Abstract
Curcuma (Curcuma alismatifolia cv. Gagnep.), a tropical flowering plant known as “Siam tulip”, were cultivated in a pot with vermiculite and supplied with different levels of nitrogen (N). Rhizomes with storage roots were harvested at 215 days after planting. Results indicated that a high level of N supply increased flower numbers and promoted continuous new rhizome formation, but storage root growth was depressed. The N supply to the plants increased the N concentrations both in the rhizomes and in the storage roots. The predominant nitrogenous compounds related to total N increase were proteins in the rhizomes. The N of the insoluble fraction of 80% ethanol or the N of the soluble fraction of 10% trichloroacetic acid was the predominant fraction of N that accumulated in the storage roots. A lack of N supply increased the starch concentration both in the rhizomes and in the storage roots. These results suggested that a high level of N supply to the curcuma plant increased new rhizome formation because of increased flower numbers, but depressed new storage root formation because of reduced starch accumulation.
INTRODUCTION
Curcuma alismatifolia cv. Gagnep. belongs to the family Zingiberaceae and is a tropical flowering plant known as “Siam tulip” or Pathumma that is grown in tropical and sub-tropical areas (CitationApavatjrut et al. 1999). In 1997, Thailand exported approximately 480,000 rhizomes to Japan, the Netherlands, New Zealand, the USA and Singapore, and this number increased to 1.3 million rhizomes in 1998 (Ruamrungzri et al. 2005).
The underground part of C. alismatifolia consists of rhizomes with storage roots. New shoot sprouts from rhizomes and storage roots are consumed by shoot growth. After planting, adventitious roots elongate from the rhizomes. Together with shoot growth, a new rhizome is formed at the basal position of the shoot. The storage roots are morphologically changed from adventitious roots after the shoots begin to grow old and they have a thick root with a spherical-ball shape at the end. The formation of storage roots is strongly influenced by day length (CitationTakano and Azuma 1996). Increases in storage root numbers are enhanced by early sprouting and an increase in flower number per rhizome, while shoot height and leaf number are not influenced (CitationTakano and Azuma 1996).
A number of the growers in Thailand cultivate curcuma in soil-less cultures to produce good quality rhizomes free from soil-borne pathogens (CitationRuamrungsri and Apavatjrut 2003). Growers apply a high level of chemical fertilizer, such as 15 g of N–P–K [21–7−14] per plant per month (CitationRuamrungsri et al. 2006). CitationRuamrungsri and Apavatjrut (2003) reported that N plays an important role in the growth and quality of curcuma rhizomes and flowers. Nitrogen-deficient plants were stunted and the quality of the flowers and rhizomes decreased. In this report we investigated the effect of different levels of N on the formation of flowers, new rhizomes and storage roots and on the accumulation of storage compounds in rhizomes and storage roots of curcuma grown in Japan.
MATERIALS AND METHODS
Plant cultivation
Rhizomes of C. alismatifolia were planted in a 1/5000 a pot filled with vermiculite on 2 May 2002 in a glass house at Niigata University, Niigata, Japan (one plant per pot). Rhizomes with three storage roots were fed with three levels of N in nutrient solutions, 50-N (50 mg N L−1), 25-N (25 mg N L−1) and 0-N (0 mg N L−1) solutions. The ratio of ammonium-N to nitrate-N was 1:4 in both the 50-N and 25-N treatments. The concentrations of the other nutrient elements were as follows (mg L−1): P, 25; K, 50; Ca, 25; Mg, 25; Fe, 5; Mn, 0.5; Zn, 0.025; Cu, 0.015; Mo, 0.015; B, 1.0. The plants were harvested at the harvesting stage on 2 December 2002, 215 days after planting. The experimental design was a completely randomized design with three pots per treatment. The numbers of flowers were counted until the harvested stage and the numbers of rhizomes were counted at harvest. Samples were washed with deionized water three times then frozen in liquid nitrogen. Each organ was weighted after being lyophilized and the analytical procedures were done in 2003.
Analysis of N and related compounds
The N concentration of the total, the soluble and insoluble fractions of 80% ethanol extraction was determined by a modified indophenol method using a Kjeldahl digested solution (CitationOhyama et al. 1991). The free amino acid concentration in 80% ethanol extract was determined using a modified ninhydrin method (CitationTakahashi et al. 1993).
Soluble protein was extracted with a 0.1 mol L−1 potassium phosphate buffer (pH7.0) and the concentration was measured using the Lowry method (CitationLowry et al. 1951) and subjected to sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Samples for SDS-PAGE were prepared as previously described (CitationOhtake et al. 1997). Proteins (10 µg) were separated using SDS-PAGE with 14% of the acrylamide separation gel concentration according to the method of CitationLaemmli (1970) and stained with Coomassie brilliant blue R-250. Protein gels were scanned with an EPSON GT-9500 scanner and the density was analyzed using the National Institute of Health (NIH) Image program ver. 1.6.1 (Epson, Tokyo, Japan).
Soluble proteins of potassium phosphate buffer extracts were precipitated using a trichloroacetic acid (TCA) solution (final TCA concentration adjusted to 10%[w/v]). The N concentrations of the potassium phosphate buffer extracts, its residues and the 10% TCA soluble and insoluble fractions were determined using the modified indophenol method.
Carbonhydrate analysis
Starch was extracted partially digested by perchloric acid (CitationOhsaki 1990) as follows. In brief, 100 mg of 80% ethanol (v/v) extracted residue was soaked in 5 mL of water and incubated in boiling water for 15 min and then 6.5 mL of 8.14 mol L−1 HClO4 was added and mixed with a glass rod for 5 min. This mixture was further incubated for 15 min in boiling water with vortex every 5 min. Twenty milliliters of water was added and the mixture was centrifuged at 795 g for 10 min. The residue was mixed with 5 mL of water and 6.5 mL of 8.14 mol L−1 HClO4, then incubated for 15 min in boiling water with vortex every 5 min. This mixture was re-centrifuged at 795 g for 10 min. The supernatants were filled up to 100 mL with water. The total sugar in this partially digested sample was determined using the anthron method (CitationTrevelyan and Harrison 1952). The 80% ethanol extract was used for determining the soluble sugar concentration using the phenol-hydrocloride method (CitationDubois et al. 1956).
RESULTS AND DISCUSSION
shows the plants in pots with three levels of N treatment at the flowering stage on 22 September 2002. Different levels of N supply affected plant growth, and the number of plants per cluster increased with higher N supply (50-N > 25-N > 0-N). Curcuma plants form one new rhizome at the basal position of one shoot. Some shoots form a flower stalk and flower at the top position. In this experiment, all shoots made a flower so the number of flowers and rhizomes was the same (). The number and dry weight (DW) of new rhizomes, organs for the new generation, increased with the N feeding level (). In contrast, the DW of the storage roots decreased with N supply (, ).
shows the concentration of various compounds in the rhizomes and storage roots with each treatment. The total N concentrations in the rhizomes and storage roots were significantly higher in the 50-N and 25-N treatments compared with the 0-N treatment. Approximately 5.54% of the total N in the rhizomes and 9.41% of the total N in the storage roots is in 80% ethanol soluble fractions. This indicates that the majority of the
Table 1 Dry weight of rhizomes and storage roots and the number of flowers and rhizomes
Figure 1 (A) Effects of N supply on plants grown on 22 September 2002 and harvested on 2 December 2002. Underground parts from the (B) 50-N, (C) 25-N and (D) 0-N treatments.
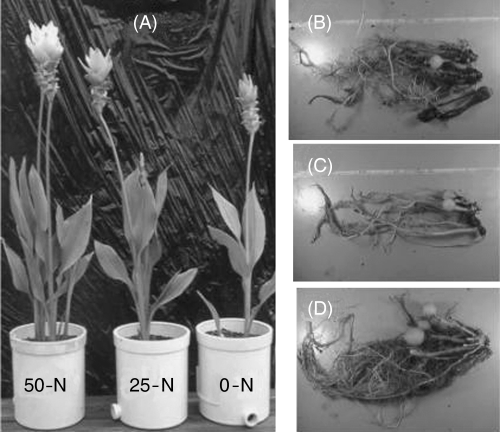
Table 2 Nitrogen, amino acid, protein and carbohydrate concentration in rhizomes and storage roots under different N treatments
Nitrogen application affected not only the level of nitrogenous compounds but also the starch concentration in the rhizomes and storage roots. The starch concentration decreased in the rhizomes and storage roots of N-supplied plants compared with 0-N plants.
In addition, N supply affected the protein concentration in the rhizomes and storage roots. The rhizomes and storage roots in the 50-N treatment accumulated approximately 197.4 mg g DW−1 and 46.7 mg g DW−1 proteins, respectively. Lack of N (0-N treatment) decreased protein concentrations in both the rhizomes and the storage roots. The protein profiles in rhizomes separated by SDS-PAGE are shown in . The
Figure 2 Sodium dodecylsulfate-polyacrylamide gel electrophoresis profiles and the density level of rhizomes and storage roots treated with different levels of N supply.
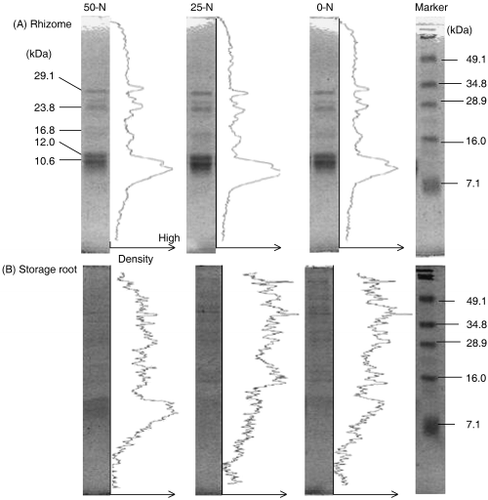
In the storage roots, the total N concentration was almost the same as that in the rhizomes and was mostly fractionated into 80% ethanol insoluble fractions. In contrast to the rhizomes, the protein concentration was lower in storage roots (). To partially characterize what compound(s) were accumulated in storage roots, the N of the 25-N samples was investigated. The N compound(s) of the storage roots was soluble in a potassium phosphate buffer (PSB) buffer (80.3% of total N) and fractionated in 10% TCA soluble fractions (92.1% of PBS soluble N). These results indicate that these compounds have a high hydrophilic character unusual in proteins.
Cassava and Pachyrhizus are perennial crops in which the tuberous roots act as storage organs for carbohydrates rather than as propagules. In contrast, the tubers of yam bean, sweet potato and potato act as propagules and contain storage proteins for mobilization during sprouting and plant re-growth (CitationForsyth and Shewry 2002). The same roles might apply to curcuma; rhizome as propagule and storage root as storage organ for carbohydrates. Increasing N supply enhanced flower and rhizome number, resulting in enhanced plant growth, while the formation of a storage root might be influenced by more complex nutrient conditions, such as the C and N balance. From the results obtained in this experiment, we demonstrated that high N supply to curcuma plants depressed storage root growth and highly enhanced protein accumulation in the rhizome. These results suggest that the carbons from photosynthesis were predominantly used not for starch synthesis in the storage root but for protein synthesis in the rhizome, thus preventing a morphological change in storage root formation.
By investigation of the N and carbohydrate constituents in rhizomes and storage roots, it was reported that the rhizome is the principal organ for N storage and the storage root is the major organ for carbohydrate storage, such as starch and soluble sugar (CitationRuamrungsri et al. 2001). In the present study, the application of N enhanced both the N concentration in the storage roots and the rhizomes to the same level. It is well known that plant species store N as protein and/or amino acids. Tulip species, in particular, store N as 4-methlyeneglutamine (CitationOhyama et al. 1985, Citation1986). The curcuma plant mainly stores N as protein in the rhizomes, while the storage roots of curcuma plants accumulate N differently from amino acids or protein compound(s) and this corresponds to the N fertilization level.
In this study, we demonstrated that N fertilization of curcuma plants increased their growth and produced new rhizomes, while a high N supply reduced storage roots. Furthermore, the N-containing compound(s) in the storage roots increased with N application and these compound(s) may play an important role in the storage of N in the storage root.
ACKNOWLEDGMENT
We thank the Hitachi Scholarship Foundation for supporting Dr Soraya Ruamrungsri to do research work in Japan.
REFERENCES
- Apavatjrut , P , Somboon , A , Puangpen , S and Chiara , A . 1999 . Molecular markers in the identification of some early flowering curcuma L. (Zingiberaceae) species . AnnBot , 84 : 529 – 534 .
- Takano , K and Azuma , A . 1996 . Studies on the flowering control of Curcuma alismatifolia Hort. III. The function and formation process of storage root . BullKouchi AgricResCent , 5 : 66 – 71 . (in Japanese)
- RuamrungsriS ApavatjrutP2003 Effect of nutrient deficiency on the growth and development of Curcuma alismatifolia Gagnep . Proc. of the 3rd Symposium on the family Zingiberaceae . July 7–12 . pp. 98104 Khon KaenThailand .
- Ruamrungsri , S , Ohtake , N , Sueyoshi , K and Ohyama , T . 2006 . Determination of uptake and utilization of nitrogen in Curcuma alismatifolia Gagnep. using 15N isotope . Soil SciPlant Nutri , 52 : 221 – 225 .
- Ohyama , T , Ito , M Kobayashi , K . 1991 . Analytical procedures of N, P, K contents in plant and manure materials using H2SO4–H2O2Kjeldahl digestion method . BullFaculAgricNiigata Univ , 43 : 111 – 120 . (in Japanese)
- Takahashi , Y , Chinushi , T and Ohyama , T . 1993 . Quantitative estimation of N2fixation and absorption rate in field grown soybean plants by relative ureide method . BullFaculAgricNiigata Univ , 45 : 91 – 105 .
- Lowry , OH , Rosebrough , NJ , Farr , AL and Randall , RJ . 1951 . Protein measurement with the Folin phenol reagent . JBiolChem , 193 : 265 – 275 .
- Ohtake , N , Suzuki , M Takahashi , Y . 1997 . Differential expression of β-conglycinin genes in nodulated and non-nodulated isolines of soybean . PhysiolPlant , 96 : 101 – 110 .
- Laemmli , UK . 1970 . Cleavage of structural proteins during the assembly of the head of bacteriophage T4 . Nature , 227 : 680 – 685 .
- Ohsaki , M . 1990 . “ Analytical methods for organic compounds ” . In Experimental Methods in Plant Nutrition , Edited by: Japanese Society for Soil Science and Plant Nutrition . 204 – 216 . Tokyo : Hakuyu-sha .
- Trevelyan , WE and Harrison , JS . 1952 . Studies on yeast metabolism. 1. Fractionation and microdetermination of cell carbohydrates . BiochemJ , 50 : 298 – 303 .
- Dubois , M , Gilles , KA , Hamilton , JK , Rebers , PA and Smith , F . 1956 . Colorimetric methods for determination of sugars and related substances . AnalChem , 28 : 350 – 356 .
- Forsyth , JL and Shewry , PR . 2002 . Characterization of the major proteins of tubers of yam bean (Pachyrhizus ahia) . JAgricFood Chem , 50 : 1939 – 1944 .
- Ruamrungsri , S , Ohtake , N , Sueyoshi , K , Suwanthada , C , Apavatjrut , P and Ohyama , T . 2001 . Changes in nitrogenous compounds, carbohydrates and abscisic acid in Curcuma alismatifolia Gagnep. during dormancy . JHortSciBiotech , 76 : 48 – 51 .
- Ohyama , T , Ikarashi , T and Baba , A . 1985 . Nitrogen accumulation in the roots of tulip plants (Tulipa gesneriana) . Soil SciPlant Nutr , 31 : 581 – 588 .
- Ohyama , T , Ikarashi , T and Baba , A . 1986 . Analysis of the reserve carbohydrate in bulb scales of autumn planting bulb plant . JpnJSoilSciPlant Nutr , 57 : 119 – 125 . (in Japanese)
- Ruamrungsri , S , Ohtake , N , Sueyoshi , K , Suwanthada , C , Ohyama , T and Apavatjrut , P . 2005 . Effect of nitrogen and potassium on growth and development of Curcuma alismatifolia Gagnep . Acta Hort , 673 : 443 – 448 .