brown Raya” and “Con-1”, depicting minimum PSF, are considered to be better adapted to such conditions. The maximum increase in PUE was observed in “Brown Raya” and the minimum was observed in “Sultan Raya”, as the former produced the maximum SDM per unit P uptake and the latter produced the lowest. PUE is the ability of the plant to grow and yield well in suboptimal P-availability situations. Cultivars grouped ER (“Brown Raya” and “Con-1”) are the most desirable as per their performance at both P sources in the rooting media (). The most undesirable cultivars are the non-efficient and non-responsive (NENR) type (“Peela Raya”, “Toria Selection” and “Sultan Raya”) as per their poor performance even at adequate (control; AP) P supply. The P uptake and RDM of NENR cultivars were inferior to ER cultivars (). Phosphorus concentration was almost similar or higher in NENR cultivars than ER cultivars. This means that the greater P efficiency resulted from P-use efficiency rather than differences in P concentration. The P concentration and uptake in shoots had a highly significant and positive correlation with RDM and SDM, suggesting that the cultivars with higher RDM accumulated higher amounts of shoot P and produced higher SDM at TCP. Thus, under P stress, better P acquisition and PUE by the efficient cultivars for biomass synthesis collectively formed the basis of higher SDM production, revealing that P uptake and PUE are important plant traits for selecting low-P tolerant cultivars.
In experiment 2, the substantial differences exhibited by cultivars in terms of root and shoot growth and plant P acquisition could be explained on the basis of RSR, PUE and P and Ca contents, as these parameters were statistically significant with biomass production. TCP was used as the P source with the objective of buffering the P concentration of nutrient solutions at deficient levels throughout the growth period. Although mean P concentration of the solutions varied among cultivars on 12 (non-significantly) and 16 (significantly) DAT, it was well maintained on or around the level (0.015–0.020 mmol L−1) used as deficient for P in another experiment (CitationAkhtar et al. 2005). In contrast, the pH of the solutions showed substantial decrease over the starting adjusted value of 7 (). The cultivars that were efficient accumulators of biomass showed a greater decrease in the pH of the solutions containing TCP than non-efficient cultivars and the decrease in pH correlated positively with plant total P uptake (). It can be assumed on the basis of this experiment that rhizosphere acidification through proton efflux is one of the strategies adopted by Brassica cultivars to increase P availability in the rooting medium. Both Ca and P concentrations of roots of cultivars had a negative impact on SDM production (), implying that cultivars capable of smoothly translocating these ions to aboveground parts sustained higher SDM. This assumption could also be confirmed by the fact that cultivars, which depicted higher Ca and P uptake in their shoots, produced higher SDM (). As a very strong positive correlation also existed between total Ca uptake and total P uptake of cultivars (), it can be assumed that the higher P acquisition of efficient cultivars was because of their high Ca uptake. By the principal of mass action, high Ca uptake by plants caused an increase in P solubility from Ca-bound P sources – a phenomenon that can increase the availability of indigenous P in calcareous soils. Cultivars that are efficient accumulators of Ca are desirable because they can acquire higher amounts of P from otherwise P-deficient soils. Significant cultivar differences in tissue Ca concentrations and uptake under P deficiency, and their meaningful correlations with growth parameters, also suggest their testing as criteria for P efficiency of crops under P-deficiency stress.
Rock phosphate containing 0.17 mole ratio of CO3/PO4 in apatite structure was used in experiment 3. It contains no soluble P and can maintain low available P in root medium over the crop growth period. Most RPs are made of apatite-like Ca phosphates and, thus, exhibit an increasing solubility with decreasing pH as the precipitation–dissolution equilibria that govern the solubility of P minerals is under the direct dependence of the pH and the concentration of P and that of the considered metal cation:
Protons supplied by the acidulation to RP break its apatite bond and increase its solubility in the chemical P fertilizer industry. Concurrently, H+ released by ATPase pumps located in plasmalemma during nutrient uptake attack RP to solubilize P for meeting plant growth requirements. CitationRuiz (1990) measured an efflux of 18 µmol H+ h−1 g−1 of fresh roots behind the root tip of the primary root of P-deficient rape and an efflux of 9 µmol OH− h−1 g−1 for basal parts of the same root. In comparison, no acidification occurred for P-sufficient rape and an average efflux of approximately 12 µmol OH− h−1 g−1 fresh roots was found along the primary root. Such enhanced acidification of the rhizosphere might be related to an inhibition of NO−3 uptake in response to P deficiency, and to a consequent increase in the excess of cation over anion uptake, as suggested by CitationNeumann and Martinoia (2002).
Accumulation of P and biomass by six cultivars was consequently dependent on their capability to solubilize and utilize P from RP added to nutrient solution as a P source. The cultivars “Brown Raya”, “Con-1” and “Rain Bow”, releasing more H+ than the other three cultivars utilized more P from RP () and accumulated the maximum biomass. Therefore, these cultivars appear to possess the highest potential of making better growth in low P soils in descending order. The amounts of P taken up by cultivars increased linearly with decreasing pH (). This suggests that intense rhizosphere acidification in response to P stress probably determines the ability of efficient cultivars to utilize acid soluble Ca phosphates in calcareous soils or RP fertilizers. Some plant species, such as buckwheat, oilseed rape and legumes, were particularly efficient at using P from RPs, as related to their peculiar ability to release H+. In experiment 3, the contents of P in shoots of cultivars increased linearly with decreasing pH (). Efficient cultivars “Brown Raya”“Con-1” and “Rain Bow” showed a greater decrease in pH in rooting media containing RP.
In addition to root morphology and uptake activity, the release of OAs because of PEPCase induction from roots of cultivars can contribute to P acquisition and utilization through the dissolution of sparingly soluble phosphates (CitationNarang et al. 2000). A major process that contributes to root-induced pH changes in the rhizosphere is the release of charges carried by H+ or OH− to compensate for an unbalanced cation–anion uptake at the soil–root interface. The cation–anion balance does not only include the ions that are taken up by plant roots, but should include all the charges/ions that cross the root cell plasma membrane via either influx or efflux. In that respect, the release of OAs can be a major component of cation–anion balance and thereby influence the net release of H+ or OH− equivalents (CitationHinsinger et al. 2003). In white lupin, CitationNeumann and Martinoia (2002) reported that a significant increase in citrate exudation occurred under low P, which might contribute to the observed acidification of the hydroponic solution in P-deficient relative to P-sufficient plants. H+ extrusion under P-deficient conditions requires accumulation of carboxylic acids in the root tissue for intracellular pH stabilization (CitationMarschner 1995). Accordingly, P limitation enhanced the root concentration of carboxylic acids in all plant species. To maintain charge balance, the remaining OAs are either stored in the vacuoles of root tissue or may be translocated to the shoot, and in some plant species, such as chick pea, Brassica and white lupin, a proportion of carboxylates are released into the rhizosphere.
In experiment 4, the efficient cultivars “Brown Raya” and “Con-1” exuded 2–3-fold more citrate and malate than the less efficient cultivars “Toria” and “B.S.A” under low P supply. The increased exudation of OAs, particularly citrate and malate, in the cultivars “Brown Raya” and “Con-1” may enable them to acquire P from Ca–P salt complexes in neutral to alkaline soils. Elevated root citrate and malate exudation has been observed in low P-tolerant cultivars “Brown Raya” and “Con-1” and they exuded more OAs than low P-susceptible cultivars. The exudation of citrate and malate in the efficient cultivars “Brown Raya” and “Con-1” is lower compared with white lupin proteoid roots, but it is comparable with the exudation by Arabidopsis accessions reported by CitationNarang et al. (2000) and the citrate and malate exudation in cultivars “Brown Raya” and “Con-1” are accompanied by strong H+ extrusion (), whereas in Arabidopsis accessions citrate and malate was not accompanied by strong H+ extrusion and there was a net alkalization of growth medium. In contrast, tested Brassica cultivars displayed a net acidification of the growth medium when grown with TCP and RP in experiments 2 and 3. Secretion of OAs and H+ extrusion has been observed in P-efficient cultivars under P stress, which thereby contributes to the solublization and assimilation of mineral Pi in alkaline calcareous environments. It remains to be tested whether the efficient cultivars “Brown Raya” and “Con-1” display high P acquisition and PUE under field conditions.
Scavenging of Pi from extracellular sparingly sources can be enhanced by biochemical adaptations in roots such as extrusion of H+ and OAs into rhizosphere via plasamlemma H+ ATPase and anion channels triggered by P starvation. Enhanced release of H+ and OAs by efficient cultivars solublizes P from unavailable bound forms. OAs and H+ exuded from roots also make organic P esters more soluble and susceptible to acid phosphatases (CitationVance et al. 2003). Phosphorus released from sparingly soluble P sources, insoluble ligands and organic P esters, is then taken up through P transporters in the plasamlemma (CitationHammond et al. 2004). Efficient cultivars can increase their capacity to access nutrients (to convert unavailable forms into available forms) by altering root morphology (increasing surface area by growing long, thin roots with numerous and long root hairs) and enhance nutrient availability and uptake. Modification in root architecture in an efficient cultivar is an important genetic adaptation to explore and mine P from P-deficient soil environments. High P efficiency of efficient cultivars under P stress can be attributed to two types of mechanisms: internal (production of high yield per unit of absorbed P) and external (i.e. those leading to high P acquisition from P-deficient rooting media). External mechanisms largely depend on plant roots. Efficient exploitation of the growth medium through either a higher root : shoot ratio or acidification of rhizosphere lead to increased plant P acquisition, and ultimately higher crop yields under P-limiting conditions.
Conclusions
Morphological and physiological analysis revealed that cultivars differed substantially in SDM, RDM, RSR, P concentration, P uptake and PUE when grown with Ca3(PO4)2 (TCP) and rock P (RP), indicating the existence of useful genetic differences among Brassica cultivars for solublization of P from sparingly soluble P sources. Biomass production correlated significantly with the P uptake and PUE of cultivars at TCP and RP, showing that cultivars efficient in P solublization accumulated more biomass. Higher P acquisition depicted by efficient cultivars at TCP was because of their high Ca uptake. Efficient cultivars “Brown Raya”, “Con-1” and “Rain Bow” showed a decrease in pH to the tune of two points at both P sources and the amount of P taken up by cultivars increased linearly with decreasing pH in rooting media. Low P-tolerant cultivars “Brown Raya” and “Con-1” exuded 2–3-fold more OAs than low P-sensitive (“Toria” and “B.S.A.”) cultivars; however, the exudation rate of both sensitive and resistant cultivars decreased with time. Rhizosphere acidification by efficient cultivars because of H+ extrusion and secretion of OAs triggered by P starvation was an important biochemical adaptation for mobilization and exploitation of Pi from extracellular sparingly soluble P sources.
ACKNOWLEDGMENT
The principal author Akhtar M. Shahbaz gratefully acknowledges the Ministry of Education, Science, Sports and Culture, Japan, for awarding a PhD scholarship that enabled him to pursue this research work.
REFERENCES
- Vance , CP , Uhde-Stone , C and Allan , DL . 2003 . Phosphorus acquisition and use: critical adaptations by plants securing a nonrenewable resource . New Phytol , 157 : 423 – 457 .
- Rausch , C and Bucher , M . 2002 . Molecular mechanisms of phosphate transport in plants . Planta , 216 : 23 – 37 .
- Narang , RA , Bruene , A and Altmann , T . 2000 . Analysis of phosphate acquisition efficiency in different Arabidopsis accessions . Plant Physiol , 124 : 1786 – 1799 .
- Wu , C , Wei , X , Sun , HL and Wang , ZQ . 2005 . Phosphate availability alters lateral root anatomy and root architecture of Fraxinus mandshuricarupr. seedlings . JIntegrative Plant Biol , 47 : 292 – 301 .
- Rengel , Z . 2002 . Genetic control of root exudation . Plant Soil , 245 : 59 – 70 .
- Hinsinger , P , Plassard , C , Tang , C and Jaillard , B . 2003 . Origins of root-induced pH changes in the rhizosphere and their responses to environmental constraints: a review . Plant Soil , 248 : 43 – 59 .
- Hinsinger , P , Gobran , GR , Gregory , PJ and Wenzel , WW . 2005 . Rhizosphere geometry and heterogeneity arising from root-mediated physical and chemical processes . New Phytol , 168 : 293 – 303 .
- Hammond , JP , Broadley , MR and White , PJ . 2004 . Genetic responses to phosphorus deficiency . AnnBot , 94 : 323 – 332 .
- Hinsinger , P . 2001 . Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes . Plant Soil , 273 : 173 – 195 .
- Neumann , G and Martinoia , E . 2002 . Cluster roots – an underground adaptation for survival in extreme environments . Trends Plant Sci , 7 : 162 – 167 .
- Rengel , Z and Marschner , P . 2005 . Nutrient availability and management in the rhizosphere of plant genotypes . New Phytol , 168 : 305 – 312 .
- Hoffland , E , Findenegg , GR and Nelemans , JA . 1989 . Utilization of rock phosphate by rape . Plant Soil , 113 : 155 – 160 .
- Chapmann , HD and Pratt , PF . 1961 . Methods of Analysis for Soils, Plants and Waters , 160 – 170 . Berkeley, USA : Div. Agric. Sci. Pub. 4034, Univ. California .
- Akhtar , MS , Oki , Y and Adachi , T . 2005 . “ Phosphorus nutrition of Brassicacultivars under deficient and adequate levels in solution culture ” . In Plant Nutrition for Food Security, Human Health and Environmental Protection , Edited by: Li , CJ , Zhang , FS Dobermann , A . 236 – 237 . Beijing : Tsinghua University Press .
- Steel , RGD and Torrie , JH . 1980 . Principles and Procedures of Statistics , New York : McGraw Hill Book Company .
- Gomez , KA and Gomez , AA . 1984 . Statistical Procedures for Agricultural Research , New York : John Willey and Sons .
- Ruiz , L and Arvieu , JC . 1990 . Measurement of pH gradients in the rhizosphere . Symbiosis , 9 : 71 – 75 .
- Marschner , H . 1995 . Mineral Nutrition of Higher Plants , 2nd edn , London, , UK : Academic Press .