Abstract
Nitrous oxide (N2O) fluxes from tropical peatland soils were measured at a grassland, three croplands, a natural forest, a burned forest and a regenerated forest in Central Kalimantan, Indonesia. Only croplands received fertilization (665–1278 kg N ha−1 year−1). Mean annual N2O emissions from croplands were 21–131 kg N ha−1 year−1 in 2002–2003 and 52–259 kg N ha−1 year−1 in 2003–2004, and were significantly higher than the emissions from other comparable sites. Cropland N2O emissions were among the highest values reported from cultivated tropical, temperate and boreal organic soils. Mean annual N2O emissions were 7.1 (2002–2003) and 23 (2003–2004) kg N ha−1 year−1 from grassland, and were significantly higher than in natural, regenerated and burned forests (0.62, 0.40 and 0.97 kg N ha−1 year−1 in 2002–2003 and 4.4, 4.0 and 1.5 kg N ha−1 year−1 in 2003–2004, respectively). Annual N2O emissions did not differ significantly between forests in 2002–2003, but were significantly lower in burned forest in 2003–2004. Annual N2O emission was significantly correlated between years. Regression analysis revealed that annual N2O emissions in 2003–2004 were 1.9-fold the corresponding 2002–2003 value (annual precipitation of 2339 and 1994 mm, respectively). N2O fluxes were higher during the rainy season than during the dry season at all sites except the regenerated forest. N2O fluxes in cropland and grassland were significantly lower when the water-filled pore space (WFPS) was less than 60–70%, and increased with an increase in soil NO3–N concentration when WFPS exceeded this threshold. Thus, changes in soil moisture were important in controlling seasonal changes in N2O emission. Our results suggest that changing land use from forestry to agriculture will increase N2O production. The effect of forest fires on N2O emission from these soils was not clear.
INTRODUCTION
Nitrous oxide (N2O) is a major greenhouse gas that contributes approximately 6% of the total global warming (CitationIntergovernmental Panel on Climate Change 2001) and leads to the destruction of the ozone layer in the stratosphere (CitationCrutzen 1981). N2O is produced in soils through nitrification and denitrification. The estimated N2O emissions from tropical wet forest soils (3 Tg N year−1) and agricultural soils (3.5 Tg N year−1) account for a large proportion of total global emissions (16.2 Tg N year−1; CitationIntergovernmental Panel on Climate Change 1996). Tropical peatlands contain a large amount of organic C and N under waterlogged conditions and could, therefore, be an important source of N2O and carbon dioxide (CO2) after drying (CitationBouwman 1990; CitationKasimir-Klemedtsson et al. 1997; CitationMosier et al. 1998) owing to accelerated decomposition of the peat. The total area of tropical peatlands is estimated at 33–50 Mha, which is 8.6–12% of the global peatland area (CitationMaltby and Immirzi 1993). Most tropical peatlands are located in Indonesia, where they cover 17–27 Mha (CitationMaltby and Immirzi 1993). A large area of tropical peatland in Indonesia has been affected by forest fires (CitationPage et al. 2002) and agricultural exploitation (CitationMuhanmad and Rieley 2002). CitationPage et al. (2002) estimated that 27.6% of the total area of peat swamp forest (1.37 million ha) burned in Central Kalimantan during the 1997 El Niño Southern Oscillation event using satellite images of a 2.5 million ha study area. In 1995, more than 1 million ha of tropical peatland in Central Kalimantan was reclaimed by the “Mega Rice Project” (CitationMuhanmad and Rieley 2002).
The cultivation of tropical peatlands has been considered to be a large source of N2O emissions and the CitationIntergovernmental Panel on Climate Change (2000) estimated that direct N2O emissions from cultivated organic soils, totaling 16 kg N ha−1 year−1, resulted from the mineralization of soil organic nitrogen. However, this value was determined using emission data collected only from boreal and temperate regions. There have been few reports on N2O emissions from tropical peatlands (e.g. CitationHadi et al. 2000; CitationInubushi et al. 2003; CitationMelling et al. 2005). Therefore, there is large uncertainty in estimating N2O emissions from tropical peatlands. Moreover, the factors that control N2O emission from these sites are not well understood. We conducted the present study to: (1) quantify the amount of N2O emissions, (2) investigate the factors that control N2O emissions from these sites, (3) investigate the effects of forest fire and agriculture on N2O emissions from tropical peatlands.
MATERIALS AND METHODS
Site description
The study sites were located at Kalampangan village, near Palangka Raya (2°S, 114°E) in Central Kalimantan, Indonesia (). We established experimental plots at three forest sites and four agricultural sites. The forest sites consisted of a natural forest (NF: 2°21′S, 114°02′E) and two fire-degraded forests designated as regenerated forest (RF: 2°21′S, 114°02′E) and burned forest (BF: 2°19′S, 114°01′E). The natural forest had not been affected by forest fire. The other two forests (regenerated and burned forest) were degraded by forest fire in 1997. The trees and the surface peat soils (maximum at a depth of 30 cm) in the burned and regenerated forests burned during the fire. The regenerated forest regenerated naturally after the fire, but the burned forest did not regenerate a forest cover. Both the regenerated and burned forests were burned again by forest fire in July and September 2002, respectively. As of March 2004, no trees could be found at either site. Both forest fires in 1997 and 2002 were caused naturally during the extreme dry season owing to the El Niño Southern Oscillation event. In the natural forest, the depth of the peat soil ranges from 3.50 to 4.85 m (CitationTuah et al. 2001). This forest consists mainly of deciduous
Figure 1 Locations of the study sites in Kalampangan, Central Kalimantan. “Agricultural lands” represent the grassland site (GL) and the croplands (CL) A, B and C.
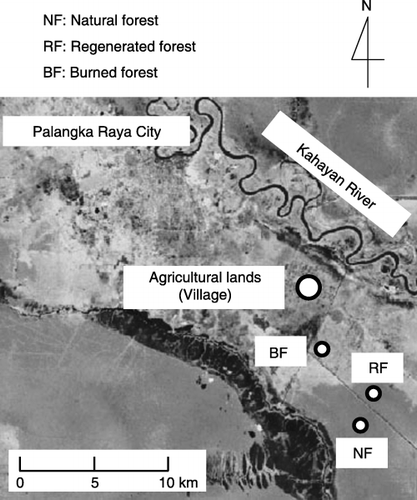
The agricultural sites (2°17′S, 114°01′E) consisted of one grassland (GL) and three croplands, designated as cropland A (CL-A), cropland B (CL-B) and cropland C (CL-C). These four sites were adjacent to each other. The grassland site was dominated by dwarf grasses and was grazed by cows every 2 months. The main crops in the croplands were maize (Zea mays L.), spinach (Spinacia oleracea L.) and cassava (Manihot esculenta
Table 1 Amount of nitrogen applied at the cropland sites
Figure 2 Daily air temperature and precipitation recorded near the natural forest (at 41.7 and 41 m above the ground, respectively).
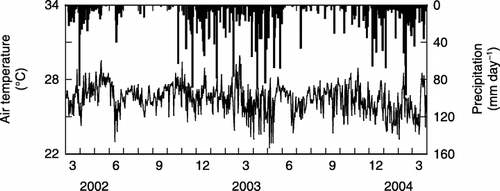
The mean daily air temperature ranged from 22 to 28°C (). In this region, the dry season normally begins in June and ends in October (CitationTakahashi et al. 2002). Precipitation during the dry season was greater in 2003 (349 mm) than in 2002 (274 mm). Total annual precipitation was 2339 mm from April 2003 to March 2004 and 1994 mm from April 2002 to March 2003 ().
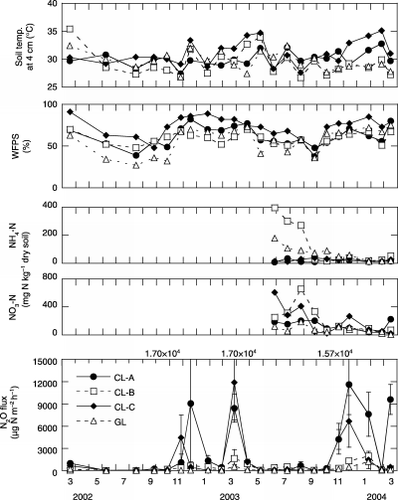
The soil temperature at a depth of 4 cm in the natural, regenerated and burned forests ranged from 25.6 to 30.2°C, 25.7 to 31.9°C and 28.4 to 34.1°C, respectively (). The mean soil temperature at a depth of 4 cm during the rainy season was significantly (P < 0.05) higher in both the burned forest (30.6 ± 1.4°C) and the regenerated forest (29.7 ± 1.6°C) than in the natural forest (27.0 ± 0.9°C) (). The mean monthly value for water-filled pore space (WFPS) (, ) at the natural forest site showed a clear seasonal change, and was significantly (P < 0.05) lower in the dry season (28 ± 16%) than in the rainy season (46 ± 5.5%). The WFPS in natural forest during the 2002 dry season was lower (<20%) than in 2003 because of the lower precipitation in 2002 (). During the 2003 rainy season, WFPS in the regenerated forest increased rapidly and remained high compared with levels in the natural forest (). The WFPS values in the regenerated and burned forests were significantly (P < 0.05) higher than in the natural forest in the rainy season (). This may have resulted from decreased water uptake by plants because of the absence of trees on these sites. The regenerated and burned forests were sometimes flooded late in the rainy season (). The mean monthly soil temperature at a depth of 4 cm at the four agricultural sites () was almost constant and had a similar pattern. The mean soil temperatures during the dry and rainy season ranged from 28.2 to 29.9°C and 29.5 to 32.1°C, respectively (). The monthly WFPS in the four agricultural lands also showed a similar pattern, and was significantly (P < 0.05) lower during the dry season (, ). The average depths to the groundwater table at the agricultural sites (croplands A, B and C were 68, 72 and 76 cm, respectively, vs 62 cm for the grassland) were lower than at the forest sites (natural, regenerated and burned forest were 51, 23 and 52 cm, respectively).
Gas sampling for flux measurement
Nitrous oxide flux measurements were carried out once every 3 months from March to August 2002, and monthly from August 2002 to March 2004. During the late rainy season we were unable to conduct N2O flux measurements on several occasions because of flooding in the regenerated forest (March, April and December 2003 as well as January 2004) and in the burned forest
Table 2. Soil chemical and physical properties at the study sites
Figure 3 Seasonal changes in soil temperature, water-filled pore space (WFPS), soil NH4–N, soil NO3–N and N2O fluxes at the three forest sites. When the regenerated and burned forests were flooded, WFPS was assumed to reach its maximum value of 100%. Arrows indicate the timing of forest fires. Positive N2O fluxes indicate net emissions; negative values indicate net uptake. Error bars show standard deviations. BF, burned forest; NF, natural forest; RF, regenerated forest.
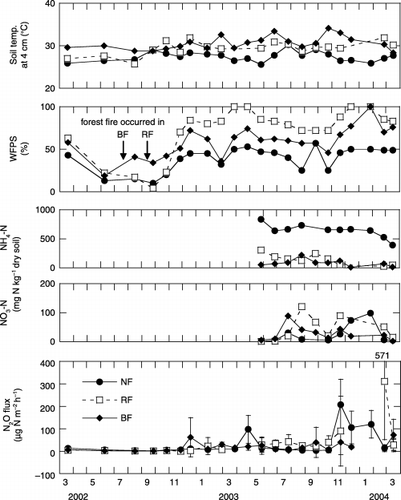
Table 3 Mean soil temperatures, water-filled pore space (WFPS) values and N2O fluxes at the study sites during the dry (June to October) and rainy (November to May) seasons
Soil temperature, soil moisture and meteorological data
The soil temperature at a depth of 4 cm, the volumetric soil water content from 0 to 6 cm in depth, and the depth of the water table were measured during the gas flux measurements. The soil temperature was measured using a thermistor thermometer (Checktemp 1C, Hanna Instruments). An amplitude domain reflectometry (ADR, ML2 Theta Probe Delta-Y Devices, Cambridge, UK) was used to measure the volumetric soil water content. These values were calibrated using the volumetric soil water content measured in 200-mL soil core samples taken from a depth of 0–10 cm. The calibration curves for each site were fitted using third-order equations (r 2 = 0.97 to 1.00, P < 0.01). The resulting volumetric soil water content was converted into a value for WFPS, which represents the ratio of the volumetric water content to the total porosity of the soil, by assuming that the porosity measured in February 2005 was consistent throughout the measurement period. The measurement of porosity is described in the “Soil sampling and analysis” section. The porosity of the regenerated forest was not measured because of flooding, and was replaced by the value for the burned forest site in the WFPS calculation. There were three replications per chamber for the soil temperature and volumetric soil water content measurements. To measure the water table depth, perforated PVC pipes (4 cm in diameter) were inserted into the peat soil at each site.
Air temperature and precipitation were measured every half hour using a 50-m-tall micrometeorological tower established inside the forest (CitationHirano et al. 2005). The air temperature was measured at 41.7 m above the ground with a platinum resistance thermometer (HMP45, Vaisala, Helsinki, Finland) and the precipitation was measured at a height of 41 m with a tipping-bucket rain gauge (TE525, Campbell Scientific. Inc., Logan, UT, USA) (CitationHirano et al. 2005).
Soil gas sampling
The following procedure was used to measure N2O concentrations in the soil gas (CitationMorishita et al. 2003). Three stainless-steel pipes (8 mm in diameter) were installed at each of five depths (5, 10, 20, 40 and 80 cm). A 50-mL sample of air was removed from each pipe, and all gas samples collected from the same depth were mixed in a 1-L sampling bag (Tedlar bag, OMI Odorair Service, Shiga, Japan). Simultaneously, air was sampled at a height of less than 5 cm above the soil surface with a sampling bag to provide a 0-cm (surface) sample. A 10-mL vacuum vial with a butyl rubber stopper was used to sample 20 mL of each of these gas samples.
When only water was collected from the pipes, the dissolved N2O concentrations in the water were measured using a modified version of the method of CitationSawamoto et al. (2002). Dissolved N2O concentrations were measured using the head-space method (CitationSawamoto et al. 2002), with a single equilibration using ambient air, and were then converted into N2O concentrations in the soil gas, which were equilibrated to the sampled water.
Analysis of gas concentration and calculation of flux
The N2O concentrations were analyzed with a gas chromatograph (GC-14B, Shimadzu, Kyoto, Japan) equipped with an electron capture detector (ECD). The minimum detectable concentration of N2O was 0.007 p.p.m.v determined by the deviation obtained by repeatedly analyzing the concentration of an N2O standard
Figure 4 Seasonal changes in soil temperature, water-filled pore space (WFPS), soil NH4–N, soil NO3–N and N2O fluxes at the agricultural sites. Error bars show standard deviations. CL, cropland; GL, grassland.
where F is the flux (µg N m−2 h−1); ρ is the gas density of N2O (1.978 × 109 µg m−3); V is the volume of the chamber (m3); A is the cross-sectional area of the chamber (m2); Δc/Δt is the ratio of change in the gas concentration (c) inside the chamber per unit time (t) during the sampling period (m3 m−3 h−1); T is the air temperature (°C) and α is a conversion factor from N2O to N (=28/44).
The annual N2O emissions were calculated from the monthly mean flux values as follows:
where F i is the mean gas flux (kg N ha−1 day−1) between two sampling times (i.e. for time interval i), D i is the number of days in the sampling interval and n is the number of sampling times. Missing values in the regenerated and burned forests because of flooding were replaced with flux data from the water surface, which was measured using a tall chamber (30 cm × 30 cm × 60 cm darkened acryl chamber) in February 2005. The mean N2O fluxes (flooding water depth) from the regenerated and burned forests in February 2005 were 4.7 µg N m−2 h−1 (at a depth of 20 cm in flooding water) and −1.6 µg N m−2 h−1 (at a depth of 10 cm in flooding water), respectively.
Soil sampling and analysis
General soil properties
Disturbed soil samples used for chemical analysis were taken from a depth of 0–10 cm at each site in 2002. The forest soils were divided into a litter layer and a peat layer. In the regenerated and burned forests, soil samples were taken both before and after the forest fire in 2002.
Sieved (2 mm), air-dried soil samples were used for the chemical analysis, except for NH4–N content. Soil pH (H2O basis) was measured with a glass electrode pH meter (pH meter F-22, Horiba, Kyoto, Japan) in a 1:20 soil : deionized water mixture. The concentrations of NO3–N were also measured in this suspension using ion chromatography (Dionex QIC Analyzer, Dionex Japan, Osaka, Japan).
Sieved (2 mm), air-dried soil samples were extracted with 1 mol L−1 ammonium acetate (pH 7), washed with 80% ethanol (pH 7) and subsequently extracted with 1 mol L−1 KCl. Cation exchange capacity (CEC) was determined by analyzing NH4–N concentrations using a 1 mol L−1 KCl extraction. Exchangeable cations were determined by analyzing their concentrations in a 1 mol L−1 ammonium acetate (pH 7) extraction (CitationKamewada 1997). Concentrations of Ca2+ and Mg2+ were determined using atomic-absorption spectrometry and those of K+ and Na+ were determined using atomic-emission spectrometry (Z5010, Hitachi, Tokyo, Japan). Base saturation was calculated by dividing the sum of the exchangeable cations by the CEC.
After grinding the air-dried samples using an agate pestle and mortar, total C and N content were determined using an N/C analyzer (NC-1000, Sumika Chemical Analysis Service, Osaka, Japan).
Fresh samples that had not been allowed to dry were extracted with 2 mol L−1 KCl solution (1:20 dried soil : water) and NH4–N concentrations in the extracted solution were determined using colorimetry (the indophenol-blue method) with a UV-VIS spectrophotometer (UV mini 1240, Shimadzu, Kyoto, Japan) following the method of CitationHidaka (1997).
Undisturbed 100-cm3 core samples were collected in February 2005 for measurement of the air ratio using a soil volume analyzer (DIK-1110, Daiki Rika Company, Saitama, Japan). The cores were then oven-dried at 105°C for 48 h and reweighed to determine their bulk density and volumetric water content. Porosity equaled the sum of the air ratio and the volumetric water content.
Temporal changes in soil NH4–N and NO3–N
Disturbed soil samples were taken from a depth of 0–3 cm at each site simultaneously with the gas flux measurements from June 2003 to March 2004. Soil NH4–N and NO3–N contents were measured as described above.
Statistical analysis
One-way anova followed by a Steel–Dwass test was used to compare the means of soil temperature, WFPS, soil NH4–N, soil NO3–N and N2O flux at each site. An unpaired t-test was used to compare the means of soil temperature, WFPS and N2O flux between the dry and rainy seasons. A least significant difference (LSD) test was used to compare the annual N2O emissions between sites. All statistical analyses were conducted using Excel statistics ver. 5.0 (Esumi Company, Tokyo, Japan).
RESULTS
Soil properties
General soil properties
The pH values in the peat layer of the agricultural sites (4.4–6.0) were higher than those in the soils of the forest sites (3.4–3.9). Soil NO3–N levels at the agricultural sites (225–292 mg N kg−1 dry soil) were higher than those at the forest sites (0.43–127 mg N kg−1 dry soil). In contrast, soil NH4–N levels at the agricultural sites (17–67 mg N kg−1 dry soil) were lower than those at the forest sites (22–511 mg N kg−1 dry soil) (). The soil bulk density was similar for all croplands (0.40–0.42 Mg m−3) and was higher than that in the grassland (0.30 Mg m−3), natural forest (0.14 Mg m−3) and burned forest (0.22 Mg m−3) (). Porosity values for the soils of the natural forest, burned forest, grassland and croplands were 90%, 84%, 73% and 76–77%, respectively ().
Seasonal changes in soil NH4–N and NO3–N
The soil NH4–N contents (0–3 cm depth, mean ± SD) in the natural forest (643 ± 117 mg N kg−1 dry soil) were significantly (P < 0.01) higher than those at other sites (,). The mean soil NH4–N contents in the regenerated forest, burned forest, grassland and croplands A, B and C were 146 ± 94, 83 ± 60, 70 ± 51, 17 ± 7, 118 ± 146 and 24 ± 9 mg N kg−1 dry soil, respectively (,).
The soil NO3–N contents (0–3 cm depth, mean ± SD) were significantly (P < 0.05) higher in the croplands
(croplands A, B and C, 136 ± 68, 201 ± 195 and 191 ± 197 mg N kg−1 dry soil) than in the natural forest (26 ± 34 mg N kg−1 dry soil) and burned forest (28 ± 26 mg N kg−1 dry soil) (,). The soil NO3–N contents in the regenerated forest and grassland were 43 ± 42 and 81 ± 52 mg N kg−1 dry soil, respectively (,). The soil NO3–N contents in the grassland were constantly low, whereas those in the three croplands during the dry season were initially high and decreased during the rainy season ().N2O fluxes
N2O fluxes from forest sites
The highest N2O fluxes from forest sites were mainly found during the rainy season (). The dry season N2O fluxes (mean ± SD) from the natural, regenerated and burned forests were 5.0 ± 2.8, 19 ± 17 and 8.5 ± 12 µg N m−2 h−1, respectively (, ). While the rainy season N2O fluxes (mean ± SD) from the natural, regenerated and burned forests were 49 ± 63, 55 ± 100 and 26 ± 22 µg N m−2 h−1, respectively (, ). The mean N2O fluxes in the natural and burned forests were significantly (P < 0.05) higher in the rainy season than in the dry season ().
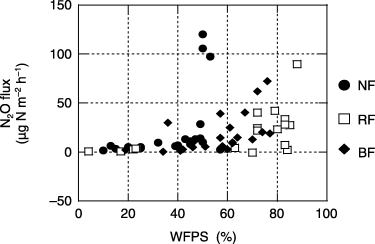
The relationships between WFPS and N2O flux from the forest sites are shown in . High N2O fluxes
(>50 µg N m−2 h−1) were found when WFPS levels in the natural, regenerated and burned forests were 45–55, 85–90 and 70–80%, respectively.N2O fluxes from agricultural sites
N2O fluxes (mean ± SD) from all croplands and from the grassland were significantly (P < 0.05) higher during the rainy season than during the dry season (grassland 257 ± 335 vs 50 ± 55 µg N m−2 h−1; cropland A 4.30 × 103± 4.30 × 103 vs 163 ± 219 µg N m−2 h−1; cropland B 637 ± 642 vs 154 ± 188 µg N m−2 h−1; cropland C 2.44 × 103± 3.55 × 103 vs 94 ± 62 µg N m−2 h−1) (, ).
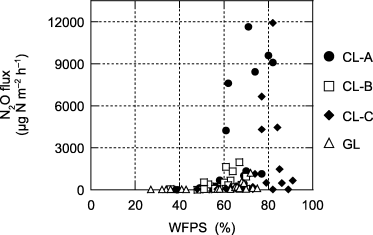
The relationships between WFPS and N2O flux from the agricultural sites are shown in . Significantly (P < 0.05) higher N2O fluxes were found when WFPS was greater than a threshold value of 60% for grassland and croplands A and B, and greater than 70% for cropland C. However, N2O fluxes decreased at the highest levels of WFPS (approximately 90%) in cropland C ().
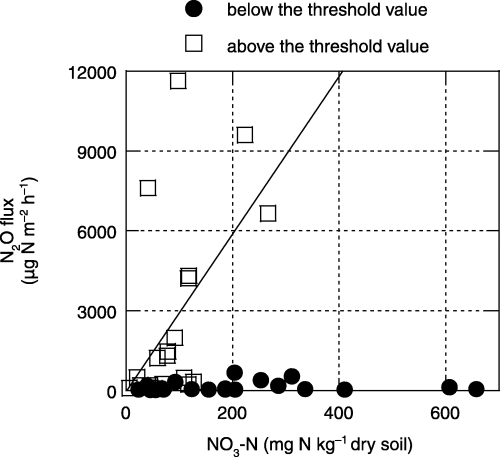
The relationships between the soil NO3–N concentration (0–3 cm depth) and the N2O flux from agricultural sites at WFPS values below and above the threshold value (60% for grassland and croplands A and B and 70% for cropland C are shown in ). N2O fluxes from all croplands and the grassland were significantly (P < 0.05) lower when WFPS was below the threshold value, regardless of the soil NO3–N concentration ().
Table 4 Annual N2O emissions from the study sites
Figure 7 The relationship between soil NO3–N (0–3 cm in depth) and N2O flux from soils of agricultural lands below and above the threshold value of water-filled pore space (WFPS) (60% for grassland and croplands A and B, and 70% for cropland C; Fig. 6). A significant correlation was found (n = 20, r = 0.536, P < 0.05) for the values above the threshold value of WFPS.
N2O concentration in the soil gas
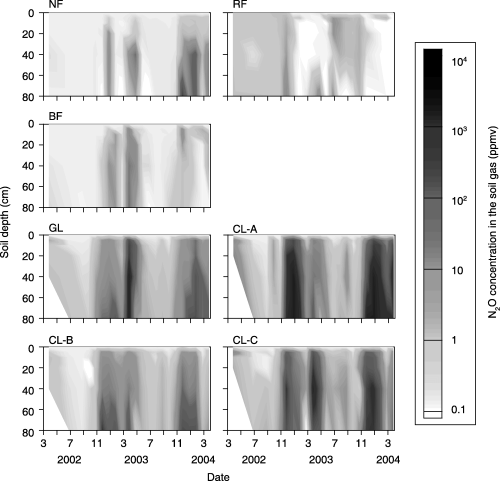
The N2O concentration was higher at greater depths in the soil, and the maximum N2O concentration was found deeper in the soil (), indicating that there was a source of N2O in deeper soil horizons. The maximum N2O concentration was higher in soils of the agricultural sites (988–5.01 × 103 p.p.m.v) than in the soils of the forest sites (31.3–842 p.p.m.v). In both groups of sites, N2O concentration increased during the rainy season and decreased during the dry season.
Annual N2O emissions
Annual N2O emissions from croplands (mean ± SD) were 131 ± 59 kg N ha−1 year−1 for cropland A, 21 ± 5.4 kg N ha−1 year−1 for cropland B and 83 ± 26 kg N ha−1 year−1 for cropland C in 2002–2003, and were 259 ± 44, 52 ± 8.2 and 151 ± 36 kg N ha−1 year−1, respectively, in 2003–2004. These values were significantly (P < 0.05) higher than those from all other sites (). Annual N2O emissions from unfertilized grassland were 7.1 ± 1.2 (2002–2003) and 23 ± 9.8 (2003–2004) kg N ha−1 year−1, and these values were also significantly (P < 0.05) higher than those from the forest sites (). There were no significant differences in annual N2O emissions among the forest sites (0.62 ± 0.11 kg N ha−1 year−1 for natural forest, 0.40 ± 0.32 kg N ha−1 year−1 for regenerated forest and 0.97 ± 0.65 kg N ha−1 year−1 for burned forest) in 2002–2003. However, the annual N2O emission from burned forest (1.5 ± 0.70 kg N ha−1 year−1) was significantly lower than that from natural forest (4.4 ± 1.2 kg N ha−1 year−1) and regenerated forest (4.0 ± 1.9 kg N ha−1 year−1) in 2003–2004 (). There was a significant difference in annual N2O emission between years for all sites except for burned forest and cropland C (). As a whole, annual N2O emission was significantly correlated between years for each site (r = 0.998, P < 0.01; ). From the slope of the regression line, the annual N2O emission in 2003–2004 was 1.9-fold the value in 2002–2003.
DISCUSSION
Temporal variation in N2O emissions
N2O fluxes were significantly higher during the rainy season (November to May) than during the dry season (June to October) at all sites except the regenerated forest site (). N2O emission from both forests and agricultural lands was strongly stimulated by high soil moisture, expressed in terms of the WFPS value (,).
N2O fluxes in all croplands and the grassland were significantly lower when WFPS was below the threshold values (60–70%), regardless of soil NO3–N concentration (,). Consequently, changes in soil moisture conditions could be an important factor controlling seasonal changes in N2O emission from tropical peat soils at these study sites.
However, low N2O emission was also found when WFPS was higher than the threshold value, and particularly low N2O emissions were found for the soils of cropland C at WFPS values of approximately 90% (). These low N2O emissions could have been caused by the predominance of N2 as a product of denitrification processes under anaerobic conditions. This hypothesis is supported by the suggestion of CitationDavidson et al. (2000) that N2 production becomes higher than N2O production through denitrification at WFPS values >80%. In addition, the results with air-dried samples showed a significant relationship between soil NO3–N and N2O flux when WFPS was above the threshold value (). This result suggests that soil NO3–N could be a controlling factor for temporal changes in N2O flux at high soil moisture levels at these study sites.
CitationInubushi et al. (2003) investigated seasonal changes in N2O emission over a year in tropical peatlands in South Kalimantan. N2O emissions from abandoned agricultural land and secondary forest were low (ranging from −40 to 30 µg N m−2 h−1), and they found no clear seasonal changes in N2O emission. They explained this result as inhibition of N2O emission by floodwater.
Figure 8 Seasonal changes in N2O concentration in the soil gas (p.p.m.v). BF, burned forest; CL, cropland; GL, grassland; NF, natural forest; RF, regenerated forest.
Because soil moisture is controlled by precipitation, annual N2O emissions should have been significantly higher in 2003–2004, with a total annual precipitation of 2339 mm, than in 2002–2003, with a total annual precipitation of 1994 mm. In fact, this is exactly what we observed (, ).
Effect of forest fire on N2O emissions
CitationIshizuka et al. (2002) reported that N2O emissions from primary forests on Ultisols in Sumatra, Indonesia, ranged from 0.13 to 0.39 kg N ha−1 year−1, and increased after clear cutting and burning in the forest.
In our study, annual N2O emission from natural forest (0.62–4.4 kg N ha−1 year−1; ) was higher than that reported by CitationIshizuka et al. (2002). There was no significant difference in annual N2O emission among the natural, regenerated and burned forests in 2002–2003. However, the annual N2O emission from burned forest was significantly lower than the emission from the natural and regenerated forests in 2003–2004. Consequently, the effect of forest fire on N2O fluxes at our forest sites was neither consistent nor clear.
Effects of agriculture on N2O emission
Soil pH at the agricultural sites became higher than that in forest sites owing to the application of ash. CitationMurakami et al. (2005) reported that lime addition to tropical peat raised the soil pH, and subsequently enhanced the decomposition of organic matter. The organic matter decomposition rates at our agricultural sites could, thus, have been higher than those at the forest sites.
Figure 9 The relationship between N2O emission in 2002–2003 and 2003–2004 at each site. Error bars are standard deviations. A significant correlation was found (y = 1.90x + 4.16, r = 0.998, P < 0.01) between the years.
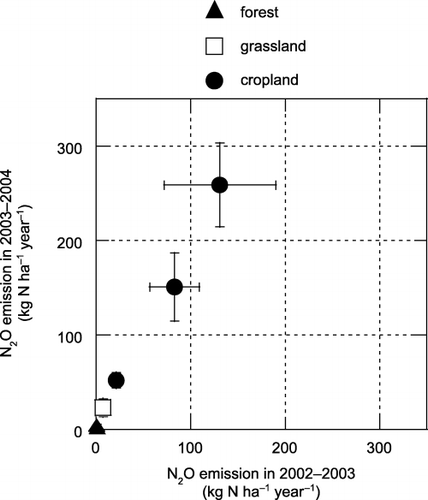
N2O emission was significantly higher in the croplands than in the unfertilized grassland, and was significantly higher in the grassland than at the three forest sites (). This indicates that agricultural practices could increase N2O emissions from tropical peatland. The N2O emissions from cropland sites were also higher than those in cultivated boreal and temperate organic soils (8.3–11.0 kg N ha−1 year−1, CitationMaljanen et al. 2004; 2–38 kg N ha−1 year−1, CitationKasimir-Klemedtsson et al. 1997) and those in tropical peatland soils in South Kalimantan (−1.1 to −0.37 kg N ha−1 year−1, CitationInubushi et al. 2003) and Sarawak, Malaysia (1.2–3.3 kg N ha−1 year−1, CitationMelling et al. 2005). Annual N2O emission from the unfertilized grassland (average 15 kg N ha−1 year−1) was close to the emission factor proposed by the CitationIntergovernmental Panel on Climate Change (2000) for N2O emission from cultivated organic soils (16 kg N ha−1 year−1). Only a weak and non-significant correlation was found between applied N () and N2O emissions () in all croplands and the grassland (y = 0.065x + 46.5, r = 0.348, P = 0.398). This suggests that there are high variations in fertilizer-induced N2O emission. We calculated the fertilizer-induced N2O emission factor, which was expressed as a percentage of N2O emission against applied N by assuming that N2O emission from the grassland represented the value for an unfertilized soil. The emission factor in croplands ranged from 1.8 to 36%, and these values were considerably higher than the value of 1.25% for N2O emission induced by nitrogen fertilizer that was proposed by the CitationIntergovernmental Panel on Climate Change (2000). However, high N2O emission from the unfertilized grassland indicated that N2O emission owing to peat decomposition could be high and also might cause this high variation in N2O emission from these agricultural soils. Therefore, quantification of N2O emission owing to peat decomposition is needed to accurately estimate the N2O emission factor in these soils. It is also important to elucidate the reason for the high emission factors that we observed and the factors responsible for the high variation in future research.
The relationship between WFPS and N2O flux suggested that denitrification might be an important process for N2O emission from these agricultural land sites (). While inorganic nitrogen was supplied to these soils as NH4–N by urea fertilization and organic matter decomposition. Therefore, nitrification also played an important role in N2O emission from these soils as a direct N2O source and a NO3–N supply for denitrification. Nitrifier denitrification (CitationWrage et al. 2001) might also contribute to high N2O emission from these soils. Further study is required to clarify the mechanism of considerably high N2O emission associated with agricultural land-use change in tropical peatland.
Our results suggest that changing land use from forestry to agriculture and agricultural practices, such as drainage, fertilization, manuring and liming, may change the microenvironmental conditions in the tropical peat soil to promote N2O production.
Conclusions
In our study area, N2O emission from tropical peatland soils was mainly controlled by a combination of soil moisture conditions and land use (i.e. forestry vs grassland vs agriculture). The effect of forest fires on N2O emission from these soils was not clear. Agricultural practices may increase N2O emission from tropical peatland soils as a result of nitrogen application and changes in the N2O production potential of the soil.
ACKNOWLEDGMENTS
We thank Dr Hanny Wijaya (Bogor Agricultural University) and the staff of the University of Palangka Raya for their support during our research. We also thank Dr T. Hirano (Hokkaido University) for providing climatic data. This study was partly supported by the JSPS-LIPI Core University Program and a Japanese Grant-in-Aid for Science Research from the Ministry of Education, Culture, Sports, Science and Technology (No. 13574012).
REFERENCES
- Intergovernmental Panel on Climate Change . 2001 . Climate Change 2001: The Scientific BasisContribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change , Edited by: Houghton , JT , Ding , Y Griggs , DJ . Cambridge : Cambridge University Press .
- Crutzen , PJ . 1981 . “ Atmospheric chemical processes of the oxides of nitrogen, including nitrous oxide ” . In Denitrification, Nitrification and Atmospheric Nitrous Oxide , Edited by: Delwiche , CC . 17 – 44 . New York : John Wiley .
- Intergovernmental Panel on Climate Change . 1996 . Climate Change 1995: The Science of Climate ChangeContribution of Working Group I to the Second Assessment Report of the Intergovernmental Panel on Climate Change , Edited by: Houghton , JT , Filho , LG Meira , Callander , BA , Harris , N , Kattenberg , A and Maskell , K . Cambridge : Cambridge University Press .
- Bouwman , AF . 1990 . “ Exchange of greenhouse gases between terrestrial ecosystems and the atmosphere ” . In Soils and the Greenhouse Effect , Edited by: Bouwman , AF . 61 – 127 . New York : John Wiley .
- Kasimir-Klemedtsson , A , Klemedtsson , L , Berglund , K , Martikainen , P , Silvola , J and Oenema , O . 1997 . Greenhouse gas emissions from farmed organic soils: a review . Soil Use Manage. , 13 : 245 – 250 .
- Mosier , A , Kroeze , C , Nevison , C , Oenema , O , Seitzinger , S and van Cleemput , O . 1998 . Closing the global N2O budget: nitrous oxide emissions through the agricultural nitrogen cycle – OECD/IPCC/IEA phase II development of IPCC guidelines for national greenhouse gas inventory methodology . Nutr. Cycl. Agroecosyst. , 52 : 225 – 248 .
- Maltby , E and Immirzi , P . 1993 . Carbon dynamics in peatlands and other wetland soils. Regional and global perspectives . Chemosphere , 27 : 999 – 1023 .
- Page , SE , Slegert , F , Rieley , JO , Boehm , HDV , Jaya , A and Limin , S . 2002 . The amount of carbon released from peat and forest fires in Indonesia during 1997 . Nature , 420 : 61 – 65 .
- Muhanmad , NZ and Rieley , JO . Management of tropical peatlands in Indonesia: Mega reclamation project in Central Kalimantan Peatlands for People, Natural Resources Function and Sustainable Management . Proceedings of the International Symposium on Tropical Peatlands . August 22–23 2001 , Jakarta, Indonesia. Edited by: Rieley , JO and Page , SE . pp. 155 – 160 . Jakarta : BPPT and Indonesian Peat Association .
- Intergovernmental Panel on Climate Change . 2000 . Good Practice Guidance and Uncertainty Management in National Greenhouse Gas Inventories , Edited by: Penman , J , Kruger , D Galbally , I . Hayama : IPCC/OECD/IEA/IGES .
- Hadi , A , Inubushi , K , Purnomo , E , Razie , F , Yamakawa , K and Tsuruta , H . 2000 . Effect of land-use changes on nitrous oxide (N2O) emission from tropical peatlands . Chemosphere-Global Change Sci. , 2 : 347 – 358 .
- Inubushi , K , Furukawa , Y , Hadi , A , Purnomo , E and Tsuruta , H . 2003 . “ Seasonal changes of CO2 CH4and N2O fluxes in relation to land-use change in tropical peatlands located in coastal area of South Kalimantan ” . In Chemosphere Vol. 52 , 603 – 608 .
- Melling , L , Hatano , R and Goh , KJ . 2005 . Global warming potential from soils in tropical peatland of Sarawak, Malaysia . Phyton (Horn, Austria) , 45 ( 4 ) : 275 – 284 .
- Tuah , SJ , Osaki , M Matsubara , T . 2001 . “ Study on leaf elemental characteristics of native plants grown in a distinct ecosystem of tropical peatland in Central Kalimantan ” . In Environmental Conservation and Land Use Management of Wetland Ecosystem in Southeast Asia, Annual Report of JSPS-LIPI Core University Program for April 2000–March 2001 , 69 – 84 . Sapporo : Graduate School of Environmental Earth Science, Hokkaido University .
- Tuah , SJ , Jamal , YM and Limin , SH . 2003 . Nutritional characteristics in leaves of plants native to tropical peat swamps and heath forests of Central Kalimantan, Indonesia . Tropics , 12 : 221 – 245 .
- Hirano , T , Segah , H Limin , S . 2005 . Energy balance of a tropical peat swamp forest in Central Kalimantan, Indonesia . Phyton (Horn, Austria) , 45 ( 4 ) : 67 – 71 .
- Takahashi , H , Shimada , S , Ibie , BF , Usep , A and Yunda, Limin , SH . Annual changes of water balance and a drought index in a tropical peat swamp forest of Central Kalimantan, Indonesia Peatlands for People, Natural Resources Function and Sustainable Management . Proceedings of the International Symposium on Tropical Peatlands . August 22–23 2001 , Jakarta, Indonesia. Edited by: Rieley , JO and Page , SE . pp. 63 – 67 . Jakarta : BPPT and Indonesian Peat Association .
- Morishita , T , Hatano , R and Desyatkin , RV . 2003 . CH4flux in an alas ecosystem formed by forest disturbance near Yakutsk, eastern Siberia, Russia . Soil Sci. Plant Nutr. , 49 : 369 – 377 .
- Sawamoto , T , Kusa , K , Hu , R and Hatano , R . 2002 . Dissolved N2O, CH4 and CO2in pipe drainage, seepage, and stream water in a livestock farm in Hokkaido, Japan . Soil Sci. Plant Nutr. , 48 : 433 – 439 .
- Kamewada , K . 1997 . “ V. 6 CEC, AEC ” . In Analysis Methods of Soil Environment , Edited by: Konno , T . 208 – 215 . Tokyo : Hakuyu-sha . (in Japanese)
- Hidaka , S . 1997 . “ V. 9 Nitrogen (Ammonium nitrogen) ” . In Analysis Methods of Soil Environment , Edited by: Konno , T . 241 – 245 . Tokyo : Hakuyu-sha . (in Japanese)
- Davidson , EA , Keller , M , Erickson , HE , Verchot , LV and Veldkamp , E . 2000 . Testing a conceptual model of soil emissions of nitrous and nitric oxides . Bioscience , 50 : 667 – 680 .
- Ishiduka , S , Tsuruta , H and Murdiyarso , D . 2002 . An intensive field study on CO2 CH4and N2O emissions from soils at four land-use types in Sumatra, Indonesia . Global Biogeochem. Cycles , 16 ( 3 ) : 1049 doi:10.1029/2001GB001614
- Murakami , M , Furukawa , Y and Inubushi , K . 2005 . Methane production after liming to tropical acid peat soil . Soil Sci. Plant Nutr. , 51 : 697 – 699 .
- Maljanen , M , Komulainen , VM , Hytönen , J , Martikainen , PJ and Laine , J . 2004 . Carbon dioxide, nitrous oxide and methane dynamics in boreal organic agricultural soils with different soil characteristics . Soil Biol. Biochem. , 36 : 1801 – 1808 .
- Wrage , N , Velthof , GL , van Beusichem , ML and Oenema , O . 2001 . Role of nitrifier denitrification in the production of nitrous oxide . Soil Biol. Biochem. , 33 : 1723 – 1732 .